Abstract
The New Zealand (NZ) lizard fossil record is currently limited to late Quaternary remains of modern taxa. The St Bathans Fauna (early Miocene, southern South Island) extends this record to 19–16 million years ago (Myr ago). Skull and postcranial elements are similar to extant Oligosoma (Lygosominae) skinks and Hoplodactylus (Diplodactylinae) geckos. There is no evidence of other squamate groups. These fossils, along with coeval sphenodontines, demonstrate a long conservative history for the NZ lepidosaurian fauna, provide new molecular clock calibrations and contradict inferences of a very recent (less than 8 Myr ago) arrival of skinks in NZ.
Keywords: Reptilia, palaeontology, biogeography, Gondwana, Squamata, Miocene
1. Introduction
The Recent New Zealand (NZ) lizard fauna, like many insular terrestrial biotas, comprises endemic radiations of few lineages. There are only two groups: skinks (Eugongylus group Lygosominae) and geckos (Diplodactylinae). NZ skinks represent a single endemic radiation (Smith et al. 2007; Chapple et al. 2009), all recently placed in Oligosoma (Chapple et al. 2009; cf. Hardy 1977). NZ geckos are also monophyletic (Kluge 1967; Chambers et al. 2001), with Hoplodactylus being a primitive grade with respect to Naultinus (Chambers et al. 2001). There has been no direct evidence for the antiquity of this fauna, with fossils currently limited to the Quaternary (Worthy 1987, 1991). However, independent molecular dating suggested that NZ skinks diverged from their New Caledonian relatives as early as more than 20 Myr ago (Hickson et al. 2000) or as recently as 7.9 Myr ago (Smith et al. 2007). A more extensive study by Chapple et al. (2009), calibrated using preliminary announcements of St Bathans lizards (e.g. Worthy et al. 2006), is not independent of the current study. Molecular dating acknowledged as coarse (Chambers et al. 2001) estimates that the most recent common ancestor of living NZ geckos existed 22.8 Myr ago. Other nodes were undated, but branch lengths in the published tree suggest that NZ geckos diverged from their sister clade (mainly New Caledonian) approximately 30 Myr ago.
The St Bathans Fauna (Bannockburn Formation, Manuherikia Group, late Early Miocene, 19–16 Myr ago) provides the only information on NZ terrestrial vertebrates during the Tertiary (e.g. Worthy et al. 2007; Jones et al. 2009). Numerous specimens recovered help elucidate the history of NZ's distinctive lizard fauna and independently test molecular clock inferences.
2. Material and methods
The general stratigraphy of St Bathans, and excavation methods, is described in Worthy et al. (2007). Appendix A in the electronic supplementary material lists precise site details and squamate specimens retrieved. Because material invariably was similar to extant NZ skinks and geckos, comparisons focused on these forms and extralimital taxa from Australasia and New Caledonia (appendix B, electronic supplementary material).
3. Results
All diagnostic lizard material (table 1) was very similar to (if not indistinguishable from) corresponding elements from either NZ skinks or geckos, the only two indigenous lineages of squamates. They also differ greatly from coeval sphenodontines (Jones et al. 2009) in shape as well as size (e.g. fused frontals, pleurodonty, low blade-like neural spines and gracile limb elements). It is thus neither necessary nor parsimonious to assume that other groups are represented. Few traits consistently separate the highly diverse and globally widespread skinks and geckos (e.g. distinguishing limb features are elusive because both include limbless taxa). However, characters outlined below differ consistently between NZ skinks and geckos. Only diagnostic traits are noted, but for every element, the overall form is very similar to living NZ representatives of the relevant group. Abbreviations in parentheses refer to labels in figures 1 and 2.
Table 1.
Squamate elements from St Bathans referable to either Scincidae or Gekkota.
