Abstract
Avian timing of reproduction is strongly affected by ambient temperature. Here we show that there is an additional effect of sunspots on laying date, from five long-term population studies of great and blue tits (Parus major and Cyanistes caeruleus), demonstrating for the first time that solar activity not only has an effect on population numbers but that it also affects the timing of animal behaviour. This effect is statistically independent of ambient temperature. In years with few sunspots, birds initiate laying late while they are often early in years with many sunspots. The sunspot effect may be owing to a crucial difference between the method of temperature measurements by meteorological stations (in the shade) and the temperatures experienced by the birds. A better understanding of the impact of all the thermal components of weather on the phenology of ecosystems is essential when predicting their responses to climate change.
Keywords: timing of reproduction, sunspots, birds, Parus major, Cyanistes caeruleus
1. Introduction
Timing of reproduction is a major component of fitness in many organisms. It has often been shown that birds start egg laying earlier in years with a warmer spring (Crick et al. 1997; McCleery & Perrins 1998; Dunn 2004) and that laying date has advanced over recent decades owing to climate change (Parmesan & Yohe 2003). These shifts in phenology are, together with range shifts, the most obvious ecological consequences of climate change (Parmesan 2006). The shifts in phenology can lead to directional selection for earlier laying, owing to a mismatch between food demands and food availability (Visser et al. 1998; Nussey et al. 2005) and, as a consequence of this mistiming, to population declines (Both et al. 2006). A better understanding of how climate, in all its aspects, affects organisms is crucial for our predictions of the effects of climate change.
Timing of reproduction is strongly affected by ambient temperatures as measured by meteorological stations. However, it remains to be shown that these are the most relevant temperatures as experienced by free living organisms. For example, it is well known that birds actively seek places in the sun during winter (Carrascal et al. 2001), which may be substantially warmer than those measured in the shade (as done by meteorological stations). Solar activity, where measurements are sunspot numbers, is often correlated with population densities (Sinclair & Gosline 1997; Klvana et al. 2004; Selas et al. 2004). Sunspots influence both the Sun's luminosity and the Earth's climate (Foukal et al. 2006). The extent to which sunspots affect Earth's climate is still largely debated, but it is believed that an increase in sunspots can reduce the amount of energy and light distributed to the Earth (Foukal et al. 2006). Here, we will correlate sunspot numbers with laying dates in five Dutch long-term studies on great and blue tit (Parus major and Cyanistes caeruleus) populations, correcting for temperatures as measured by meteorological stations.
2. Material and methods
Annual mean population laying dates have been recorded in five study populations of the Netherlands Institute of Ecology (1955–2006; 472 year–population–species combinations; table S1, electronic supplementary material). Nest-boxes were checked at least weekly, and laying dates of first clutches were calculated assuming that one egg per day is laid (n = 20 357). We have used these populations as independent measures for each species, but were interested in the effect of solar activity at a species level. Data on the phenology and maximum abundance of food during the chick-feeding period were collected by sampling caterpillar frass in the Hoge Veluwe area (Visser et al. 2006).
Daily ambient temperature (°C) was obtained from the Royal Netherlands Meteorological Institute's meteorological station in De Bilt (www.knmi.nl). Temperature was measured 1.5 m above the ground level in naturally ventilated radiation shields (Brandsma et al. 2003). Daily number of sunspots was obtained from the National Geophysical Data Center (ftp://ftp.ngdc.noaa.gov/STP/SOLAR_DATA). Daily total solar irradiance (TSI; W m−2; amount of radiant energy over all wavelengths that fall each second on one square metre outside the Earth's atmosphere) was obtained from the Physikalisch-Meteorologisches Observatorium Davos (ftp://ftp.pmodwrc.ch/pub/data/irradiance/composite; no data available prior to 1976; Fröhlich & Lean 2004). Daily solar radiation (J cm−2) at the ground level was obtained from the meteorological station in De Bilt (1958–2006). The mean of these variables was calculated for the period 16 March to 20 April (the period for which the laying dates correlate best with temperature (Visser et al. 2006)).
We fitted a general linear mixed model with mean population laying date as the response variable and as explanatory variables species and population (as discrete factors) and ambient shade temperature, sunspots and year (as continuous covariates) as well as all two-way interactions. Year (as a factor), population, species and their interactions were also included as random effects to account for the fact that all populations were exposed to the same annual abiotic variables (see Visser et al. 2003, for a similar approach). Only significant terms are presented. Analyses were weighted using the standard error of the laying date (1/(s.e.)2). The degrees of freedom of the fixed effects were adjusted using Satterthwaite's procedure (Satterthwaite 1946).
