Abstract
A new approach to maximize data recovery from siderite-hosted fossils is presented. Late Carboniferous trigonotarbids (Arachnida: Trigonotarbida) from Coseley, UK, were chosen to assess the potential of high-resolution X-ray micro-tomography (XMT). Three-dimensional computer reconstruction visualizes the animals at 20 µm or better resolution, resolving subtle and previously unseen details. Novel data recovered includes (possibly plesiomorphic) retention of endites on leg coxae of Cryptomartus hindi (Anthracomartidae) and highlights further similarities between this family and the Devonian Palaeocharinidae. Also revealed is a flattened body with robust anterior limbs, implying a hunting stance similar to modern crab spiders (Thomisidae). Eophrynus prestvicii (Eophrynidae) had more gracile limbs but a heavily ornamented body, with newly identified upward-pointing marginal spines on the opisthosoma. Its habitus is comparable with certain modern laniatorid harvestmen (Opiliones). These findings demonstrate the potential of XMT to revolutionize the study of siderite-hosted Coal Measures fossils.
Keywords: Trigonotarbida, Coal Measures, computed tomography, X-ray micro-tomography, siderite
1. Introduction
Many Coal-Measures Lagerstätten host fossils within early-diagenetic siderite (FeCO3) concretions (Allison & Briggs 1991). Examples include Coseley (UK), Montceau-les-Mines (France) and Mazon Creek (USA). Early incorporation into concretions typically prevents compaction; hence well-preserved, three-dimensional fossils are common, but extracting maximal information from them has long been problematic. Traditional approaches rely on splitting concretions and studying the portion of the fossil thus revealed; morphological data recovery is typically incomplete. In arachnids, for example, the hydraulically extended legs (Parry & Brown 1959) curl underneath the body after death owing to the absence of haemolymph pressure and their distal ends are typically hidden within the matrix in three-dimensionally preserved fossils. The preparation of latex casts may help obtain hidden morphological data (e.g. Petrunkevitch 1949, figs 200–202), but rarely recovers all available details and risks damaging delicate structures.
X-ray micro-tomography (XMT) and related techniques have the potential to achieve better results (Sutton 2008). To test whether XMT could recover complete, three-dimensional morphologies of siderite-hosted fossils, well-preserved examples of trigonotarbids from the Coseley Lagerstätte were chosen. This extinct arachnid order is the most basal member of the Pantetrapulmonata clade (Shultz 2007), which also includes spiders, whip spiders and whip scorpions. Superficially spider-like, trigonotarbids lack spinnerets and characteristically possess opisthosomal tergites divided into median and lateral plates. They range from the Late Silurian to Early Permian (ca 414–290 Myr ago; Dunlop & Brauckmann 2006). Around 70 species are known, among which the exceptionally preserved Devonian palaeocharinids from the Rhynie and Windyfield Cherts (Scotland) yield valuable morphological data (Hirst 1923; Fayers et al. 2005). However, the majority of trigonotarbid species—and thus the largest potential data source—come from the Late Carboniferous of Europe and North America (Petrunkevitch 1949). If XMT-based ‘virtual palaeontology’ can recover new information from such a well-studied group, it has the potential to transform the study of all three-dimensionally preserved siderite-hosted fossils.
2. Material and methods
A specimen of the anthracomartid Cryptomartus hindi (Pocock, 1911) (Natural History Museum, London, NHM In22841) was scanned. Also selected for study was a specimen of the eophrynid Eophrynus prestvicii (Buckland, 1837) (Lapworth Museum of Geology, Birmingham, BU699). Both originate from the Clay croft open-cast works of the Coseley Lagerstätte, Staffordshire, in the 10 foot ironstone measures, Duckmantian in age (ca 311 Ma; Wilson 2005). They are typical of the period, consisting of partially kaolanite-infilled voids within siderite nodules.
Specimens were scanned on a Metris X-Tek HMX-ST scanner (NHM, London) with a tungsten reflection target at 200 mA and 225 kV, 0.17–1 s exposure times for 3142 projections and a 1 mm copper filter. The 4MP (2000 × 2000) Perkin Elmer detector panel provided a voxel size (resolution) of 15–25 µm. Three-dimensional models were created from the tomographic datasets using the custom SPIERS software suite developed by one of us (M.D.S.), implementing the methods described by Sutton et al. (2002). Threshold images were created for each slice (all pixels darker than a user-defined grey-level considered fossil), and data were manually cleaned. Distinct structures were assigned to ‘masks’, allowing each to be rendered as an individual isosurface (Sutton 2008). This approach requires occasional arbitrary termination of features such as limbs once indistinguishable from the body, but allows false colouring of structures and removal of artefacts such as cracks. Through visualization and iterative improvement of the masks and editing, accurate models of the fossils were created. Publication-quality images were generated using raytracing application Blender (blender.org).
