Abstract
Lacking the capacity for thermogenesis, most ectotherms inhabiting thermally heterogeneous environments rely instead upon exploiting that ambient heterogeneity. In many cases they maintain body temperatures within a narrow range despite massive spatial and temporal variation in ambient conditions. Reliance on diverse thermal opportunities is reflected in specific terms for organisms that bask in sunlight to regulate their temperature (heliotherms), or that press their bodies against warm substrates to facilitate heat flow (thigmotherms), or that rely on large body mass to maintain thermal constancy (gigantothermy). We propose an additional category of thermoregulators: kleptotherms, which regulate their own temperature by ‘stealing’ heat from other organisms. This concept involves two major conditions: the thermal heterogeneity created by the presence of a warm organism in a cool environment and the selective use of that heterogeneity by another animal to maintain body temperatures at higher (and more stable) levels than would be possible elsewhere in the local area. Kleptothermy occurs in endotherms also, but is usually reciprocal (rather than unilateral as in ectotherms). Thermal monitoring on a small tropical island documents a possible example of kleptothermy, based on high stable temperatures of a sea snake (Laticauda laticaudata) inside a burrow occupied by seabirds.
Keywords: ectothermy, thermoregulation, thigmothermy, reptile, snake
Reliance on other animals to create the thermal heterogeneity exploited by behavioural thermoregulation is widespread. In some cases, conspecifics are exploited to increase effective mass (as in tightly clustered groups of lizards (Shah et al. 2003) and snakes (Myres & Eells 1968; Aubret & Shine 2009)). Similar clustering (or huddling) behaviour is also widespread in gregarious endotherms (e.g. Brown & Foster 1992; Arends et al. 1995; Ancel et al. 1997). Such behaviour enables a group of individuals to increase its thermal inertia, retard heat loss and/or reduce the per capita metabolic expenditure needed to maintain stable body temperatures (Myres & Eells 1968; Canals et al. 1989, 1998). In other cases, physical contact with warmer conspecifics enables cold animals to achieve higher temperatures; such an advantage may explain the phenomenon of female mimicry in Canadian garter snakes, Thamnophis sirtalis (Shine et al. 2001). In this system, males that have recently emerged from hibernation attract courtship from other males, gaining significant heat transfer in the process (Shine et al. 2001).
The most dramatic examples of kleptothermy (heat-stealing), however, should come from cases where ectotherms regulate their own temperatures by exploiting the high and constant body temperatures exhibited by endothermic species. Especially if the endotherm is ensconced within a well-insulated site (such as a nest or a burrow), any animal sharing that habitat probably will experience higher and/or constant ambient temperatures than would otherwise be the case. The endotherms involved are not only mammals and birds; termites maintain high and constant temperatures within their mounds, providing otherwise-unavailable thermal regimes that are exploited by a wide array of lizards, snakes and crocodilians (Vanzolini 1948; Leloup 1984; Magnusson et al. 1985; Ehmann et al. 1991). However, many cases of kleptothermy involve ectotherms sheltering inside the burrows used by endotherms. For example, seabird burrows are common retreat-sites for reptiles as diverse as Australian tiger snakes (Notechis scutatus, Worrell 1958) and New Zealand tuatara (Sphenodon punctatus, Newman 1987), and mammal burrows are used by geckos (Finlayson 1941).
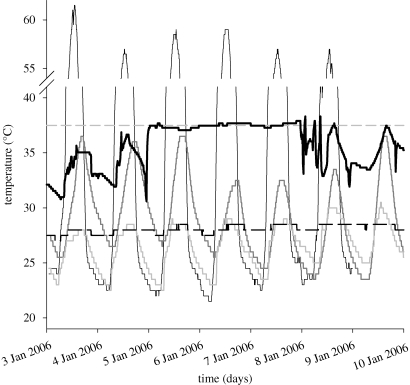
While conducting ecological studies on amphibious sea snakes (blue-banded sea kraits, Laticauda laticaudata) in New Caledonia, we documented a possible example of the thermal benefits of kleptothermy (figure 1). One adult male snake (snout-vent length 94.5 cm, mass 217 g), to which we had attached a time-depth recorder (that also records temperature, TDR LTD-1110, Lotek Wireless Inc. Canada, sampling rate 450s, ±0.1°C; see Brischoux et al. 2007 for implantation methodology), spent three days within a burrow occupied by a pair of seabirds (wedge-tailed shearwater, Puffinus pacificus) that were incubating their chick. The snake's body temperature over this period was higher and more constant (37.5 ± 0.2°C, range 37.1–37.9°C, figure 1) than at any other time during the period we monitored the animal (or during periods when we have monitored other sea kraits), and very different from the thermal regimes available in any other microhabitat available to the snake on this small island (figure 1). In particular, the diel range of body temperatures of this snake was more stable (coefficient of variation (CV) = 0.005) during the period it occupied the shearwater burrow, compared with 31.7 ± 3.7°C (CV = 0.117) when in other available microhabitats (figure 1). The critical role of the birds in maintaining the high burrow temperature is evident from the low, stable temperature of an unoccupied burrow (28.1 ± 0.4°C, range 27.0–28.5°C, figure 1).
