Abstract
The duration of startles provides an inverse measure of motivation to resume the previous activity. Here, we use a novel method in which one convict cichlid fish (Amatitlania nigrofasciata) of a competing pair was startled independently of the opponent. Fish were given various opponents and the mean startle duration determined. This mean was negatively correlated with the mean use of highly escalated ‘frontal activities’ such as biting and frontal display, but not the less escalated lateral displays or tail beating. Thus the startle duration was a reliable surrogate measure of the most escalated components of aggressive interactions. That is, it provided a motivational probe for aggressiveness of individual fish. Fight motivation is often determined in terms of fight duration or physiological costs for losers, who reveal the costs they are prepared to pay. We discuss various potential advantages of the motivational probe over previous measures, particularly with respect to winners and losers and different times during the interactions.
Keywords: motivation, aggression, cichlid, startle
1. Introduction
The motivational state of competing animals is typically inferred by variation in the costs they are prepared to pay in terms of contest duration, vigour, injury or physiological change, such as lactate accumulation or glucose depletion (Arnott & Elwood 2008). However, measuring the costs at the termination of fights only gives information about the motivation of the loser, because the winner does not reveal the full cost it was prepared to pay. Moreover, this approach provides a poor measure of loser motivation because it is not possible to know whether the costs relate to the total the loser is able to pay, as determined by its resource holding potential (RHP), or whether they relate to what the loser was willing to pay, reflecting motivational state. However, motivational state may be investigated by the application of a novel, potentially startling stimulus, with the speed of recovery reflecting the motivation to continue the previous activity (Culshaw & Broom 1980; Elwood et al. 1998). The technique has the benefit of providing a measure that is independent of ongoing behaviour, which is useful because current behaviour may not accurately reflect motivational state, especially in fights where it may be important not to signal future intentions (Elwood et al. 1998). Essentially this technique examines the trade-off between two motivational systems, one involving fighting, the other predator avoidance. A major advantage of this motivational probe is that it could be applied at any time in the contest and, unlike other methods, its application would not be limited to the eventual loser. Furthermore, by applying the probe at different times the potential exists to plot motivational change as the contest progresses (Briffa & Elwood 2001).
To date, however, the probe has only been applied in hermit crab (Pagurus bernhardus) contests (Elwood et al. 1998; Briffa & Elwood 2001). Attackers that could effect a high gain (i.e. improving their shell quality by taking the defenders' shell) showed shorter startles than those with low gain, indicating higher fight motivation. A defender withdraws into its shell and only the attacker is open to being startled. Thus the attacker's response is not dependent on the actions of the opponent. However, for other animal contests, where both opponents fight in a similar way, a technique is required so that just one opponent is subject to the novel startling stimulus. Here, we suggest a novel method that allows a startle to be induced in one of two cichlid fish when displaying to each other from adjacent tanks. This enables us to probe the motivation of the contestant of our choice, independent of having an effect on the opponent's behaviour. Correlations between the duration of the startle response and of key agonistic activities are used to determine if (i) motivation may be probed and (ii) particular activities are indicative of a high aggressive motivation.
2. Material and Methods
Male Amatitlania nigrofasciata (standard length, 48.3–69.9 mm) were obtained from Grosvenor Tropicals (Belfast) and housed in groups of 6 to 18 in aquaria (54–90 l) at 27 ± 2°C and 12∶12 h light:dark cycles. Chemical and biological filtration, substantial aeration and a 6-cm gravel substrate with plants, bogwood and terracotta pots as refuges were provided. Fish were fed ‘Hi-grow (JMC)’ granules once a day.
Following acclimation for >6 days, fish were placed one per 12 l tank. These were equipped with a heater, thermometer, air-driven filter (all of which were removed during contest observations) and gravel substrate. Visual isolation for 48 h was achieved by aligning tanks and separating them with opaque plastic dividers. The absence of social interaction mitigates the behavioural effects of previous winning or losing (Hsu et al. 2006; Prenter et al. 2008) and also heightens aggression (Gomez-Laplaza & Morgan 2000). The duration of isolation was carefully selected (in accordance with previous contest studies in A. nigrofasciata, such as Reddon & Hurd 2009) to balance the need to mitigate previous social experience and enable territory formation, while trying to minimize the stress associated with isolation.