| taxon and element | museum of NZ number (right/left element) | details |
|---|---|---|
| Scincidae | ||
| frontal | S42688, S44217, S51260 | posterior and interorbital region |
| maxilla | S42620(R), S43074(R), S44218(R), S50707(L) | central portion |
| compound mandibular element | S42689(R) | largely complete, missing part of the retroarticular process and the anterior tip |
| vertebrae | S42618, S42728, S44001, S44302, S44216, S50022, S50024, S50388, S50819, S50943, S51262, S50708 | all isolated dorsals except: 42618 (2 elements), 44216 (3 elements), 51262 (3 elements), 50708 (isolated dorsal and two sacrals missing distal ends of all sacral ribs) |
| humerus | S44002(R), S50938(R) | distal portion with attached epiphysis |
| femur | S50945(R), S50810(R) | 50945 is a proximal end, including capitellum; 50810 is almost complete, lacking capitellum and distal epiphysis |
| Gekkota | ||
| frontal | S43124 | most of posterior portion |
| pterygoid | S44154(L) | central portion |
| maxilla | S51004(L), S44152(R) | central portion |
| dentary | S42296(L), S51324(L) | 42296 central portion, 51324 anterior portion |
| compound mandibular element | S42341(L), S42342(L), S42577(L), S42731(L), S44003(R), S44153(L), S44338(R) | posterior portion |
| vertebrae | S42343, S42616, S42687, S42730, S43125, S51269 | all are dorsals except for 51269, which appears to be caudal based on transverse processes in the middle region (broken), and an articulatory surface for the chevron |
| humerus | S44072(L) | proximal portion (with attached epiphysis) and most of shaft |
| femur | S50709(R) | distal portion (with attached epiphysis) and part of shaft |
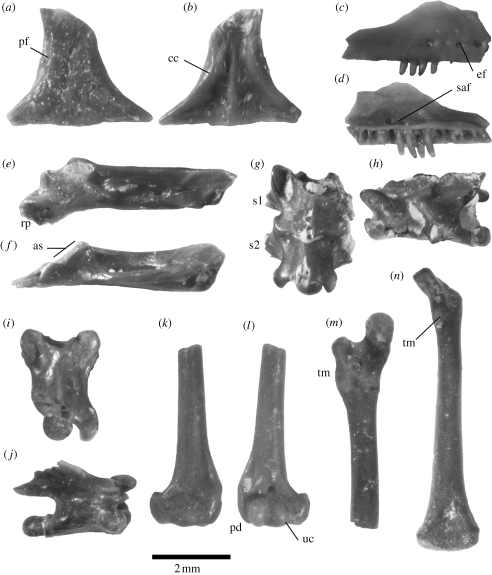
Figure 1.
Scincid material; for colour version, see appendix C in the electronic supplementary material. (a) Dorsal and (b) ventral view of frontal NMNZ S42688. (c) Lateral and (d) medial view of right maxilla 44218. (e) Dorsal and (f) medial view of right compound lower jaw element 42689. (g) Dorsal and (h) left lateral view of sacral vertebrae 50708. (i) Dorsal and (j) right lateral view of dorsal vertebra 50708. (k) Dorsal and (l) ventral view of distal end of right humerus 44002. (m) Postaxial (posterior) and (n) ventral view of right femur 50945 and 50810, respectively. Abbreviations in main text and table 1.
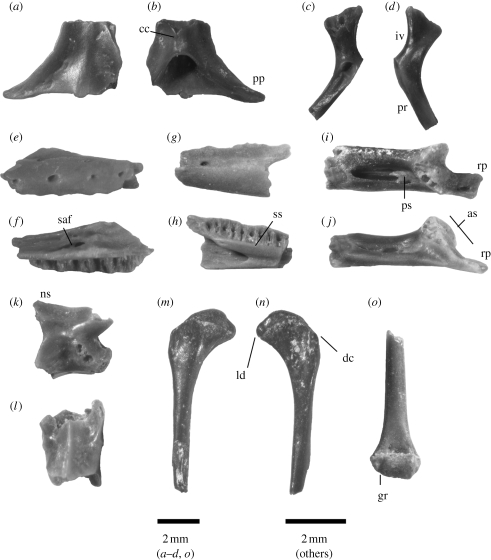
Figure 2.
Gekkotan material; for colour version, see appendix C in the electronic supplementary material. (a) Dorsal and (b) ventral view of frontal NMNZ S43124. (c) Lateral and (d) medial view of right left pterygoid 44154. (e) Lateral and (f) medial view of left maxilla 51004. (g) Lateral and (h) medial view of left dentary 42296. (i) Dorsal and (j) medial view of right compound bone of lower jaw 44338. (k) Right lateral and (l) dorsal view of dorsal vertebra 42730. (m) Dorsal and (n) ventral view of proximal portion of left humerus 44072. (o) Ventral view of distal end of right femur 50709. Abbreviations in main text and table 1.
(a). Scincidae
The elements are of moderate size, typically intermediate between the living Oligosoma zelandicum and Oligosoma infrapunctatum (60–90 mm snout-vent length (SVL); Gill & Whitaker 1996). The cryptozoic Cyclodina is nested within, and now synonymized with, the surface-active Oligosoma (Chapple et al. 2009). The frontal, compound and sacrals show similarities to taxa examined (appendix B, electronic supplementary material) with the depressed ‘Oligosoma morphotype’ rather than the cylindrical ‘Cyclodina morphotype’. The remaining elements could be from either morphotype.
