Abstract
Predator foraging may be affected by previous prey capture, but it is unknown how nutrient balance affects foraging behaviour. Here, we use a trap-building predator to test whether nutrients from previous prey captures affect foraging behaviour. We fed orb-weaving spiders (Zygiella x-notata) prey flies of different nutrient composition and in different amounts during their first instar and measured the subsequent frequency of web building and aspects of web architecture. We found that both the likelihood of web building and the number of radii in the web were affected by prey nutrient composition while prey availability affected capture area and mesh height. Our results show that both the balance of nutrients in captured prey and the previous capture rate may affect future foraging behaviour of predators.
Keywords: foraging behaviour, prey quality, trap construction
1. Introduction
Predators hunt in order to gain access to energy and nutrients, and both nutritional needs and the composition of foods should be considered in models of optimal hunting strategies (Pulliam 1975; Simpson et al. 2004). However, while energy gain has been investigated in numerous foraging studies (e.g. Stephens & Krebs 1986), there has been little focus on how nutrients affect foraging (Pulliam 1975), particularly in predatory animals (Greenstone 1979). In the face of unbalanced nutrient availability, theory predicts that foragers should rely on compensatory feeding (Cruz-Rivera & Hay 2000), change their food preference (Mayntz et al. 2005), or change foraging habitat (Belovsky 1978). In the two latter cases, the strategy is to increase the chance of finding nutritionally complementary foods that contain the deficient nutrients. Thus, Shaner et al. (2007) found that Peromyscus mice would spend more time in a foraging patch containing variable food types that could be combined into a balanced diet.
An interesting feature of spider webs is that these traps not only catch nutrients but are also built up of nutrients; the costs of foraging therefore include both the energy used for movements while building the web (Peakall & Witt 1976) as well as the nutritional materials contained in the silk (Prestwich 1977). Although biomaterial costs may be reduced by recycling web materials, nutrient-limited spiders have to trade off their allocation of nutrients between trap building and other physiological functions (Higgins & Rankin 1999).
While a number of studies have investigated how foraging efforts (webs) may vary in response to variable food availabilities (Sherman 1994; Vollrath & Samu 1997; Heiling & Herberstein 2000), responses to variable prey nutrient composition have never been assessed. Recently, however, studies have shown that prey nutrient composition may affect the chemical composition of spider silk (Craig et al. 2000; Tso et al. 2005). Furthermore, orb-web spiders may produce varying mesh sizes of their webs in response to different species of prey intercepting their webs (Schneider & Vollrath 1998; Tso et al. 2007); but the extent to which such web adjustment is caused by prey nutrient composition or by different prey behaviour is not clear.
In this study we explore how the orb-weaving spider Zygiella x-notata adjusts foraging behaviour in response to changes in nutrient balance, in both well-fed and prey-limited conditions. In accordance with previous studies, we expected prey-limited spiders to increase the catching area and the mesh size of webs to increase the chance of capturing larger prey (Sherman 1994; Vollrath & Samu 1997). We predicted that nutrient-limited spiders would reduce material allocation to webs in a way that compromised catching ability the least.
2. Material and methods
Drosophila melanogaster of low quality (LQ) were reared on Carolina Drosophila medium formula 4-24 (Carolina Biological Supply, USA); high quality (HQ) prey were produced on a mixture of Carolina medium and dog food (Techni-Cal, Canada) containing 26 per cent protein, 16 per cent fat, and a variety of vitamins and minerals. Addition of dog food to the fruitfly medium enhances the performance of the spider (Mayntz et al. 2003). Because HQ flies are often larger than LQ flies, crowding the HQ fly cultures was necessary to produce flies of equal mass (see Mayntz et al. 2003). This produced LQ prey weights of 0.99 ± 0.02 mg and HQ prey weights of 0.97 ± 0.02 mg (mean ± s.e.; t-test, t = 0.706; d.f. = 10; p = 0.49). The nitrogen content (Kjeldahl method) was 8.7 ± 0.1 per cent (mean ± s.e., n = 2) in HQ prey and 7.9 ± 0.1 per cent (n = 5) in LQ prey.
