Abstract
As sperm production is costly, males are expected to strategically allocate resources to sperm production according to mating opportunities. While sperm number adjustments have been reported in several taxa, only a few studies investigated whether sperm quality shows adaptive plasticity as well. We tested this prediction in the guppy, Poecilia reticulata. A total of 46 males were initially stripped of all retrievable sperm before being randomly allocated to one of two treatments simulating different levels of mating opportunities (visual contact with females or female deprived). After 3 days, males were stripped and sperm velocity was assayed using Computer Assisted Sperm Analysis. Males in the presence of females produced significantly faster sperm than their counterparts. Implications for the evolution of this ejaculate plasticity in the light of results of sperm competition studies are discussed.
Keywords: sperm competition, post-copulatory sexual selection, sperm priming
1. Introduction
On the basis of the low unitary cost of producing sperm, it has long been assumed that sperm are cheap and males are rarely, if ever, sperm limited. In contrast to this view, there is now ample evidence that sperm production is costly (see Wedell et al. 2002 for a review) and that sperm production is strategically adjusted according to mating opportunities and hence, expected sperm use (Shapiro et al. 1994; Vermeirssen et al. 1997; Bozynski & Liley 2003; Aspbury & Gabor 2004). Recently, it has been recognized that not only do numbers influence fertilization success, but also sperm quality traits such as viability and velocity have a great impact on male fitness (reviewed in Snook 2005). In particular, elevated sperm velocity is often associated with high fertilization success both in presence (Birkhead et al. 1999; Gage et al. 2004; Denk et al. 2005) and in the absence (Froman et al. 1999; Levitan 2000) of sperm competition. Producing sperm with enhanced swimming performances is likely to be costly, as higher sperm velocity and mobility are associated with higher ATP content (Burness et al. 2004; Locatello et al. 2007). Recently, it has been shown that sperm quality traits are adjusted in response to the perceived level of sperm competition (Kilgallon & Simmons 2005; Rudolfsen et al. 2006; Simmons et al. 2007; but see Evans 2009) and female quality (Cornwallis & Birkhead 2007). Only one study investigated the influence of the female presence on sperm quality and the authors did not find any effects on sperm motility (Liley et al. 2002).
Using the guppy (Poecilia reticulata), a freshwater fish with internal fertilization, we investigate whether sperm velocity is associated with the presence of potential mates. We measured sperm swimming velocity in two groups of males after 3 days of treatment. During this period males were maintained either in visual contact with females or completely female-deprived. We chose a 3 day treatment as adaptive plasticity in sperm production (‘sperm priming’) was demonstrated over this period in guppy males (Bozynski & Liley 2003). We predict that males maintained in the presence of females will produce faster sperm than males isolated from females.
2. Material and methods
(a). Experimental design
Guppies used in this experiment were descendants of wild-caught fish from the lower part of Tacarigua River, Trinidad. Each experimental male was stripped to completely deplete sperm reserves from ‘ready sperm’ (see Bozynski & Liley 2003; Evans 2009) before the start of each treatment. After stripping, the male was revived in a small tank with fresh water and allowed to rest for 24 h. Each male was placed individually in a small transparent tank, right next to an identical container which either contained three females (‘high mating opportunities’ treatment, HM hereafter) or was empty (‘low mating opportunities’ treatment, LM hereafter). After 3 days of exposure, the male was stripped again and sperm were collected for subsequent velocity assay (see below). At the same time, each male was photographed with a digital camera (Nikon Coolpix 4300) and images were analyzed using Image Tool software (v. 3.0, UTHSCSA). Body size and the area of colour spots (divided in orange, black and iridescent) were measured.