(i). Frontal (figure 1a,b)
Identifiable as Scincidae by adpressed cephalic osteoderms (Scincoidea apomorphy: Estes et al. 1988) and shallow crista cranii (cc; subolfactory processes) that do not meet ventrally (figure 1b). The long prefrontal facet (pf) indicates the prefrontal extended posteriorly past the centre of the orbit, a trait found in living NZ skinks, but absent in extralimital taxa examined. Finally, the cristae cranii are very shallow and attenuate posteriorly, as in the Oligosoma morphotype but unlike the more prominent structures in the Cyclodina morphotype.
(ii). Maxilla (figure 1c,d)
Identifiable as Scincidae by the anterior position of the internal (superior alveolar) foramen (saf) for the maxillary nerve and artery and the absence of lateral maxillary foramina on the posterior margin. There are approximately 14 alveoli in the best-preserved element. Teeth are peg-shaped and not recurved. Five closely spaced external foramina (ef) are present anteriorly (others probably present on the missing end).
(iii). Compound element (figure 1e,f)
Identifiable as Scincidae by a wide flaring retroarticular process (rp), and an articular surface (as) that is more horizontally than vertically orientated. The retroarticular process is distinctly angled medially (Scincoidea apomorphy: Estes et al. 1988), more than in the Cyclodina morphotype and resembling the Oligosoma morphotype.
(iv). Vertebra (figure 1i,j)
Identifiable as Scincidae by procoelous articulations. In the sacrals (figure 1g,h), the first rib (s1) is more gracile than the second (s2), as in the Oligosoma morphotype but unlike the Cyclodina morphotype.
(v). Humerus (figure 1k,l)
Identifiable as Scincidae by the large ulna condyle (uc) and the absence of a notch on the preaxial distal corner (pd).
(vi). Femur (figure 1m,n)
Identifiable as Scincidae by the trochanter major (tm), which tapers gradually in height distally.
(b). Gekkota
Elements recovered are mostly from large geckos intermediate in size between Hoplodactylus maculatus and Hoplodactylus duvaucelii (100–140 mm SVL; Gill & Whitaker 1996). Two forms, differing in adult size, are represented (see Compound). Taxa can be assigned to Gekkonidae sensu lato based on the presence of apomorphies with Gekkota (Gekkonidae+Pygopodidae) but the absence of apomorphies of Pygopodidae (e.g. limb reduction). The frontal shows similarities to Hoplodactylus rather than Naultinus.
(i). Frontal (figure 2a,b)
Identifiable as Gekkonidae by the absence of adpressed cephalic osteoderms, and deep, ventrally fused cc, a Gekkota apomorphy (Estes et al. 1988; Evans 2008). More similar to Hoplodactylus than to Naultinus in possessing long, delicate posterolateral processes (pp), with the caveat that this trait is highly variable across Gekkota.
(ii). Pterygoid (figure 2c,d)
Identifiable as Gekkonidae by a narrow, rod-like posterior ramus (pr) and a concave margin bordering a large interpterygoid vacuity (iv).
(iii). Maxilla (figure 2e,f)
Identifiable as Gekkonidae by the posterior position of the internal (superior alveolar) foramen (saf) for the maxillary nerve and artery.
(iv). Dentary (figure 2g,h)
Identifiable as gekkonidae by the sublingual shelf (ss), which is straight rather than dorsally concave in medial/lingual view.
(v). Compound (figure 2i,j)
Identifiable as Gekkonidae by a narrow retroarticular process (rp), resulting in a ‘lateral notch’ (Evans 2008) where it joins the wider, main portion of the lower jaw (Gekkota apomorphy: Estes et al. 1988), and an articular surface (as) that is more vertically than horizontally oriented. The adductor fossa bears a large ossified posterior shelf (ps), a distinctive trait found in some NZ geckos (e.g. H. duvaucelii and Naultinus elegans) but poorly developed or absent in extralimital taxa examined. One specimen (NMNZ S44338) is presumably adult with no trace of the surangular-articular suture; this represents a moderate-sized gecko approximately 20 per cent larger than living H. maculatus. Three specimens (42342, 44003 and 44153) are markedly larger than 44338 yet retain this suture, suggesting that they are immature individuals of a larger taxon that, when fully grown, was at least as large as living H. duvaucelii.
(vi). Vertebrae (figure 2k,l)
Identifiable as Gekkonidae based on amphicoelous articulations, an apomorphic reversal in squamates widespread in Gekkota (Estes et al. 1988).