Zygiella spiders are characterized by their web containing a free sector without spiral threads and from the hub, access to a retreat is allowed via a single silk thread. Hatchlings from 28 egg sacs were distributed into four treatments: (i) HQ prey ad libitum, (ii) LQ prey ad libitum, (iii) HQ prey in limited amounts, and (iv) LQ prey in limited amounts. Prey was supplied three times weekly to unlimited fed spiders while prey-limited spiders received one prey item per week. Spiders were kept in 70 ml plastic tubes at 24 ± 1°C on a 12 L : 12 D photoperiod. After moulting into instar II, spiderlings (n = 265) were weighed and placed in 15 cm2 frames (Zschokke & Herberstein 2005) and web building was observed over the following three 24 h periods. Webs were removed every evening to allow the building of new webs. Photographs were taken of the second web and the following parameters were measured: (i) number of radii, (ii) number of spiral turns in east–west (EW) and north–south (NS) axis, (iii) web diameter (distance between the two outermost spirals of both the EW axis and the NS axis), (iv) free space diameter (distance between the two innermost spirals of the EW axis and the NS axis), and (v) the angle of the free sector.
From the measured variables, mesh height was estimated as: ½ × ((NSweb diameter − NSfree space diameter)/(no. of spiral turns NS)) + ½ × ((EWweb diameter − EWfree space diameter)/(no. of spiral turns EW)). Capture areas were estimated by placing a transparency with known grid density above the projection image of a web and counting the number of grid crosses within the capture area (cf. Gundersen et al. 1988). A validation showed sufficiently high accuracy of this method (n = 10; maximum error: 2.81%; mean error: 0.99 ± 1.01% s.d.).
(a). Statistical analyses
We used nominal logistic regression to test whether food availability or prey nutrient composition affected web production on the first day in instar II. To test web production over the first 3 days in instar II, we used ordinal logistic regression with number of webs produced as an ordinal variable. Analysis of covariance was used to test parameters of web architecture with spider body mass as covariate (Heiling & Herberstein 1998). The assumption of equal slopes was tested in a model including the interaction term between the covariate and the factors. All analyses were performed using JMP 6.0 (SAS Institute Inc., Cary, USA).
Results
(a). Web-building frequency
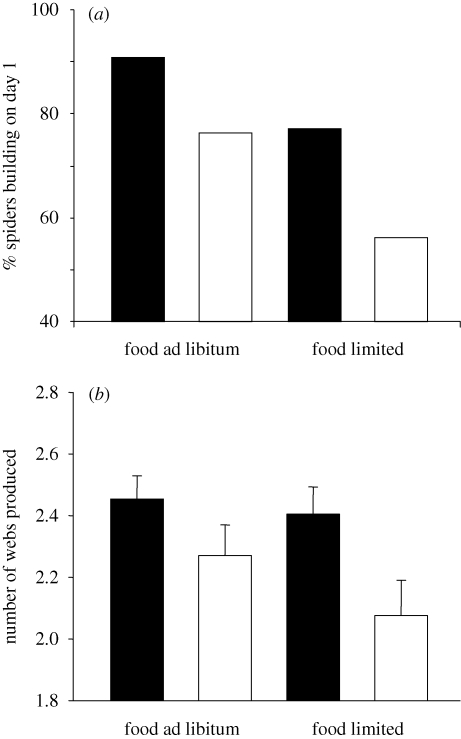
There was a significant effect of prey nutrient composition on the proportion of spiders building a web on the first day of instar II (table 1). Spiders fed HQ flies were more likely to build a web when compared with spiders fed LQ flies under both limited and unlimited conditions (figure 1a). We also found a significant effect of prey availability on web building at the first day in instar II (table 1) and spiders were most likely to build a web if they had been fed prey in unlimited amounts.
Table 1.
Tendency to build a web in the beginning of instar II. Nominal logistic regression (log. reg.) on the proportion of spiders building a web on the first day of instar II. Ordinal logistic regression of the number of webs produced over the first 3 days of instar II.
| nominal log. reg. |
ordinal log. reg. |
|||||
|---|---|---|---|---|---|---|
| source of variation | d.f. | χ2 | p | d.f. | χ2 | p |
| prey nutrient quality (PQ) | 1 | 11.4 | 0.0007 | 1 | 5.69 | 0.017 |
| prey availability (PA) | 1 | 10.7 | 0.0011 | 1 | 0.52 | 0.47 |
| PQ × PA | 1 | 0.07 | 0.79 | 1 | 0.53 | 0.47 |
Figure 1.