3. Results
We found that sunspot numbers and TSI were positively correlated (1976–2006; r30 = 0.85, p < 0.001). In contrast, solar radiation at the ground level (r48 = 0.24, p = 0.10) and ambient shade temperature (r51 = 0.12, p = 0.39; figure S1, electronic supplementary material) were not correlated with sunspot numbers.
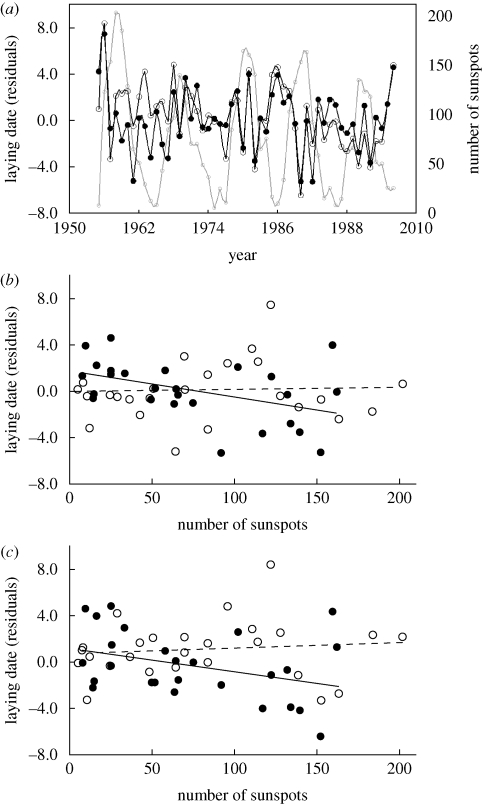
Laying dates were correlated not only with spring ambient shade temperature (Visser et al. 2006), species and population (Visser et al. 2003) but also with sunspot numbers (table 1). In years with few sunspots, the birds often start egg laying late, while in years with many sunspots, they often lay early (figure 1a). Apparently, in years with high solar activity, the birds can be held back (for reasons other than temperature, which we include in the model) and lay late, but in years with low solar activity, the birds are often late relative to what is expected from the effect of spring temperature. The sunspots effect on laying date has become stronger over our 52-year study period (table 1 and figure 1b,c; figure S2, electronic supplementary material). The same results were obtained when sunspots were replaced by TSI (table S2, electronic supplementary material).
Table 1.
Annual mean population laying dates (1955–2006) in relation to ambient temperature and sunspot numbers for two species (the great tit and the blue tit) in five Dutch populations (HV, VL, OH, LB and WB).
| variable | F-value | p-value | level | estimate (s.e.) |
|---|---|---|---|---|
| sunspots | F1,48.7 = 4.57 | 0.038 | 1.731 (0.780) | |
| sunspots * year | F1,48.6 = 4.63 | 0.036 | −0.00088 (0.00039) | |
| corrected for | ||||
| year | F1,48.7 = 0.17 | 0.682 | ||
| species * year | F1,51.6 = 18.51 | <0.001 | GT | 0.048 (0.039) |
| BT | −0.016 (0.015) | |||
| temperature | F1,47 = 93.91 | <0.001 | ||
| population * temperature | F4,12 = 4.41 | 0.020 | HV | −3.087 (0.356) |
| VL | −2.234 (0.361) | |||
| LB | −3.236 (0.361) | |||
| OH | −3.545 (0.363) | |||
| WB | −2.913 (0.393) | |||
| species * temperature | F1,42.3 = 8.37 | 0.006 | GT | −2.913 (0.393) |
| BT | −2.415 (0.171) |
Figure 1.
(a) Annual fluctuation in laying date compared with fluctuations in sunspot numbers. Laying date of (b) great and (c) blue tits versus sunspots. Because of the interaction between year (as a continuous variable) and sunspots, the data have been split solely for the purposes of illustration into those for 1955–1980 (open circle, dashed line) and for 1981–2006 (filled circle, solid line). Laying dates of both species are the residuals from the model, with population and temperature as explanatory variables.
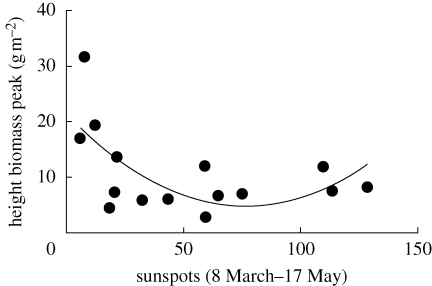
Although we have only 15 years' data on caterpillar biomass in one of the study areas, there was a sunspot effect on the height of the biomass peak (figure 2), but no effect on the date of peak food abundance (correcting for temperature): p = 0.68 for the quadratic term and p = 0.29 for the linear term.