(vii). Humerus (figure 2m,n)
Identifiable as Gekkonidae based on the indistinct deltopectoral crest (dc) and flange for the latissimus dorsi (ld); in NZ scincids, these are clearly off-set (via a concavity) from the proximal articulation.
(viii). Femur (figure 2o)
Identifiable as Gekkonidae by the groove (gr) on the preaxial region of the distal articulatory surface.
4. Discussion
The first pre-Pleistocene fossil record of squamates in NZ fills a major gap in the regional fossil record. The fossils are most parsimoniously interpreted as related to living lineages: they are extremely similar to, and share some putative-derived characters with, extant NZ diplodactyline geckos or lygosomine skinks. Early Miocene presence of both living lineages of NZ lizards, as well as sphenodontines (Jones et al. 2009), and apparent absence of all other lepidosaur groups indicate long-term conservatism of the NZ reptile fauna. Since then, there have been no new colonizing lineages and limited phenotypic divergence within each lineage; a situation contrasting greatly with the nearest continental fauna, Australia (e.g. Greer 1989). The squamate fossils cannot refute or confirm the debated Oligo-Miocene submergence of NZ (Landis et al. 2008), but the presence of many vertebrate lineages soon afterwards (e.g. see Worthy et al. 2006; Jones et al. 2009) suggests either continuous persistence of land or subsequent rapid colonization.
The lizard fossils reveal that NZ was occupied 19–16 Myr ago by at least one skink taxon resembling extant species with the Oligosoma morphotype and two gecko taxa resembling extant Hoplodactylus species. Both these morphotypes represent primitive grades in their respective NZ lineages (Chambers et al. 2001; Smith et al. 2007; Chapple et al. 2009), and it might be expected that early fossils would be attributable to these taxa. The fossils are too fragmentary to exhibit diagnostic differences from extant species of Oligosoma and Hoplodactylus and thus cannot be named. The lack of evidence for derived skinks (Cyclodina morphotype) and derived geckos (Naultinus) despite numerous fossils is consistent with these taxa having a later (post early Miocene) origin, although support consisting of absence of fossils must be acknowledged as weak.
These fossils are also important given the rarity of robust fossil calibration points within modern scincids and gekkotans for molecular clock analyses (e.g. Skinner et al. 2008). They provide the first minimum age constraints for the divergence of the endemic NZ scincid and gekkotan clades from their extralimital sister groups (Chapple et al. 2009). Molecular divergence dating using other calibration points has thus far yielded highly disparate dates for the age of NZ skinks, suggesting that they diverged from New Caledonian relatives 20 Myr ago (Hickson et al. 2000) or either 22.9–19.9 or 7.9 Myr ago, depending on calibrations and genes employed (Smith et al. 2007). For geckos, the only study suggests NZ geckos diverged from (primarily) New Caledonian relatives about 30 Myr ago (Chambers et al. 2001; see §1). The fossils described here demonstrate that both endemic NZ lizard lineages were already present 16 Myr ago and thus refute the younger molecular estimates for skinks. Deep phylogeographic divergences found within NZ skink species complexes also suggest early origins (Greaves et al. 2007; Chapple et al. 2009). Further, the 7.9 Myr date was inferred by applying mtDNA substitution rates for agamids to skinks. Because agamids have approximately twice the rate of mtDNA evolution of skinks (e.g. Kumazawa 2007), this extrapolation would underestimate (by half) divergence dates within skinks. Thus, the deeper molecular estimates for NZ skink origins (more than 20 Myr ago: Hickson et al. 2000; Chapple et al. 2009) are best supported by the fossil record, phylogeography and methodological considerations.
Acknowledgements
We thank Ann and Euan Johnstone and Jack Enright for site access, numerous field volunteers (appendix A, electronic supplementary material) and Michael Caldwell and two anonymous referees for helpful comments. Supported by Australian Research Council Grant DP0770660 and UNSW Strategic Initiative Funding Scheme to S. Hand, M.A. and T.H.W.