3. Results
General descriptions of material examined here can be found in Petrunkevitch (1949). We outline these for both species and then focus our attention on novel features revealed (or confirmed) by tomography. Results (figure 1) are also available as movies (electronic supplementary material).
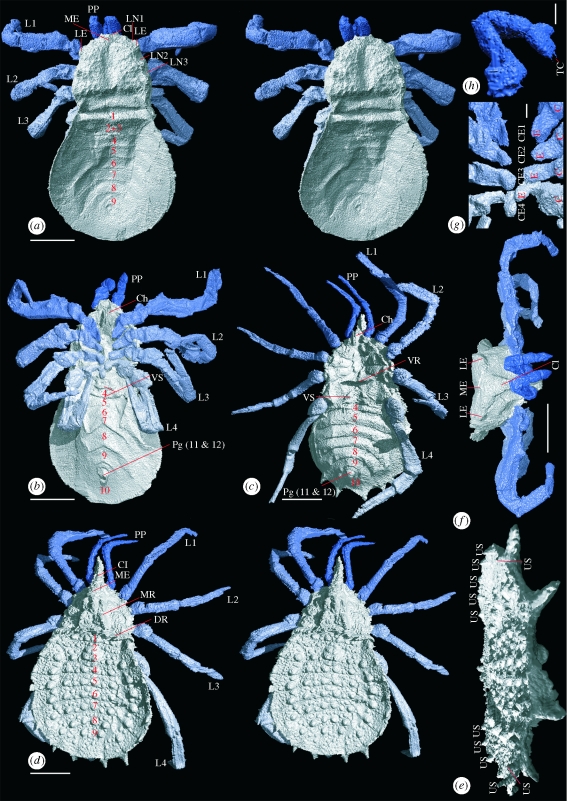
Figure 1.
Digital visualizations of Carboniferous trigonotarbids. (a) Stereo-pair showing Cryptomartus hindi in dorsal view (NHM In22841); (b) C. hindi ventral view; (c) Eophrynus prestivicii ventral view (BU699); (d) E. prestivicii stereo-pair dorsal view; (e) E. prestivicii posterior dorsal view, legs removed; (f) C. hindi anterior view; (g) C. hindi endites, body removed; (h) C. hindi pedipalps. (a–d,f): scale bar, 5 mm; (g,h): scale bar, 1 mm. (e) Maximum width 15 mm. TC, tarsal claw; US, upward pointing spines; LE, lateral eye tubercles; ME, median eye tubercle; L1–L4, limbs; Cl, clypeus; LN1–3, lateral notches; Ch, chelicerae; VS, ventral sacs; Pg, pygidium; VR, ventral ridge; DR, dorsal ridge; MR, lobed median ridge; C, coxa; E, endite; CE1–4, coxal endites; 1–12, segment number.
Cryptomartus hindi (figure 1a,b) is a large trigonotarbid; this specimen is 23 mm long and 14 mm wide, although smaller examples are known. The carapace is box-like, with a granular cuticle texture and a strong, ventrally projecting clypeus anteriorly. The chelicerae comprise two articles; they hang vertically and paraxially from the ventral surface (‘palaeognath’ orientation). The flattened opisthosoma is oval, with tergites divided ex-sagittally into one median, two lateral and two marginal sections and the posterior-most, number nine, divided longitudinally (figure 2). Two and three form a diplotergite. The sternites become chevron-shaped posteriorly, and the almost-triangular segment 10 bears a prominent pygidium. Anterior to sternite five are ventral sacs—two lobes anterior to a transverse, gently curved ridge.
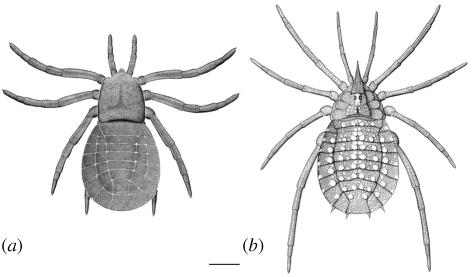
Figure 2.
Idealized reconstructions based on digital visualizations. Dorsal view: (a) C. hindi and (b) E. prestvicii. Scale bar, 5 mm.