Web-building frequency of Zygiella x-notata spiderlings in the beginning of instar II (n = 265). During instar I, spiderlings were fed prey of high (black bars) or low (white bars) nutrient quality in limited or unlimited amounts. (a) Percentage of spiders building a web the first day in instar II. (b) Number of webs produced over the first 3 days of instar II. Means and s.e.'s are shown here for clarity, but statistical analyses were conducted with web counts as an ordinal variable (table 1).
The number of webs built over three consecutive 24 h periods was significantly affected by prey nutrient composition but not by prey availability (table 1). Spiders fed HQ prey built more webs than spiders fed LQ prey under both prey-limited and prey-unlimited conditions (figure 1b).
(b). Web architecture
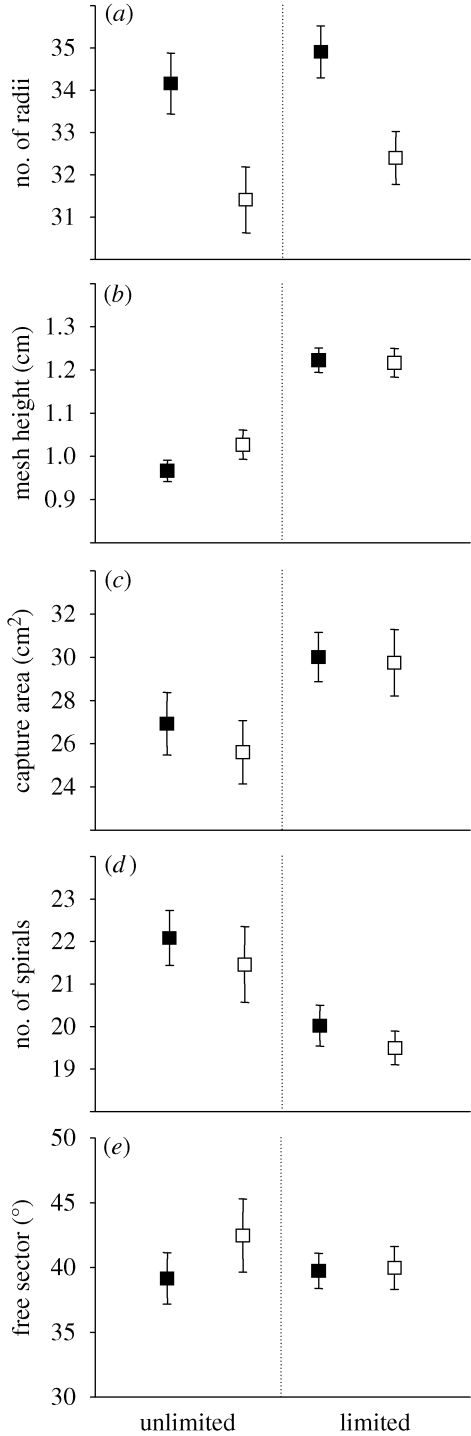
Prey nutrient quality was a highly significant factor influencing the number of radii (table 2), with spiders fed HQ flies making more radii than spiders fed LQ flies (figure 2a). Prey limitation increased the average mesh height and total capture area of webs (table 2), and prey-limited spiders produced larger and more open webs (figure 2b,c). Although the number of spirals appeared lower in the webs of prey-limited spiders (figure 2d), prey availability was not a significant factor when body mass was added as a covariate (table 2). Of all measured web parameters, only the free sector angle was unaffected by nutritional factors (table 2; figure 2e).
Table 2.