References
- Chambers G. K., Boon W. M., Buckley T. R., Hitchmough R. A.2001Using molecular methods to understand the Gondwanan affinities of the New Zealand biota: three case studies. Aust. J. Bot. 49, 377–387 (doi:10.1071/BT00021) [Google Scholar]
- Chapple D. G., Ritchie P. A., Daugherty C. H.2009Origin, diversification and systematics of the New Zealand skink fauna (Reptilia: Scincidae). Mol. Phyl. Evol. 52, 470–487 (doi:10.1016/j.ympev.2009.03.021) [DOI] [PubMed] [Google Scholar]
- Estes R., de Queiroz K., Gauthier J.1988Phylogenetic relationships within Squamata. In Phylogenetic relationships of the lizard families (eds Estes E., Pregill G.), pp. 119–281 Stanford, CA: Stanford University Press [Google Scholar]
- Evans S. E.2008The skull of lizards and Tuatara. In Biology of the Reptilia, Morphology H, vol. 20 (eds Gans C., Gaunt A. S., Adler K.), pp. 1–347 Ithaca, NY: SSAR [Google Scholar]
- Gill B. T., Whitaker A. H.1996New Zealand frogs and reptiles Albany, New Zealand: David Bateman Ltd [Google Scholar]
- Greaves S. N. J., Chapple D. G., Gleeson D. M., Daugherty C. H., Ritchie P. A.2007Phylogeography of the spotted skink (Oligosoma lineoocellatum) and green skink (O. chloronoton) species complex (Lacertilia: Scincidae) in New Zealand reveals pre-Pleistocene divergence. Mol. Phyl. Evol. 45, 729–739 (doi:10.1016/j.ympev.2007.06.008) [DOI] [PubMed] [Google Scholar]
- Greer A. E.1989The biology and evolution of Australian lizards Chipping Norton, New South Wales: Surrey Beatty [Google Scholar]
- Hardy G. S.1977The New Zealand Scincidae (Reptilia: Lacertilia): a taxonomic and zoogeographic study. NZ J. Zool. 4, 221–325 [Google Scholar]
- Hickson R. E., Slack K. E., Lockhart P.2000Phylogeny recapitulates geography, or why New Zealand has so many species of skinks. Biol. J. Linn. Soc. 70, 415–433 (doi:10.1111/j.1095-8312.2000.tb01232.x) [Google Scholar]
- Jones M. E. H., Tennyson A. J. D., Worthy J. P., Evans S. E., Worthy T. H.2009A sphenodontine (Rhynchocephalia) from the Miocene of New Zealand and palaeobiogeography of the tuatara (Sphenodon). Proc. R. Soc. B 276, 1385–1390 (doi:10.1098/rspb.2008.1785) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluge A.1967Systematics, phylogeny, and zoogeography of the lizard genus Diplodactylus Gray (Gekkonidae). Aust. J. Zool. 15, 1007–1108 (doi:10.1071/ZO9671007) [Google Scholar]
- Kumazawa Y.2007Mitochondrial genomes from major lizard families suggest their phylogenetic relationships and ancient radiations. Gene 388, 19–26 (doi:10.1016/j.gene.2006.09.026) [DOI] [PubMed] [Google Scholar]
- Landis C. A., Campbell H. J., Begg J. G., Mildenhall D. C., Paterson A. M., Trewick S. A.2008The Waipounamu erosion surface: questioning the antiquity of the New Zealand land surface and terrestrial fauna and flora. Geol. Mag. 145, 173–197 (doi:10.1017/S0016756807004268) [Google Scholar]
- Skinner A., Lee M. S. Y., Hutchinson M. N.2008Rapid and repeated limb loss in a clade of scincid lizards. BMC Evol. Biol. 8, 1–23 (doi:10.1186/1471-2148-8-310) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. A., Sadlier R. A., Bauer A. M., Austin C. C., Jackman T.2007Molecular phylogeny of the scincid lizards of New Caledonia and adjacent areas: evidence for a single origin of the endemic skinks of Tasmantis. Mol. Phyl. Evol. 43, 1151–1166 (doi:10.1016/j.ympev.2007.02.007) [DOI] [PubMed] [Google Scholar]
- Worthy T. H.1987Osteological observations of the larger species of skink Cyclodina and the subfossil occurrence of these and the gecko Hoplodactylus duvaucelii in the North Island, New Zealand. NZ J. Zool. 14, 219–229 [Google Scholar]
- Worthy T. H.1991Fossil skink bones from Northland, New Zealand, including a description of a new Cyclodina, Scincidae. J. R. Soc. NZ 21, 329–348 [Google Scholar]
- Worthy T. H., Hand S. J., Archer M. A., Tennyson A. J. D.2006The St Bathans Fauna: first insight into Neogene terrestrial vertebrate faunas in New Zealand. Geol. Soc. NZ Misc. Publ. 121, 35–36 [Google Scholar]
- Worthy T. H., Tennyson A. J. D., Jones C., McNamara J. A., Douglas B. J.2007Miocene waterfowl and other birds from Central Otago, New Zealand. J. Syst. Palaeontol. 5, 1–39 (doi:10.1017/S1477201906001957) [Google Scholar]