Figure 2.
Data on peak caterpillar biomass for 15 years at the HV population. There is a significant negative effect of sunspot numbers (linear term: F1,12 = 7.31, p = 0.019; quadratic term: F1,12 = 5.33, p = 0.039) on biomass. There is no effect of temperature (F1,11 = 1.92, p = 0.19). Both the mean temperature and the number of sunspots were calculated for the period 8 March to 17 May (i.e. the period for which caterpillar phenology correlates best with mean temperature (Visser et al. 2006)).
4. Discussion
We show for the first time that not only population densities but also seasonal timing are statistically related to sunspots (Sinclair & Gosline 1997; Klvana et al. 2004; Selas et al. 2004). The same conclusion is drawn when the analyses are performed per species (table S3a,b, electronic supplementary material), showing that our finding is robust. But how would sun activity affect timing? We explore two hypotheses.
One hypothesis is that ambient temperatures as measured by the meteorological services differ from that experienced by birds (Carrascal et al. 2001) and that solar activity affects this difference. Although in high sunspot years more radiation enters the atmosphere (Foukal et al. 2006), there is no relationship between solar activity and the radiation reaching the surface. This makes it less likely that sun activity directly affects the differences between experienced temperatures and temperatures measured in the shade. However, the perception of the thermal component of weather results from the integral effects of all meteorological parameters relevant for heat exchange between the organism's body and its environment. The animals' heat flow is affected directly by air temperature, vapour pressure, wind velocity and mean radiant temperature of the surroundings (Höppe 1999). Therefore, a genuine climate index considering the influence of all these parameters should be used to understand how climate affects organisms in a physiologically relevant way. A different climate index had been proposed, such as the physiological equivalent temperature (Höppe 1999; Matzarakis et al. 1999) for the biometeorological assessment of the thermal environment, and thus it remains to be studied how sunspot number or TSI may affect this thermal environment.
An alternative hypothesis to explain the effect of solar activity on avian timing is that there is an effect of solar activity on food abundance for the birds (Selas et al. 2004), for instance, on the abundance of food during the laying period. Potentially, a relationship between sunspots and this food abundance would be able to explain the stronger sunspot effect on laying dates over time: over the past 30 years, there has been increasing selection on early laying in great tits (Visser et al. 1998, 2006), and this may have led to birds having become more constrained during the laying period. In years with many insects, this constraint may be lifted. However we have no data on food availability during this period. Alternatively, sun activity may affect food abundance during chick feeding. For insectivorous birds, the abundance of arthropods at the time of maximum food requirement of their young is a crucial determinant of fitness (Lack 1968). In Norway, caterpillar abundance was negatively related to sunspots (Selas et al. 2004). High UV-B radiation in periods of low solar activity reduces the herbivore resistance of trees and thus increases the survival of caterpillars (Selas et al. 2004). In the present study, the abundance of caterpillars (Visser et al. 2006) is indeed negatively correlated with sunspots (figure 2). But there is, however, no effect of sun activity on the date at which caterpillar biomass peaks, and hence it is unlikely that sun activity affects avian timing via an effect on the phenology of the nestlings' food.
To assess the ecological consequences of climate change, it is essential to predict the rate at which species can adapt (Visser 2008). To predict changes in timing, we need to use the ecologically most relevant temperatures. We suggest that the temperatures measured by meteorological stations may not be the most ecologically relevant. Hence, we need to take into account also other thermal components such as sunspots, radiation reaching the ground and wind speed near ground level, which affect the deviation of the temperatures as perceived by the organisms from the temperatures as reported by these meteorological stations.
Acknowledgements
We thank Jan Visser, Piet de Goede and the late Huybert van Eck for their efforts in the field and Jan Visser for careful management of the great tit data that form the basis of this paper. We thank the board of the National Park ‘de Hoge Veluwe’, the State Forestry Service at Vlieland and Breda (Liesbos), het Gelders Landschap (Warnsborn) and Barones van Boetzelaer van Oosterhout for permission to work in their woodlands for all these years. We thank the reception of the irradiance dataset (ext_composite_d41_62_0901) from PMOD/WRC. J.J.S. was supported by the project GCL2007-61395/BOS and M.E.V. by an NWO-VICI grant.