Two-way analysis of covariance on web architecture parameters of the second web built in instar II with log (spider body mass) as a covariate (n = 174).
| no. of radii |
no. of spiralsa |
capture area |
mesh height |
free sector angle (°) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| source of variation | d.f. | F | p | F | p | F | p | F | p | F | p |
| prey nutrient quality (PQ) | 1 | 14.70 | 0.0002 | 2.36 | 0.13 | 0.97 | 0.33 | 1.27 | 0.26 | 0.53 | 0.47 |
| prey availability (PA) | 1 | 2.23 | 0.14 | 0.05 | 0.83 | 11.81 | 0.0007 | 45.64 | <0.0001 | 0.16 | 0.69 |
| PQ × PA | 1 | 0.02 | 0.90 | 0.74 | 0.39 | 0.37 | 0.54 | 1.21 | 0.27 | 0.68 | 0.41 |
| log (spider body mass) | 1 | 0.70 | 0.41 | 17.44 | <0.0001 | 6.80 | 0.01 | 0.19 | 0.66 | 0.02 | 0.88 |
a1/X-transformed to secure equal variances—Levene test.
Figure 2.
Web architecture of the second web in instar II by Zygiella x-notata spiderlings (n = 174). During the first instar, spiderlings were fed prey of high (black squares) or low (white squares) nutrient quality in limited or unlimited amounts.
Discussion
Our results show that nutrient composition of previous prey captures, and thus the nutrient balance of a predator, can alter foraging behaviour. Nutrient balance affected predatory behaviour when spiders were fed both in limited or unlimited amounts. Thus, prey nutrient quality changed the foraging behaviour of the predators independent of energy status. As we controlled both prey species and prey mass, we argue that the nutritional composition of the prey provides the most plausible explanation for these effects.
The reduced number of radii in webs built by spiders fed LQ flies is interesting from a functional point of view. The radii provide the scaffolding of the web, while the sticky spiral threads retain the prey. Thus, spiders fed sub-optimal nutrients may reduce their investment in the web by reducing the number of radii without seriously affecting the amount of sticky spiral silk. Whether this is an optimal strategy under nutrient deficiency may depend on the types of prey that are available in the habitat. A web with fewer radii is less resistant to strong forces, e.g. fast-flying prey, rain or wind (Craig 1987; Eberhard 1990). The nitrogen content of HQ flies and LQ flies was 8.7 per cent and 7.9 per cent, respectively. However, the dog food added to the HQ fly medium contains many nutrients, which may even have been altered during passage through the digestive tract of the fly. Therefore, other candidate nutrients might have been responsible for the observed variation in web building, for example the balance of amino acids (Craig et al. 2000; Tso et al. 2005).
Our results regarding the effects of prey availability confirm previous findings of increased foraging activity and enlarged webs under food-limited situations (Sherman 1994; Vollrath & Samu 1997). Thus, it appears that the two types of nutritional stress lead to opposite directed responses: reduced prey availability leads to larger webs and nutritional imbalance leads to reduced material investment and fewer webs. All web parameters (except the free sector angle) were affected by either spider body mass or one of the two nutritional factors. As the free sector angle was the only parameter without any expected influence on the capture rate, our results support the view that the orb web is highly plastic and adapts to current foraging requirements (Sherman 1994; Heiling & Herberstein 2000).
