Abstract
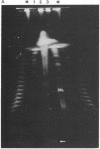
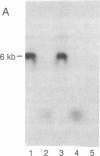
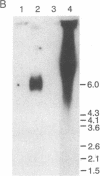
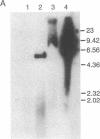
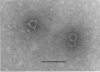
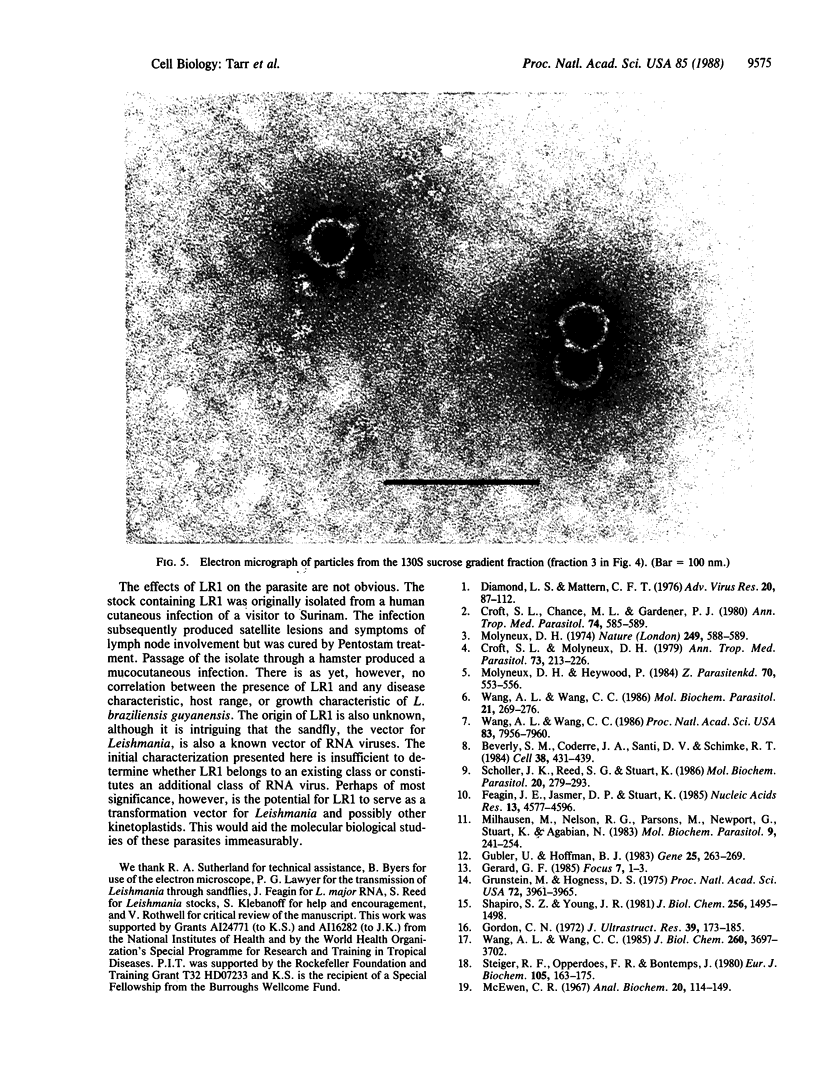
Although viruses are important biological agents and useful molecular tools, little is known about the viruses of parasites. We report here the discovery of a candidate for an RNA virus in a kinetoplastid parasite. This potential virus, which we term LR1, is present in the promastigote form of the human pathogen Leishmania braziliensis guyanensis CUMC1-1A but not in 11 other stocks of Leishmania that were examined nor in Trypanosoma brucei. The candidate viral RNA has a size of approximately 6000 nucleotides, is single-stranded, and is largely, if not exclusively, located in the cytoplasm. No homologous LR1 sequences are detected in genomic DNA. The candidate viral RNA is associated with a spherical particle 32 nm in diameter that has a sedimentation coefficient of approximately 130 S. There is as yet no evident effect of this potential virus on parasite physiology or the disease caused by the parasite.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beverley S. M., Coderre J. A., Santi D. V., Schimke R. T. Unstable DNA amplifications in methotrexate-resistant Leishmania consist of extrachromosomal circles which relocalize during stabilization. Cell. 1984 Sep;38(2):431–439. doi: 10.1016/0092-8674(84)90498-7. [DOI] [PubMed] [Google Scholar]
- Croft S. L., Chance M. L., Gardener P. J. Ultrastructural and biochemical characterization of stocks of Endotrypanum. Ann Trop Med Parasitol. 1980 Dec;74(6):585–589. doi: 10.1080/00034983.1980.11687391. [DOI] [PubMed] [Google Scholar]
- Croft S. L., Molyneux D. H. Studies on the ultrastructure, virus-like particles and infectivity of Leishmania hertigi. Ann Trop Med Parasitol. 1979 Jun;73(3):213–226. doi: 10.1080/00034983.1979.11687251. [DOI] [PubMed] [Google Scholar]
- Diamond L. S., Mattern C. F. Protozoal viruses. Adv Virus Res. 1976;20:87–112. doi: 10.1016/s0065-3527(08)60502-3. [DOI] [PubMed] [Google Scholar]
