Abstract
Predators influence prey through consumption, and through trait-mediated effects such as emigration in response to predation risk (risk effects). We studied top-down effects of (sub-) adult wolf spiders (Lycosidae) on arthropods in a meadow. We compared risk effects with the overall top-down effect (including consumption) by gluing the chelicers of wolf spiders to prevent them from killing the prey. In a field experiment, we created three treatments that included either: (i) intact (‘predation’) wolf spiders; (ii) wolf spiders with glued chelicers (‘risk spiders’); or (iii) no (sub-) adult wolf spiders. Young wolf spiders were reduced by their (sub-) adult congeners. Densities of sheetweb spiders (Linyphiidae), a known intraguild prey of wolf spiders, were equally reduced by the presence of risk and predation wolf spiders. Plant- and leafhoppers (Auchenorrhyncha) showed the inverse pattern of higher densities in the presence of both risk and predation wolf spiders. We conclude that (sub-) adult wolf spiders acted as top predators, which reduced densities of intermediate predators and thereby enhanced herbivores. Complementary to earlier studies that found trait-mediated herbivore suppression, our results demonstrate that herbivores can be enhanced through cascading risk effects by top predators.
Keywords: Araneae, Auchenorrhyncha, conservation biological control, predation risk, trait-mediated effect, trophic cascade
1. Introduction
Predators affect prey through direct consumption. In addition, prey can respond to predation risk through behavioural changes such as reduced feeding time or emigration (Abrams 1995; Griffin & Thaler 2006). Such defensive tactics can lead to reduced growth, maturation rates, survivorship, fecundity or population density (Werner & Peacor 2003; Bolnick & Preisser 2005). Their overall impact on prey demography appears to be at least as strong as direct consumption (Werner & Peacor 2003; Preisser et al. 2005). The quantification of risk effects is hence important for our understanding of trophic interactions and biological pest control.
A further process that complicates trophic interactions is omnivory, feeding on more than one trophic level. Terrestrial communities have a high diversity of generalist predators, which feed to variable degrees on herbivores and other predators (Polis & Strong 1996). The resulting intraguild interference affects the impact of generalist predators on herbivore populations (Straub et al. 2008). To understand the role of generalist predators in food webs, the influence of intraguild interference on the strength of top-down effects on herbivores has to be compared. Strong intraguild interference can limit the ability of generalist predators to control herbivore densities (Finke & Denno 2005; Straub et al. 2008).
The variable role of predators in food webs is exemplified by wolf spiders (Lycosidae). Wolf spiders are widespread freely hunting predators in open habitats such as arable fields and grasslands. Wolf spiders often act on the third trophic level through preying on herbivores (Chase 1996; Denno et al. 2004). However, wolf spiders can act as intraguild predators or competitors of other entomophagous arthropods, including their own offspring (Nyffeler 1999). This can enhance herbivory (Finke & Denno 2004).
We evaluated the effects of wolf spiders on other arthropods in grassland and distinguished between consumptive and non-consumptive effects. Effects of intact wolf spiders were compared with the effect of predation risk, which was implemented through wolf spiders with glued chelicers (Schmitz et al. 1997). We expected that: (i) wolf spiders reduce other predators; (ii) wolf spiders reduce herbivorous plant- and leafhoppers; and (iii) wolf spiders with glued chelicers have smaller effects on other arthropods than unmanipulated wolf spiders.
2. Material and methods
We installed 39 quadratic enclosures of 0.36 m2 in a recently mown meadow. The enclosures prevented migration of (sub-) adult wolf spiders, but did not prevent movement in and out of the plots by insects and spiders that are able to fly (e.g. ballooning spiders), and/or have good climbing abilities (e.g. plant- and leafhoppers, young wolf spiders). Thirteen replicates of three treatments were distributed randomly among the 39 enclosures: predation spides—four unmanipulated (sub-) adult wolf spiders per enclosure; risk spiders—four (sub-) adult wolf spiders with glued chelicers per enclosure; spiders removed—removal of (sub-) adult wolf spiders prior to the experiment. The experiment lasted for 8 days. Then, we collected arthropods from the experimental areas. See methods in the electronic supplementary material for details.
In the laboratory, we tested possible side-effects of gluing chelicers on spider longevity. We filled each of 36 plastic pots (diameter: 10 cm, height: 15 cm) with 2 cm of soil and placed them in 25 cm high-gauze cages. Then, we assigned 18 glued risk spiders and 18 unmanipulated predation spiders randomly to the 36 pots. In daily observations, we noted survival time for each spider. We replaced dead spiders. In total, we examined 28 risk spiders and 19 predation spiders. The experiment was stopped after 48 days.