XMT has confirmed palaeognath chelicerae for the first time, to our knowledge, in trigonotarbids outside the Rhynie/Windyfield Cherts. It has also elucidated a number of new features; the robust prosomal appendages are seen in full for the first time. They become increasingly triangular in cross section distally, and the tarsus (terminal podomere) is chelate in the pedipalps. The termination is less well resolved in appendages one through to four. The model also reconstructs the carapace in great detail; median and lateral eye tubercles are confirmed (figure 1f), and three lateral notches on each side of the carapace margin are associated with emerging leg trochanters. A median depression runs from behind the median eye tubercle into a linear posterior lateral depression in front of the prosoma–opisthosoma boundary. Ventrally, the sternum is recessed to accommodate newly observed proximal coxal endites on each walking leg (figure 1g), which grade from dorsoventrally flattened anteriorly to more cylindrical in form posteriorly, where they almost touch medially.
Eophrynus prestvicii (figure 1c,d) is another large trigonotarbid, being 25 mm in length, with a maximum width of 15 mm. The subtriangular carapace is drawn into a long and spine-like anteriorly directed clypeus. A strongly lobed median ridge bears the median eye tubercle, with three raised, semicircular lobes, separated by strong transverse suture lines to either side. The coxo-sternal region reveals prominent triangular coxae increasing in size posteriorly. The dorsal opisthosoma is heavily ornamented; most tergites bear six large tubercles (four on the median plate, one on each lateral plate), and sternites eight and nine have outward-directed spines. The ventral surface of the flattened opisthosoma features a pygidium enclosed by sternite 10 and ventral sacs (two lobes anterior to sternite four). The untuberculated sternites show a slight posterior recurvature.
Among fossil arachnids, E. prestvicii has achieved an almost iconic status; its images are regularly reproduced in both technical and popular palaeontological literature and it has been extensively studied. Nonetheless, our XMT study of a single specimen has revealed new detail. Palaeognath chelicerae are confirmed, and for the first time, to our knowledge, the legs and pedipalps revealed in full. They are long and slender and possess an almost spherical trochanter. XMT reveals high and conical lateral tubercles and dorsally directed spines on the postero-lateral margins of sternites five to seven (figure 1e). Also newly resolved are transverse ridges marking the posterior margin of the prosoma on both the dorsal and ventral surfaces.
4. Discussion
This study demonstrates the potential of XMT for reconstructing complete three-dimensional organisms from siderite-concretion fossils (figure 1). Morphological features that continue into the matrix, such as appendages, can now be studied in detail. The height and thickness of specimens can be more effectively visualized. Resolutions better than 20 µm are achievable, resolving fine details such as claws or spines. The ability to digitally manipulate models as ‘virtual fossils’ provides a powerful means of studying their morphology, facilitating accurate idealized reconstructions (figure 2).
Our approach has yielded the most complete morphological overview yet obtained of a ‘typical’ anthracomartid. This model also allows us to infer the likely stance of C. hindi. The anterior legs are preserved rotated with their prolateral side uppermost and held slightly forward and aloft, as if the bodyweight was borne principally on the posterior limbs (figure 1f). Such a ‘laterigrade’ stance is comparable to that used by modern crab spiders (Thomisidae). Assuming that this limb orientation was typical of anthracomartids (as implied by our unpublished observations of other material), they can be inferred to have been ambush predators; grabbing prey with their outstretched, prolaterally orientated forelimbs. The dorsoventral thinness of the opisthosoma is unusual. However, the study by one of us (R.G.) of conspecific specimens in the NHM London suggests that small gaps between posterior opisthosomal sclerites are typical. We suggest that these gaps represent flexible membranes and that the opisthosoma could expand when the animal was well fed or (in females) gravid. The flexibility of this region would have offered less resistance to flattening during burial.
These results highlight important similarities between the Anthracomartidae and the (probably more basal) Palaeocharinidae. Both have a ‘box-like’ carapace with an anterior clypeus and similarly proportioned leg and pedipalp articles (Dunlop 1996). Although Petrunkevitch (1949) regarded anthracomartids as blind, the carapace in both groups actually bears median and lateral eye tubercles (figure 1f). Palaeocharinids also have chevron-shaped sternites (Fayers 2005) and an opisthosomal diplotergite. A significant anthracomartid character resolved here is the presence of coxal endites. Although not formally described from trigonotarbids before, they have been illustrated in Rhynie palaeocharinids (Hirst 1923, fig. 5, pl. 14b). Comparable endites are not known from other arachnids (living or extinct). While their presence could be interpreted as autapomorphic for Trigonotarbida, their location on the inner face of the leg coxa is equivalent to the position of the spinous gnathobases used to masticate prey in arachnid outgroups such as Xiphosura and Eurypterida (Shultz 2007, character 52). Loss of the spiny pedal gnathobases sensu stricto was regarded by Shultz as a possible arachnid synapomorphy, although our results suggest that vestiges of these structures may have been retained as the mesally expanded coxae of at least some (possibly basal) trigonotarbids. The chelate pedipalp termination in C. hindi (figure 1h) could also be significant, as post-cheliceral chelae have recently been identified in Palaeocharinidae (Dunlop et al. 2009), with implications for the position of Trigonotarbida within Arachnida. While a full analysis of the relationship between the Palaeocharinidae and Anthracomartidae is beyond the scope of this paper, these shared characters suggest that it may be close.