References
- Belovsky G. E.1978Diet optimization in a generalist herbivore: the moose. Theor. Popul. Biol. 14, 105–134 (doi:10.1016/0040-5809(78)90007-2) [DOI] [PubMed] [Google Scholar]
- Craig C. L.1987The ecological and evolutionary interdependence between web architecture and web silk spun by orb web weaving spiders. Biol. J. Linn. Soc. 30, 135–163 (doi:10.1111/j.1095-8312.1987.tb00294.x) [Google Scholar]
- Craig C. L., Riekel C., Herberstein M. E., Weber R. S., Kaplan D., Pierce N. E.2000Evidence for diet effects on the composition of silk proteins produced by spiders. Mol. Biol. Evol. 17, 1904–1913 [DOI] [PubMed] [Google Scholar]
- Cruz-Rivera E., Hay M. E.2000Can food quantity replace food quality? Food choice, compensatory feeding, and the fitness of marine mesograzers. Ecology 81, 201–219 [Google Scholar]
- Eberhard W. G.1990Function and phylogeny of spider webs. Annu. Rev. Ecol. Syst. 21, 341–372 (doi:10.1146/annurev.es.21.110190.002013) [Google Scholar]
- Greenstone M. H.1979Spiders feeding behaviour optimises dietary essential amino acid composition. Nature 282, 501–503 (doi:10.1038/282501a0) [Google Scholar]
- Gundersen H. J. G., et al. 1988Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. Apmis 96, 379–394 [DOI] [PubMed] [Google Scholar]
- Heiling A. M., Herberstein M. E.1998The web of Nuctenea sclopetaria (Araneae, Araneidae): relationship between body size and web design. J. Arachnol. 26, 91–96 [Google Scholar]
- Heiling A. M., Herberstein M. E.2000Interpretations of orb-web variability: a review of past and current ideas. Ekol. Bratislava 19, 97–106 [Google Scholar]
- Higgins L., Rankin M. A.1999Nutritional requirements for web synthesis in the tetragnathid spider Nephila clavipes. Physiol. Entomol. 24, 263–270 (doi:10.1046/j.1365-3032.1999.00135.x) [Google Scholar]
- Mayntz D., Toft S., Vollrath F.2003Effects of prey quality and availability on the life history of a trap-building predator. Oikos 101, 631–638 (doi:10.1034/j.1600-0706.2003.12408.x) [Google Scholar]
- Mayntz D., Raubenheimer D., Salomon M., Toft S., Simpson S. J.2005Nutrient-specific foraging in invertebrate predators. Science 307, 111–113 (doi:10.1126/science.1105493) [DOI] [PubMed] [Google Scholar]
- Peakall D. B., Witt P. N.1976Energy budget of an orb web-building spider. Comp. Biochem. Phys. A 54, 187–190 (doi:10.1016/S0300-9629(76)80094-1) [DOI] [PubMed] [Google Scholar]
- Prestwich K. N.1977Energetics of web-building in spiders. Comp. Biochem. Phys. A 57, 321–326 (doi:10.1016/0300-9629(77)90199-2) [Google Scholar]
- Pulliam H. R.1975Diet optimization with nutrient constraints. Am. Nat. 109, 765–768 (doi:10.1086/283041) [Google Scholar]
- Schneider J. M., Vollrath F.1998The effect of prey type on the geometry of the capture web of Araneus diadematus. Naturwissenschaften 85, 391–394 (doi:10.1007/s001140050521) [Google Scholar]
- Shaner P.-J., Bowers M., Macko S.2007Giving-up density and dietary shifts in the white-footed mouse, Peromyscus leucopus. Ecology 88, 87–95 (doi:10.1890/0012-9658(2007)88[87:GDADSI]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- Sherman P. M.1994The orb web: an energetic and behavioral estimator of a spiders dynamic foraging and reproductive strategies. Anim. Behav. 48, 19–34 (doi:10.1006/anbe.1994.1208) [Google Scholar]
- Simpson S. J., Sibly R. M., Lee K. P., Behmer S. T., Raubenheimer D.2004Optimal foraging when regulating intake of multiple nutrients. Anim. Behav. 68, 1299–1311 (doi:10.1016/j.anbehav.2004.03.003) [Google Scholar]
- Stephens D. W., Krebs J. R.1986Foraging theory Princeton, NJ: Princeton University Press [Google Scholar]
- Tso I. M., Wu H. C., Hwang I. R.2005Giant wood spider Nephila pilipes alters silk protein in response to prey variation. J. Exp. Biol. 208, 1053–1061 (doi:10.1242/jeb.01437) [DOI] [PubMed] [Google Scholar]
- Tso I. M., Chiang S. Y., Blackledge T. A.2007Does the giant wood spider Nephila pilipes respond to prey variation by altering web or silk properties? Ethology 113, 324–333 (doi:10.1111/j.1439-0310.2007.01318.x) [Google Scholar]
- Vollrath F., Samu F.1997The effect of starvation on web geometry in an orb-weaving spider. Bull. Br. Arachnol. Soc. 10, 295–298 [Google Scholar]
- Zschokke S., Herberstein M. E.2005Laboratory methods for maintaining and studying web-building spiders. J. Arachnol. 33, 205–213 (doi:10.1636/CT04-72.1) [Google Scholar]