We first tested for general effects of wolf spiders by comparing the pooled risk and predation spider treatments with the wolf spider removal. Then, differences between risk and predation spider treatments were tested. Numbers of arthropod individuals per plot were tested using generalized linear models with ‘quasi-Poisson’ family in R v. 2.6.1 (R Development Core Team 2007). Averages ± 1 s.e. are given in the text and tables.
3. Results
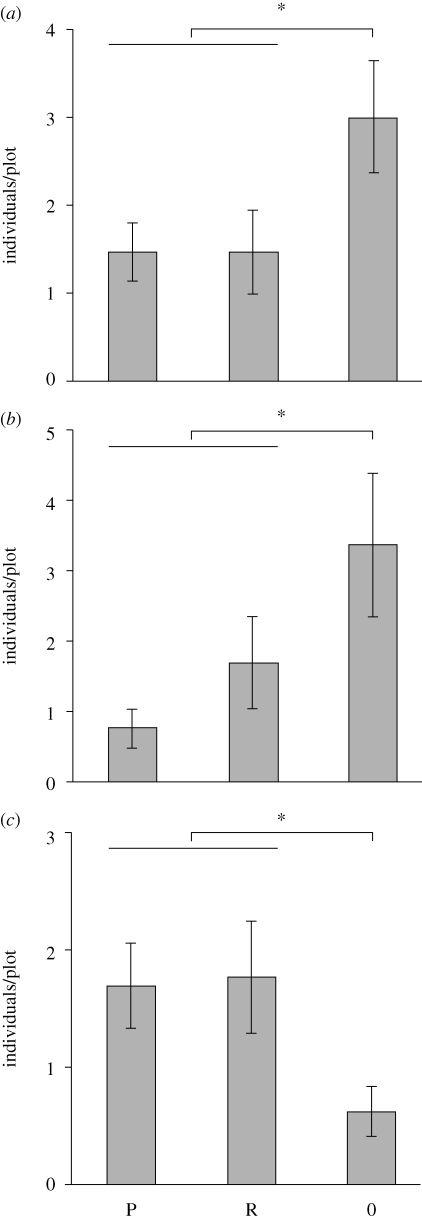
Sheetweb spiders had 47 per cent lower numbers in the two wolf spider addition treatments compared with the wolf spider removal (figure 1a; t(1,37) = 2.1, p = 0.038), and young wolf spiders were reduced by 64 per cent by their (sub-) adult congeners (figure 1b; t(1,37) = 2.4, p = 0.019). Surprisingly, sheetweb spider densities showed no difference between the risk and the predation spider treatment (t(1,24) = 0.0, p = 1.0), and the difference for young wolf spiders was not significant (t(1,24) = 1.3, p = 0.19). Plant- and leafhoppers showed the inverse pattern to sheetweb spiders (figure 1c). Plant- and leafhopper numbers were almost tripled by the presence of risk and predation spiders compared with the wolf spider removal (t(1,37) = 2.5, p = 0.019). Again, there was no significant difference between the risk and the predation spider treatment (t(1,24) = 0.13, p = 0.90). The remaining arthropod groups were less abundant, and showed no significant response to the wolf spider treatment (see table S1 of the electronic supplementary material).
Figure 1.
Effects of wolf spider manipulation on densities of (a) sheetweb spiders, (b) young wolf spiders, and (c) plant- and leafhoppers at the end of the experiment (individuals per enclosure; P = predation spiders, R = risk spiders and 0 = spiders removed; *p < 0.05).
By the end of the experiment, the wolf spider addition plots retained 7.2 times higher densities of (sub-) adult wolf spiders than the removal (t(1,37) = 3.0, p = 0.005). However, while almost three of the four introduced predation spiders were recaptured per plot on average, the average number of recovered risk spiders was less than one (t(1,24) = 4.1, p < 0.001; table S1 of the electronic supplementary material). Laboratory trials revealed that the survival of risk spiders was significantly reduced compared with unmanipulated predation spiders (F(1,46) = 55.1; p < 0.001). After 8 days under laboratory conditions, 46 per cent of the risk spiders were alive compared with 94 per cent of the predation spiders.