(b). Sperm velocity assay
Immediately after stripping, ejaculate was collected with a Drummond 3 µl micropipette. In this species, sperm are packaged into oval-shaped bundles (i.e. spermatozeugmata). Each bundle, one at a time, was placed on a glass slide previously coated with silicone spray to reduce sperm sticking to the glass surface. Once the bundle was set, 10 µl of activating solution (150 mM of KCl and 2 mg ml−1 bovine serum albumin, see Billard & Cosson 1990) was added, and gently covered with a coverslip. Sperm were analysed using a Ceros Sperm Tracker (v. 12.3; Hamilton Thorne Research, Beverly, MA, USA). For each male, sperm velocity was measured in two different spermatozeugmata and the average was used in the analysis. Measurements of sperm velocity, taken blind of the experimental group, were based on 178.8 ± 13.90 (mean ± SE) motile sperm (range: 37–399) and include average path velocity (VAP) and curvilinear velocity (VCL). The threshold values defining static cells were predetermined at 25 µm s−1 (Locatello et al. 2006; Evans 2009). The within-male repeatability estimated following Becker (1984) was: 0.70 ± 0.08 SE (p < 0.001) for VAP and 0.58 ± 0.11 SE (p < 0.001) for VCL. These values are comparable to analogous studies of repeatability of sperm velocity within the same ejaculate (Locatello et al. 2006). Statistical analyses were performed using SPSS v. 16.0.
3. Results
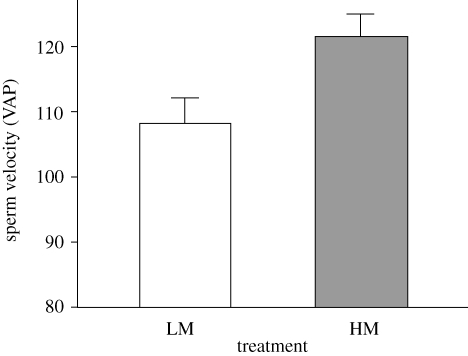
Males that were randomly assigned to one of two treatments, did not differ in any of morphological traits we considered, such as body size and coloration (ANOVA, all differences between treatments: p > 0.249). According to prediction, males produced significantly faster sperm in the presence of females (VAP, HM males: 121.82 ± 3.35 n = 23; LM males: 108.35 ± 3.94, n = 23; F1,45 = 6.784; p = 0.012; see figure 1; VCL, HM: 146.76 ± 2.61 n = 23; LM: 137.48 ± 3.62, n = 23; F1,45 = 4.337, p = 0.043). Sperm velocity remained significantly different between treatments also after controlling for morphological male traits (table 1).
Figure 1.
Sperm swimming velocity (VAP; mean±SE) in males maintained for 3 days in conditions simulating low (LM) and high mating opportunities (HM).
Table 1.
Results from ANCOVA for sperm swimming velocity according to experimental treatment (high versus low mating opportunities) and male phenotypic traits.
| VAP |
VCL |
|||||
|---|---|---|---|---|---|---|
| F-value | p-value | b ± SE | F-value | p-value | b ± SE | |
| treatmenta | 5.912 | 0.020 | −13.52 ± 5.56 | 4.247 | 0.046 | −9.02 ± 4.38 |
| orange coloration | 2.772 | 0.104 | 1.66 ± 1.00 | 9.288 | 0.004 | 2.39 ± 0.78 |
| black coloration | 0.008 | 0.927 | −0.41 ± 4.42 | 0.621 | 0.436 | −2.74 ± 3.48 |
| iridescent coloration | 1.266 | 0.268 | −0.95 ± 0.85 | 1.281 | 0.265 | −0.75 ± 0.67 |
| body size (area) | 0.106 | 0.747 | 0.09 ± 0.27 | 0.266 | 0.609 | −0.11 ± 0.21 |
aHM treatment as factor's reference level.