- Feagin J. E., Jasmer D. P., Stuart K. Apocytochrome b and other mitochondrial DNA sequences are differentially expressed during the life cycle of Trypanosoma brucei. Nucleic Acids Res. 1985 Jun 25;13(12):4577–4596. doi: 10.1093/nar/13.12.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- McEwen C. R. Tables for estimating sedimentation through linear concentration gradients of sucrose solution. Anal Biochem. 1967 Jul;20(1):114–149. doi: 10.1016/0003-2697(67)90271-0. [DOI] [PubMed] [Google Scholar]
- Milhausen M., Nelson R. G., Parsons M., Newport G., Stuart K., Agabian N. Molecular characterization of initial variants from the IsTat I serodeme of Trypanosoma brucei. Mol Biochem Parasitol. 1983 Nov;9(3):241–254. doi: 10.1016/0166-6851(83)90100-7. [DOI] [PubMed] [Google Scholar]
- Molyneux D. H. Virus-like particles in Leishmania parasites. Nature. 1974 Jun 7;249(457):588–589. doi: 10.1038/249588a0. [DOI] [PubMed] [Google Scholar]
- Scholler J. K., Reed S. G., Stuart K. Molecular karyotype of species and subspecies of Leishmania. Mol Biochem Parasitol. 1986 Sep;20(3):279–293. doi: 10.1016/0166-6851(86)90108-8. [DOI] [PubMed] [Google Scholar]
- Shapiro S. Z., Young J. R. An immunochemical method for mRNA purification. Application to messenger RNA encoding trypanosome variable surface antigen. J Biol Chem. 1981 Feb 25;256(4):1495–1498. [PubMed] [Google Scholar]
- Steiger R. F., Opperdoes F. R., Bontemps J. Subcellular fractionation of Trypanosoma brucei bloodstream forms with special reference to hydrolases. Eur J Biochem. 1980 Mar;105(1):163–175. doi: 10.1111/j.1432-1033.1980.tb04486.x. [DOI] [PubMed] [Google Scholar]
- Wang A. L., Wang C. C. A linear double-stranded RNA in Trichomonas vaginalis. J Biol Chem. 1985 Mar 25;260(6):3697–3702. [PubMed] [Google Scholar]
- Wang A. L., Wang C. C. Discovery of a specific double-stranded RNA virus in Giardia lamblia. Mol Biochem Parasitol. 1986 Dec;21(3):269–276. doi: 10.1016/0166-6851(86)90132-5. [DOI] [PubMed] [Google Scholar]