4. Discussion
Wolf spiders acted as top predators, which reduced densities of intermediate predators and thereby enhanced herbivores. The augmentation of plant- and leafhoppers may be due to reduction of sheetweb spiders and/or young wolf spiders. Accordingly, wolf spiders acted mostly on the fourth trophic level by reducing more important predators of plant- and leafhoppers. Denno et al. (2004) found adverse effects of wolf spiders on sheetweb spiders, but wolf spiders nevertheless reduced planthopper populations. In accordance with our results, Sanders & Platner (2007) found that the δN15 signature of adult wolf spiders is one trophic level above young wolf spiders and sheetweb spiders. As wolf spiders are common in arable fields (Samu & Szinetár 2002), their potential to either enhance or reduce herbivores is important for biological pest control and deserves further study. Notably, sheetweb spiders avoid field edges where densities of wolf spiders are higher than in field centres (Schmidt-Entling & Döbeli in press). The low numbers of herbivores compared with predators in the current experiment may be a consequence of the recent disturbance of the habitat. In particular, summer cuttings are known to reduce plant- and leafhopper densities (Morris 1981). In addition, generalist predators such as wolf and sheetweb spiders can be sustained by prey from the decomposer food web, enabling them to maintain high densities even when herbivores are rare (Nyffeler 1999; Wise et al. 1999).
The impact of risk and predation wolf spiders on sheetweb spiders and herbivores was not distinguishable, indicating a dominant role of non-consumptive risk effects by wolf spiders. The high importance of risk effects is in accordance with Schmitz et al. (1997), who found that the influence of nursery web spiders (Pisauridae) on plants was largely determined by behavioural changes of their grasshopper prey. Enhanced plant growth resulting from non-consumptive effects of predators on herbivores has been reported from terrestrial and aquatic systems (Schmitz et al. 2004; Thaler & Griffin 2008). However, our study may be the first demonstration of risk effect cascading from top predator to herbivore among terrestrial invertebrates. The transmission of wolf spider predation risk may occur via mechanical, visual and/or chemical cues. The presence of wolf spiders can be sensed chemically by herbivores (Storm & Lima 2008) and by other wolf spiders (Persons & Rypstra 2001). Given the short duration of our experiment and the ability of sheetweb spiders and young wolf spiders to enter and leave the plots, it is likely that migration has caused the observed differences in their density. Also plant- and leafhoppers were able to cross the enclosures, but the nature of their response to sheetweb spiders and/or young wolf spiders could not be determined with the current experimental setup. However, regardless of whether plant- and leafhoppers were consumed or not, their densities were equally enhanced by risk and predation wolf spiders.
(a). Side-effects of chelicer manipulations
Gluing of chelicers drastically reduced wolf spider longevity in our experiment. This is in contrast with Schmitz et al. (1997) who found that gluing chelicers of nursery web spiders (Pisauridae) did not alter spider movement and hunting behaviour, though they did not provide data on longevity. Possibly, the more actively hunting wolf spiders have higher energy demands and are thus more strongly affected by gluing chelicers than nursery web spiders with their sit-and-wait hunting strategy (Schmitz & Suttle 2001). We tried to delay possible starvation of the risk spiders by feeding them with a surplus of Drosophila melanogaster before gluing. The reduced longevity of wolf spiders in our experiment could be due to the prevention of water uptake through the glued chelicers, or due to moulting problems. The equally strong effects of risk and predation spiders irrespective of reduced longevity of risk spiders can have different causes. First, sheetweb spiders and young wolf spiders may have emigrated from experimental plots early after wolf spider release, when densities of risk spiders still resembled those of predation spiders. Second, chemical cues may have persisted beyond the death of the risk spiders (Barnes et al. 2002).
Acknowledgements
This manuscript benefited greatly from comments by Oswald Schmitz, Dirk Sanders and two anonymous referees. We thank Herbert Nickel for discussion and for the determination of plant- and leafhoppers. We further thank Beata Eichenberger, Hansueli Weber, John Herrmann and Peter Eberhart for help in the field. The Baumberger-Burri family kindly allowed us to perform the experiment on their land.