References
- Both C., Bouwhuis S., Lessells C. M., Visser M. E.2006Climate change and population declines in a long-distance migratory bird. Nature 441, 81–83 (doi:10.1038/nature04539) [DOI] [PubMed] [Google Scholar]
- Brandsma T., Können G. P., Wessels H. R. A.2003Empirical estimation of the effect of urban heat advection on the temperature series of De Bilt (The Netherlands). Int. J. Climatol. 23, 829–845 (doi:10.1002/joc.902) [Google Scholar]
- Carrascal L. M., Diaz J. A., Huertas D. L., Mozetich I.2001Behavioral thermoregulation by tree creepers: trade-off between saving energy and reducing crypsis. Ecology 82, 1642–1654 [Google Scholar]
- Crick H. Q. P., Dudley C., Glue D. E., Thomson D. L.1997UK birds are laying eggs earlier. Nature 388, 526 (doi:10.1038/41453) [Google Scholar]
- Dunn P.2004Breeding dates and reproductive performance. Adv. Ecol. Res. 35, 69–87 (doi:10.1016/S0065-2504(04)35004-X) [Google Scholar]
- Foukal P., Fröhlich C., Spruit H., Wigley T. M. L.2006Variations in solar luminosity and their effect on the Earth's climate. Nature 443, 161–166 (doi:10.1038/nature05072) [DOI] [PubMed] [Google Scholar]
- Fröhlich C., Lean J.2004Solar radiative output and its variability: evidence and mechanisms. Astron. Astrophys. Rev. 12, 273–320 (doi:10.1007/S00159-004-0024-1) [Google Scholar]
- Höppe P.1999The physiological equivalent temperature—a universal index for the biometeorological assessment of the thermal environment. Int. J. Biometeorol. 43, 71–75 (doi:10.1007/S004840050118) [DOI] [PubMed] [Google Scholar]
- Klvana I., Berteaux D., Cazelles B.2004Porcupine feeding scars and climatic data show ecosystem effects of the solar cycle. Am. Nat. 164, 283–297 (doi:10.1086/423431) [DOI] [PubMed] [Google Scholar]
- Lack D.1968Ecological adaptations for breeding in birds London, UK: Methuen [Google Scholar]
- Matzarakis A., Mayer H., Iziomon M. G.1999Applications of a universal thermal index: physiological equivalent temperature. Int. J. Biometeorol. 43, 76–84 (doi:10.1007/S004840050119) [DOI] [PubMed] [Google Scholar]
- McCleery R. H., Perrins C. M.1998… temperature and egg-laying trends. Nature 391 (doi:10.1038/34073) [Google Scholar]
- Nussey D. H., Postma E., Gienapp P., Visser M. E.2005Selection on heritable phenotypic plasticity in a wild bird population. Science 310, 304–306 (doi:10.1126/science.1117004) [DOI] [PubMed] [Google Scholar]
- Parmesan C.2006Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 37, 637–669 (doi:10.1146/annurev.ecolsys.37.091305.110100) [Google Scholar]
- Parmesan C., Yohe G.2003A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42 (doi:10.1038/nature01286) [DOI] [PubMed] [Google Scholar]
- Satterthwaite F. E.1946An approximate distribution of estimates of variance components. Biometrics Bull. 2, 110–114 (doi:10.2307/3002019) [PubMed] [Google Scholar]
- Selas V., Hogstad A., Kobro S., Rafoss T.2004Can sunspot activity and ultraviolet-B radiation explain cyclic outbreaks of forest moth pest species? Proc. R. Soc. Lond. B 271, 1897–1901 (doi:10.1098/rspb.2004.2811) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair A. R. E., Gosline J. M.1997Solar activity and mammal cycles in the Northern Hemisphere. Am. Nat. 149, 776–784 (doi:10.1086/286020) [Google Scholar]
- Visser M. E.2008Keeping up with a warming world; assessing the rate of adaptation to climate change. Proc. R. Soc. B 275, 649–659 (doi:10.1098/rspb.2007.0997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M. E., van Noordwijk A. J., Tinbergen J. M., Lessells C. M.1998Warmer springs lead to mistimed reproduction in great tits (Parus major). Proc. R. Soc. Lond. B 265, 1867–1870 (doi:10.1098/rspb.1998.0514) [Google Scholar]
- Visser M. E., et al. 2003Variable responses to large-scale climate change in European Parus populations. Proc. R. Soc. Lond. B 270, 367–372 (doi:10.1098/rspb.2002.2244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M. E., Holleman L. J. M., Gienapp P.2006Shifts in caterpillar biomass phenology due to climate change and its impact on the breeding biology of an insectivorous bird. Oecologia 147, 164–172 (doi:10.1007/s00442-005-0299-6) [DOI] [PubMed] [Google Scholar]