4. Discussion
Our results suggest that male guppies adjust sperm velocity according to the availability of potential mates. Males which were maintained in visual contact with females produced faster sperm than males in the absence of females. Although in this study we did not assess the number of sperm produced, previous work by Bozynski & Liley (2003) and observations from the same laboratory population (C. Gasparini & A. Pilastro 2006, unpublished results) showed that males respond to female presence by increasing the number of ‘ready sperm’. Taken together, these results suggest that male guppies show adaptive plasticity both in the quantity and in the quality of the ‘ready sperm’ in response to the perceived mating opportunities. While an increase in sperm production in the presence of females has been reported in several taxa (Olsen & Liley 1993; Vermeirssen et al. 1997; Aspbury & Gabor 2004; Olsen et al. 2006), this is the first study, to our knowledge, to demonstrate an adjustment in sperm quality in response to mating opportunities.
A previous study in the rainbow trout did not show an effect of female presence on sperm motility (Liley et al. 2002), while another study demonstrated that in the fowl Gallus gallus, males allocate faster sperm with high-quality females (Cornwallis & Birkhead 2007). An increased sperm production associated with increased mating opportunities is expected in the guppy, as males can deliver to the female, during a single copulation, up to 92 per cent of their available sperm (Pilastro & Bisazza 1999). One may wonder, however, why male guppies respond to the increased mating opportunities by increasing sperm velocity. Our result is particularly surprising, as male guppies do not adjust the number and quality of sperm that are primed in response to the perceived level of sperm competition (Evans 2009), despite sperm competition being intense in this species (Hain & Neff 2007). One explanation may be that the actual sex ratio does not predict the level of sperm competition associated with a given mating. Indeed, female guppies actively solicit mating with multiple males during the receptive period (Houde 1997). Furthermore, females are continuously exposed to forced matings, which can result in large numbers of sperm inseminated (Pilastro et al. 2002). Males do not associate permanently with a female, but after a mating usually turn their attention to other females (Houde 1997). Thus, males may not be able to predict whether a female will mate again (or has already mated) with other males (Engqvist & Reinhold 2005). Therefore, if most matings are associated with high levels of sperm competition (Neff et al. 2008), males may adjust their sperm quality in response to mating opportunities only. Experiments of artificial insemination found that both sperm number and velocity are significant predictors of sperm competition success (C. Boschetto, C. Gasparini & A. Pilastro 2006, unpublished data), suggesting that both features are important for sperm competition success in the guppy.
Interestingly, the positive association between sperm velocity (VCL) and orange coloration we found corroborates previous findings (Locatello et al. 2006), suggesting that colourful, high-quality males (Evans et al. 2004) are probably able to produce both more numerous (Pitcher & Evans 2001) and faster sperm (see also Evans et al. 2003). Whether sperm priming strategies of males are associated with their attractiveness represents an interesting avenue for future research.
Acknowledgments
The research was carried out in conformity with the relevant Italian Laws governing the care of animals in research (D.L. 116/27.01.92, C.M.S. 8/22-04-94) and was authorized by the Instituto Superiore di Sanita (Italian National Health Institute).
This research was funded by grants from the University of Padova (Grandi Attrezzature 2005) and the CARIPARO (Progetto di Eccellenza 2007) to A.P. A.V.P. was supported by the Coimbra Group Scholarships Programme.