Random pairs were created by placing a wooden observation chamber (70 cm long × 45 cm wide × 40 cm high) with a one-way mirror over two visually isolated adjacent tanks (see electronic supplementary material for a diagram of the experimental setup). The opaque divider was removed 30 min later and the tanks pushed together, enabling the contestants to interact across the glass ‘display window’ at adjacent ends of the tanks. A 6 g glass marble was dropped through a hole on top of the observation chamber 2 min after the onset of display by both fish. The marble fell a distance of 22 cm before landing with a distinct splash and sinking to the substrate within the focal fish tank. The marble landed behind a narrow vertical screen of opaque plastic (6 cm wide × 20 cm long) inside the tank on the ‘display window’ so that only the ‘focal’ fish was aware of the stimulus and startled (see electronic supplementary material). The startle duration, defined as the time the focal fish remained motionless following the marble drop, was recorded. Using the same technique, the focal fish was subjected to a second startle 5 min following resumption of display. Interactions were terminated 5 min following recovery from the second startle. All interactions were recorded using a camcorder (Sony) and analysed with a Psion Workabout hand-held computer configured as a time-event recorder using the Observer v. 3.0 software (Noldus Technology). We recorded the duration of the frontal display (the contestant faces the opponent while extending its fins and lowering its gill covers) and lateral display (similar in form to the frontal display except the contestant presents its side to the opponent), the total number of attempted bites (open-mouthed contacts with the glass) and tail beats (contestant flexes its tail and water is pushed, usually in the direction of the other fish; Enquist et al. 1990).
The opaque dividers were replaced and 24 h later the original pairs were re-tested in the same manner, but with the previously non-startled stimulus fish now being designated as the focal fish. Preliminary analysis (ANOVA) showed no dependence of data on the order of testing (F1,25 = 1.527, p = 0.228). Tanks were then rearranged and dividers put in place to create more novel pairings for testing 24 h later. The process was then repeated so that each individual was subjected to three novel pairings (n = 36). The decision to use individuals in more than one pairing was taken to gain a measure of the behaviour of each fish that was not unduly influenced by their responses to a specific opponent. Three fish did not display on one occasion, and six could not be observed for their third interaction (due to unforeseen circumstances). In these cases mean values for the two interactions were used whereas in the remainder the mean value for three was used, thus avoiding pseudoreplication. Fish were weighed (wet mass, g), and gender verified.
Pearson product–moment correlations were used to test for correlations between mean startle duration and mean of each aggressive activity. Data were tested for normality using the Kolmorogov–Smirnoff (K–S) test and log(n + 1) transformed as appropriate. Data are presented as the proportion of the interaction spent performing each display (frontal and lateral) and the rates (number per second) of tail beating and biting.
3. Results
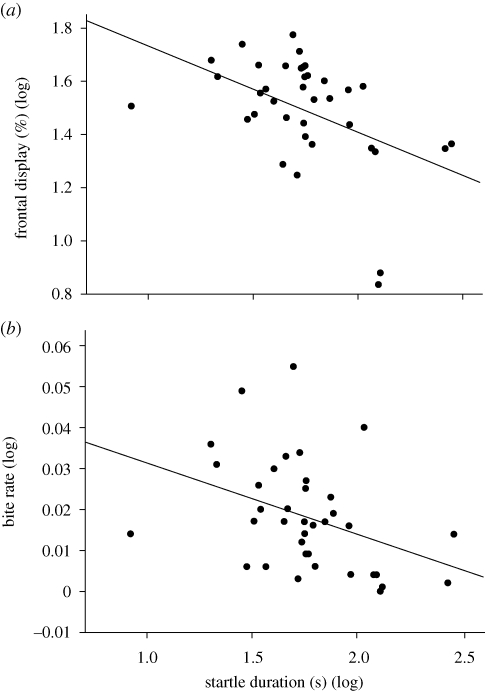
The average startle (log) duration of males was significantly negatively correlated with frontal display (r = −0.465, n = 36, p = 0.0038; figure 1a) and bite rate (r = −0.377, n = 36, p = 0.0225; figure 1b), but not with lateral display (r = 0.083, n = 36, p = 0.635) or tail beat rate (r = −0.255, n = 36, p = 0.134).