Our reconstruction of E. prestvicii provides, to our knowledge, the first accurate picture of the entire limb series, including the chelicerae in their palaeognath orientation. Fine details newly recovered include ventral and dorsal transverse ridges on the prosoma and dorsally directed spines originating from the opisthosomal margins. This robust ornament is interpreted as defensive, an adaptation to increase handling time for predators. Loman (1900) drew attention to similarities between E. prestvicii and certain modern members of the Laniatores suborder of the Opiliones (harvestmen), for Loman evidence that these groups were closely related. Trigonotarbids had book lungs and are unquestionably pantetrapulmonate arachnids (Shultz 2007), but parallels can be drawn between E. prestvicii and lanatorid harvestmen—the extinct eophrynids may have had a similar mode of life to this leaf-litter dwelling, derived harvestman lineage.
These results demonstrate the ability of XMT to differentiate the void left by the original organism's decay within sideritic host material and the power of computer-based three-dimensional visualizations of the resultant datasets as a tool for morphological analysis. Despite the small number of specimens scanned and the well-studied nature of the trigonotarbid taxa investigated, significant new morphological data were recovered, with important implications for our understanding of trigonotarbid palaeobiology. This suggests that our approach could be profitably applied to many nodule-hosted Coal Measures fossils, offering a new window to the biotas of these early forest environments.
Acknowledgements
We thank Claire Mellish (NHM) and Jon Clatworthy (BU) for the loan of material. R.G. thanks Richard Abel and Stig Walsh for training in computer tomography techniques.
References
- Allison P. A., Briggs D. E. G.1991Taphonomy: releasing the data locked in the fossil record New York, NY: Plenum Press [Google Scholar]
- Dunlop J. A.1996Systematics of fossil arachnids. Rev. suisse Zool. hors série, 173–184 [Google Scholar]
- Dunlop J. A., Brauckmann C.2006A new trigonotarbid from the Coal Measures of Hagen-Vorhalle, Germany. Foss. Rec. 9, 130–136 (doi:10.1002/mmng.200600004) [Google Scholar]
- Dunlop J. A., Kamenz C., Talarico G.2009A fossil trigonotarbid arachnid with a ricinuleid-like pedipalpal claw. Zoomorphology (doi:10.1007/s00435-009-0090-z) [Google Scholar]
- Fayers S. R., Dunlop J. A., Trewin N. H.2005A new Early Devonian trigonotarbid arachnid from the Windyfield Chert, Rhynie, Scotland. J. Syst. Palaeontol. 2, 269–284 (doi:10.1017/S147720190400149X) [Google Scholar]
- Hirst S.1923On some arachnid remains from the Old Red Sandstone (Rhynie Chert Bed, Aberdeenshire). Ann. Mag. Nat. Hist. 12, 455–474 [Google Scholar]
- Loman J. C. C.1900Ueber die geographische Verbreitung der Opilioniden. Zool. Jb. Syst. 13, 71–104 [Google Scholar]
- Parry D. A., Brown R. H. J.1959The hydraulic mechanism of the spider leg. J. Exp. Biol. 36, 423–433 [Google Scholar]
- Petrunkevitch A. I.1949A study of Palaeozoic Arachnida. Trans. Conn. Acad. Arts Sci. 37, 69–315 [Google Scholar]
- Shultz J. W.2007A phylogenetic analysis of the arachnid orders based on morphological characters. Zool. J. Linn. Soc. 150, 221–265 (doi:10.1111/j.1096-3642.2007.00284.x) [Google Scholar]
- Sutton M.2008Tomographic techniques for the study of exceptionally preserved fossils. Proc. R. Soc. B 275, 1587–1593 (doi:10.1098/rspb.2008.0263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton M. D., Briggs D. E. G., Siveter D. J., Siveter D. J., Orr P. J.2002The arthropod Offacolus kingi (Chelicerata) from the Silurian of Herefordshire, England: computer based morphological reconstructions and phylogenetic affinities. Proc. R. Soc. Lond. B 269, 1195–1203 (doi:10.1098/rspb.2002.1986) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson H. M.2005A new genus of Archipolypodan millipede from the Coseley Lagerstätte, Upper Carboniferous, UK. Palaeontology 48, 1097–1100 (doi:10.1111/j.1475-4983.2005.00496.x) [Google Scholar]