References
- Aspbury A. S., Gabor C. R.2004Differential sperm priming by male sailfin mollies (Poecilia latipinna): effects of female and male size. Ethology 110, 193–202 (doi:10.1111/j.1439-0310.2003.00963.x) [Google Scholar]
- Becker W. A.1984A manual of quantitative genetics Washington, DC: Pullman Academic Enterprises [Google Scholar]
- Billard R., Cosson M. P.1990The energetics of fish sperm motility. In Controls of sperm motility: biological and clinical aspects (ed. Gagnon C.), pp. 155–173 Boca Raton, FL: CRC Press [Google Scholar]
- Birkhead T. R., Martinez J. G., Burke T., Froman D. P.1999Sperm mobility determines the outcome of sperm competition in the domestic fowl. Proc. R. Soc. Lond. B 266, 1759–1764 (doi:10.1098/rspb.1999.0843) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozynski C. C., Liley N. R.2003The effect of female presence on spermiation, and of male sexual activity on ‘ready’ sperm in the male guppy. Anim. Behav. 65, 53–58 (doi:10.1006/anbe.2002.2024) [Google Scholar]
- Burness G., Casselman S. J., Schulte-Hostedde A. I., Moyes C. D., Montgomerie R.2004Sperm swimming speed and energetics vary with sperm competition risk in bluegill (Lepomis macrochirus). Behav. Ecol. Sociobiol. 56, 65–70 (doi:10.1007/s00265-003-0752-7) [Google Scholar]
- Cornwallis C. K., Birkhead T. R.2007Changes in sperm quality and numbers in response to experimental manipulation of male social status and female attractiveness. Am. Nat. 170, 758–770 (doi:10.1086/521955) [DOI] [PubMed] [Google Scholar]
- Denk A. G., Holzmann A., Peters A., Vermeirssen E. L. M., Kempenaers B.2005Paternity in mallards: effects of sperm quality and female sperm selection for inbreeding avoidance. Behav. Ecol. 16, 825–833 (doi:10.1093/beheco/ari065) [Google Scholar]
- Engqvist L., Reinhold K.2005Pitfalls in experiments testing predictions from sperm competition theory. J. Evol. Biol. 18, 116–123 (doi:10.1111/j.1420-9101.2004.00792.x) [DOI] [PubMed] [Google Scholar]
- Evans J. P.2009No evidence for sperm priming responses under varying sperm competition risk or intensity in guppies. Naturwissenschaften 96, 771–779 (doi:10.1007/s00114-009-0529-6) [DOI] [PubMed] [Google Scholar]
- Evans J. P., Zane L., Francescato S., Pilastro A.2003Directional postcopulatory sexual selection revealed by artificial insemination. Nature 421, 360–363 (doi:10.1038/nature01367) [DOI] [PubMed] [Google Scholar]
- Evans J. P., Kelley J. L., Bisazza A., Finazzo E., Pilastro A.2004Sire attractiveness influences offspring performance in guppies. Proc. R. Soc. Lond. B 271, 2035–2042 (doi:10.1098/rspb.2004.2815) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froman D. P., Feltmann A. J., Rhoads M. L., Kirby J. D.1999Sperm mobility: a primary determinant of fertility in the domestic fowl (Gallus domesticus). Biol. Reprod. 61, 400–405 (doi:10.1095/biolreprod61.2.400) [DOI] [PubMed] [Google Scholar]
- Gage M. J. G., Macfarlane C. P., Yeates S., Ward R. G., Searle J. B., Parker G. A.2004Spermatozoal traits and sperm competition in Atlantic salmon: relative sperm velocity is the primary determinant of fertilization success. Curr. Biol. 14, 44–47 [PubMed] [Google Scholar]
- Hain T. J. A., Neff B. D.2007Multiple paternity and kin recognition mechanisms in a guppy population. Mol. Ecol. 16, 3938–3946 (doi:10.1111/j.1365-294X.2007.03443.x) [DOI] [PubMed] [Google Scholar]
- Houde A. E.1997Sex, color, and mate choice in guppies Princeton, NJ: Princeton University Press [Google Scholar]