Figure 1.
Negative correlation between male average startle duration (log (n + 1)) and (a) the percentage of the interaction spent using frontal display and (b) the bite rate (number per second).
4. Discussion
Contesting cichlids alternate between lateral activities and head-on activities. The former include lateral displays and tail beats, which appear to be low risk in terms of injury. Frontal activities, however, include the frontal display, which leads to biting and mouth-wrestling in unrestrained fish (Enquist et al. 1990). In the present study, the fish could not contact each other so the biting at the glass was probably an attempt to either bite the opponent and/or mouth-wrestle. The latter activity can lead to significant injury, for example, jaw dislocation, and is thus considered highly escalated (Enquist et al. 1990). Startle duration did not correlate with lateral displays and tail beats, but, because these are not thought to be highly escalated (Reddon & Hurd 2009) it is perhaps unsurprising that they fail to reflect an underlying aggressive motivational state. Lateral displays and tail beating are thought to facilitate assessment of fighting ability (Enquist et al. 1990; Hurd 1997). However, startle duration showed clear negative correlations with frontal displays and biting. That is, the startle provided a surrogate measure of escalated fighting and thus appears to reflect the motivation to fight rather than display.
Displays have long been considered to occur when the animal is in an ambivalent motivational state in which the tendency to approach is balanced by the tendency to withdraw (Baerends 1975). Escalated fighting, however, clearly occurs when the tendency to withdraw is low (Maynard Smith & Parker 1976). The present data support this idea by showing that aggressive motivation, as determined by the startle, relates to escalated fight activities rather than lateral displays. Our results are also consistent with those from hermit crab contests, where a link was demonstrated between the duration of a startle and subsequent fight vigour (Elwood et al. 1998), and eventual winners had shorter startles, suggesting that they were highly motivated (Briffa & Elwood 2001). Startles are an established method of probing motivation in non-agonistic contexts, for example, in feeding and preening in chicks (Culshaw & Broom 1980), walking in locusts (Moorehouse et al. 1987) and acquiring a new shell in hermit crabs (Jackson & Elwood 1990), indicating the theoretical basis for their use in contests.
The probe provides a way of measuring aggressive motivation that is more accurate than simply observing a contest to measure duration or final intensity. It provides a means of investigating information gathering about fighting ability, because assessment is predicted to alter motivation to fight (Arnott & Elwood 2009). Moreover, if applied relatively early in an aggressive encounter it negates the need for contestants to engage in potentially lengthy, welfare-compromising contests (Huntingford 1984). However, by using it at different times in the fight it may be possible to determine how motivation changes as the fight progresses (Briffa & Elwood 2001). There is also scope to use the startle probe in studies of resource value (Elwood et al. 1998). Game theory models predict that fight motivation will increase with the value of the contested resource (Maynard Smith & Parker 1976; Enquist & Leimar 1987), and variation between startle time and resource quality in a fight can be used to infer resource assessment (Arnott & Elwood 2008). Furthermore, it may be used to provide insights into the use of specific activities at particular motivational states, as in the present study. Also, by enabling researchers to probe the motivation of the opponent of their choice, it should be possible to compare the motivation of dominants and subordinates, larger versus smaller individuals, and individuals with established territories versus intruders.
In summary, the motivational probe outlined here offers an alternative way to measure aggressive motivation, overcoming limitations of current approaches, which are only capable of revealing a poor estimate of the motivational state of the loser. Furthermore, the probe provides a means of measuring motivation that is independent of ongoing behaviour, which is useful given current behaviour may not accurately reflect motivational state, especially in fights where it may be important not to signal future intentions. We suggest this probe will have a number of useful applications and offers a further tool in the armoury of workers investigating animal contests.
Acknowledgment
All work was carried out under a UK Home Office Licence after review by the Queen's University Ethical Review Committee. The total number of fish used in this study was reduced by using each fish as both focal and stimulus fish.