References
- Abrams P. A.1995Implications of dynamically variable traits for identifying, classifying, and measuring direct and indirect effects in ecological communities. Am. Nat. 146, 112–134 (doi:10.1086/285789) [Google Scholar]
- Barnes M. C., Persons M. H., Rypstra A. L.2002The effect of predator chemical cue age on antipredator behavior in the wolf spider Pardosa milvina (Araneae: Lycosidae). J. Insect Behav. 15, 269–281 (doi:10.1023/A:1015493118836) [Google Scholar]
- Bolnick D. I., Preisser E. L.2005Resource competition modifies the strength of trait-mediated predator–prey interactions: a meta-analysis. Ecology 86, 2771–2779 (doi:10.1890/04-1249) [Google Scholar]
- Chase J. M.1996Abiotic controls of trophic cascades in a simple grassland food chain. Oikos 77, 495–506 (doi:10.2307/3545939) [Google Scholar]
- Denno R. F., Mitter M. S., Langellotto G. A., Gratton C., Finke D. L.2004Interactions between a hunting spider and a web-builder: consequences of intraguild predation and cannibalism for prey suppression. Ecol. Entomol. 29, 566–577 (doi:10.1111/j.0307-6946.2004.00628.x) [Google Scholar]
- Finke D. L., Denno R. F.2004Predator diversity dampens trophic cascades. Nature 429, 407–410 (doi:10.1038/nature02554) [DOI] [PubMed] [Google Scholar]
- Finke D. L., Denno R. F.2005Predator diversity and the functioning of ecosystems: the role of intraguild predation in dampening trophic cascades. Ecol. Lett. 8, 1299–1306 (doi:10.1111/j.1461-0248.2005.00832.x) [Google Scholar]
- Griffin C. A. M., Thaler J. S.2006Insect predators affect plant resistance via density- and trait-mediated indirect interactions. Ecol. Lett. 9, 335–343 (doi:10.1111/j.1461-0248.2005.00880.x) [DOI] [PubMed] [Google Scholar]
- Morris M. G.1981Response of grassland invertebrates to management by cutting. III. Adverse effects on Auchenorrhyncha. J. Appl. Ecol. 18, 107–123 (doi:10.2307/2402481) [Google Scholar]
- Nyffeler M.1999Prey selection of spiders in the field. J. Arachnol. 27, 317–324 [Google Scholar]
- Persons M. H., Rypstra A. L.2001Wolf spiders show graded antipredator behavior in the presence of chemical cues from different sized predators. J. Chem. Ecol. 27, 2493–2504 (doi:10.1023/A:1013679532070) [DOI] [PubMed] [Google Scholar]
- Polis G. A., Strong D. R.1996Food web complexity and community dynamics. Am. Nat. 147, 813–846 (doi:10.1086/285880) [Google Scholar]
- Preisser E. L., Bolnick D. I., Benard M. F.2005Scared to death? The effects of intimidation and consumption in predator–prey interactions. Ecology 86, 501–509 (doi:10.1890/04-0719) [Google Scholar]
- R Development Core Team 2007R: A language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing; (http://www.R-project.org). [Google Scholar]
- Samu F., Szinetár C.2002On the nature of agrobiont spiders. J. Arachnol. 30, 389–402 (doi:10.1636/0161-8202(2002)030[0389:OTNOAS]2.0.CO;2) [Google Scholar]
- Sanders D., Platner C.2007Intraguild interactions between spiders and ants and top-down control in a grassland food web. Oecologia 150, 611–624 (doi:10.1007/s00442-006-0538-5) [DOI] [PubMed] [Google Scholar]
- Schmidt-Entling M. H., Döbeli J.In press Sown wildflower areas to enhance spiders in arable fields. Agr. Ecosyst. Environ. (doi:10.1016/j.agee.2009.04.015) [Google Scholar]
- Schmitz O. J., Suttle K. B.2001Effects of top predator species on direct and indirect interactions in a food web. Ecology 82, 2072–2081 [Google Scholar]
- Schmitz O. J., Beckerman A. P., O'Brien K. M.1997Behaviorally mediated trophic cascades: effects of predation risk on food web interactions. Ecology 78, 1388–1399 [Google Scholar]
- Schmitz O. J., Krivan V., Ovadia O.2004Trophic cascades: the primacy of trait-mediated indirect interactions. Ecol. Lett. 7, 153–163 (doi:10.1111/j.1461-0248.2003.00560.x) [Google Scholar]
- Storm J. J., Lima S. L.2008Predator-naive fall field crickets respond to the chemical cues of wolf spiders. Can. J. Zool. 86, 1259–1263 (doi:10.1139/Z08-114) [Google Scholar]
- Straub C. S., Finke D. L., Snyder W. E.2008Are the conservation of natural enemy biodiversity and biological control compatible goals? Biol. Control 45, 225–237 (doi:10.1016/j.biocontrol.2007.05.013) [Google Scholar]
- Thaler J. S., Griffin C. A. M.2008Relative importance of consumptive and non-consumptive effects of predators on prey and plant damage: the influence of herbivore ontogeny. Ent. Exp. Appl. 128, 34–40 (doi:10.1111/j.1570-7458.2008.00737.x) [Google Scholar]
- Werner E. E., Peacor S. D.2003A review of trait-mediated indirect interactions in ecological communities. Ecology 84, 1083–1100 (doi:10.1890/0012-9658(2003)084[1083:AROTII]2.0.CO;2) [Google Scholar]
- Wise D. H., Snyder W. E., Tuntibunpakul P., Halaj J.1999Spiders in decomposition food webs of agroecosystems: theory and evidence. J. Arachnol. 27, 363–370 [Google Scholar]