- Kilgallon S. J., Simmons L. W.2005Image content influences men's semen quality. Biol. Lett. 1, 253–255 (doi:10.1098/rsbl.2005.0324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan D. R.2000Sperm velocity and longevity trade off each other and influence fertilization in the sea urchin Lytechinus variegatus. Proc. R. Soc. Lond. B 267, 531–534 (doi:10.1098/rspb.2000.1032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liley N. R., Tamkee P., Tsai R., Hoysak D. J.2002Fertilization dynamics in rainbow trout (Oncorhynchus mykiss): effect of male age, social experience, and sperm concentration and motility on in vitro fertilization. Can. J. Fish. Aquatic Sci. 59, 144–152 (doi:10.1139/f01-202) [Google Scholar]
- Locatello L., Rasotto M. B., Evans J. P., Pilastro A.2006Colourful male guppies produce faster and more viable sperm. J. Evol. Biol. 19, 1595–1602 (doi:10.1111/j.1420-9101.2006.01117.x) [DOI] [PubMed] [Google Scholar]
- Locatello L., Pilastro A., Deana R., Zarpellon A., Rasotto M. B.2007Pattern of variation of sperm quality traits in two gobies with alternative mating tactics. Funct. Ecol. 21, 975–981 (doi:10.1111/j.1365-2435.2007.01314.x) [Google Scholar]
- Neff B. D., Pitcher T. E., Ramnarine I. W.2008Inter-population variation in multiple paternity and reproductive skew in the guppy. Mol. Ecol. 17, 2975–2984 (doi:10.1111/j.1365-294X.2008.03816.x) [DOI] [PubMed] [Google Scholar]
- Olsen K. H., Liley N. R.1993The significance of olfaction and social cues in milt availability, sexual hormone status, and spawning behavior of male rainbow-trout (Oncorhynchus-Mykiss). Gen. Comp. Endocrinol. 89, 107–118 (doi:10.1006/gcen.1993.1014) [DOI] [PubMed] [Google Scholar]
- Olsen K. H., Sawisky G. R., Stacey N. E.2006Endocrine and milt responses of male crucian carp (Carassius carassius L.) to periovulatory females under field conditions. Gen. Comp. Endocrinol. 149, 294–302 (doi:10.1016/j.ygcen.2006.06.011) [DOI] [PubMed] [Google Scholar]
- Pilastro A., Bisazza A.1999Insemination efficiency of two alternative male mating tactics in the guppy (Poecilia reticulata). Proc. R. Soc. Lond. B 266, 1887–1891 [Google Scholar]
- Pilastro A., Evans J. P., Sartorelli S., Bisazza A.2002Male phenotype predicts insemination success in guppies. Proc. R. Soc. Lond. B 269, 1325–1330 (doi:10.1098/rspb.2002.2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher T. E., Evans J. P.2001Male phenotype and sperm number in the guppy (Poecilia reticulata). Can. J. Zool. 79, 1891–1896 (doi:10.1139/cjz-79-10-1891) [Google Scholar]
- Rudolfsen G., Figenschou L., Folstad I., Tveiten H., Figenschou M.2006Rapid adjustments of sperm characteristics in relation to social status. Proc. R. Soc. B 273, 325–332 (doi:10.1098/rspb.2005.3305) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro D. Y., Marconato A., Yoshikawa T.1994Sperm economy in a coral-reef fish, Thalassoma bifasciatum. Ecology 75, 1334–1344 (doi:10.2307/1937458) [Google Scholar]
- Simmons L. W., Denholm A., Jackson C., Levy E., Madon E.2007Male crickets adjust ejaculate quality with both risk and intensity of sperm competition. Biol. Lett. 3, 520–522 (doi:10.1098/rsbl.2007.0328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snook R. R.2005Sperm in competition: not playing by the numbers. Trends Ecol. Evol. 20, 46–53 (doi:10.1016/j.tree.2004.10.011) [DOI] [PubMed] [Google Scholar]
- Vermeirssen E. L. M., Scott A. P., Liley N. R.1997Female rainbow trout urine contains a pheromone which causes a rapid rise in plasma 17, 20 beta-dihydroxy-4-pregnen-3-one levels and milt amounts in males. J. Fish Biol. 50, 107–119 [Google Scholar]
- Wedell N., Gage M. J. G., Parker G. A.2002Sperm competition, male prudence and sperm-limited females. Trends Ecol. Evol. 17, 313–320 (doi:10.1016/S0169-5347(02)02533-8) [Google Scholar]