We thank the Department of Agriculture and Rural Development for Northern Ireland (DARDNI) for funding and two anonymous referees for valuable comments.
References
- Arnott G., Elwood R. W.2008Information-gathering and decision-making about resource value in animal contests. Anim. Behav. 76, 529–542 (doi:10.1016/j.anbehav.2008.04.019) [Google Scholar]
- Arnott G., Elwood R. W.2009Assessment of fighting ability in animal contests. Anim. Behav. 77, 991–1004 (doi:10.1016/j.anbehav.2009.02.010) [Google Scholar]
- Baerends G. P.1975An evaluation of the conflict hypothesis as an explanatory principle for the evolution of displays. In Function and evolution in behaviour (eds Baerends G., Beer C., Manning A.). Oxford, UK: Clarendon Press [Google Scholar]
- Briffa M., Elwood R. W.2001Motivational change during shell fights in the hermit crab Pagurus bernhardus. Anim. Behav. 62, 505–510 (doi:10.1006/anbe.2001.1764) [Google Scholar]
- Culshaw A. D., Broom D. M.1980The imminence of behavioural change and startle responses of chicks. Behaviour 73, 64–76 (doi:10.1163/156853980X00168) [Google Scholar]
- Elwood R. W., Wood K. E., Gallagher M. B., Dick J. T. A.1998Probing motivational state during agonistic encounters in animals. Nature 393, 66–68 (doi:10.1038/29980) [Google Scholar]
- Enquist M., Leimar O.1987Evolution of fighting behaviour: the effect of variation in resource value. J. Theor. Biol. 127, 187–205 (doi:10.1016/S0022-5193(87)80130-3) [Google Scholar]
- Enquist M., Leimar O., Ljungberg T., Mallner Y., Segerdahl N.1990A test of the sequential assessment game: fighting in the cichlid fish Nannacara anomala. Anim. Behav. 40, 1–14 (doi:10.1016/S0003-3472(05)80660-8) [Google Scholar]
- Gomez-Laplaza L. M., Morgan E.2000Laboratory studies of the effects of short-term isolation on the aggressive behaviour in fish. Marine Freshwater Behav. Physiol. 33, 63–102 (doi:10.1080/10236240009387083) [Google Scholar]
- Hsu Y. Y., Earley R. L., Wolf L. L.2006Modulation of aggressive behaviour by fighting experience: mechanisms and contest outcomes. Biol. Rev. 81, 33–74 (doi:10.1017/S146479310500686X) [DOI] [PubMed] [Google Scholar]
- Huntingford F. A.1984Some ethical issues raised by studies of predation and aggression. Anim. Behav. 32, 210–215 (doi:10.1016/S0003-3472(84)80339-5) [Google Scholar]
- Hurd P. L.1997Cooperative signalling between opponents in fish fights. Anim. Behav. 54, 1309–1315 (doi:10.1006/anbe.1997.0531) [DOI] [PubMed] [Google Scholar]
- Jackson N. W., Elwood R. W.1990Interrupting an assessment process to probe changes in the motivational state. Anim. Behav. 39, 1068–1077 (doi:10.1016/S0003-3472(05)80779-1) [Google Scholar]
- Maynard Smith J., Parker G. A.1976The logic of asymmetric contests. Anim. Behav. 24, 159–175 (doi:10.1016/S0003-3472(76)80110-8) [Google Scholar]
- Moorehouse J. E., Fosbrooke I. H. M., Ludlow A. R.1987Stopping a walking locust with sound: an analysis of variation in behavioural threshold. Exp. Biol. 46, 193–201 [PubMed] [Google Scholar]
- Prenter J., Taylor P. W., Elwood R. W.2008Large body size for winning and large swords for winning quickly in swordtail males, Xiphophorus helleri. Anim. Behav. 75, 1981–1987 (doi:10.1016/j.anbehav.2007.12.008) [Google Scholar]
- Reddon A. R., Hurd P. L.2009Differences in aggressive behavior between convict cichlid color morphs: amelanistic convicts lose even with a size advantage. Acta Ethologica 12, 49–53 (doi:10.1007/s10211-009-0054-9) [Google Scholar]