Figure 1.
Ambient temperatures on a small island (Signal Islet, New Caledonia) and body temperatures of a male sea krait (Laticauda laticaudata) on that island over a seven-day period in January 2006. Thermal regimes were monitored with data-loggers (ACR SmartButton data Loggers: ±0.5°C, sampling rate 5 min excepted for the snake, see text); all sites other than the occupied shearwater burrow provided cooler and/or more variable temperatures than did the burrow used by the snake from 5 to 8 January (open: 35.3 ± 13.4°C, ‘dry’ beach rock: 29.1 ± 3.6°C, intertidal beach rock: 26.5 ± 2.0°C, vacant sea bird burrow: 28.1 ± 0.4°C). Beach rocks and vacant bird burrows are frequently used as shelters by L. laticaudata (Bonnet et al. 2009). Thin black line indicates open; thick grey line indicates ‘dry’ beach rock; thin grey line indicates intertidal beachrock; thick broken black dashes indicate vacant seabird burrow; thick black line indicates L. laticaudata (adult male); thin broken grey dashes indicate constant 37.5°C temperature).
Although several authors have reported co-habitation of ectothermic vertebrates with endothermic organisms within well-insulated sites (see above), our dataset appears to be the first to quantify the potential thermal benefits of this phenomenon in a free-ranging ectotherm. Our data are unreplicated and preliminary, and do not tell us whether or not the snakes actively select occupied bird burrows, or whether such occupancy conveys any fitness benefits (or, indeed, costs). Nonetheless, the data do confirm that the presence of an endotherm can create thermal heterogeneity at a spatial and temporal scale relevant to a mobile ectotherm in the same area, and can do so in a way that is potentially exploitable by the ectotherm. If such conditions are frequently satisfied, then the ability to exploit endotherm-derived ‘hotspots’ may well be significant for some species. Kleptothermy may be distinctive not only in the source of heat involved (the byproduct of another organism's metabolism and/or thermoregulatory behaviour) but also in the magnitude and stability of the thermal regimes potentially achievable; alternative tactics such as heliothermy and other types of thigmothermy typically function only during daylight hours, and do not provide as much thermostability. Given the thermal dependence of locomotor and physiological processes in ectotherms (Dawson 1975; Bennett 1990; Dorcas et al. 1997), the fitness benefits of kleptothermy for such animals could be considerable.
As with almost any definitional problem in biology, the boundaries of kleptothermy are unlikely to be clearcut and will require debate and discussion. However, the phenomenon relies upon two major conditions: (i) thermal heterogeneity created by the presence of a warm organism in a cool environment; and (ii) the selective use of that heterogeneity by another animal to maintain body temperatures at higher and more stable levels than would be possible elsewhere in the local area. Importantly, the source of heat in kleptothermy involves the metabolic thermogenesis of another animal, rather than solar radiation, volcanically warmed soil, or any other non-biotic source. Kleptothermy is widespread in endotherms as well as ectotherms (huddling behaviour of juvenile endotherms or microchiropterans are clear examples) but typically is reciprocal. By contrast, kleptothermy in ectotherms generally will be unilateral. Clustering together to retard heat loss thus constitutes kleptothermy in endotherms (because the slower cooling rate is partially owing to heat production by members of the group) but not in ectotherms (because slower cooling is entirely owing to thermal inertia (i.e. gigantothermy)). Endoparasites (including oviductal embryos) are not kleptotherms, because they cannot actively move about to select specific thermal regimes within a heterogeneous environment. Future research could usefully explore the occurrence, forms, and potential fitness consequences, of this putative thermoregulatory tactic.
Acknowledgements
The study was carried out under permits no. 6024-179/DRN/ENV and no. 6024-3601/DRN/ENV.
S. Lorioux and M. De Crignis helped during fieldwork, and C. Chevillon and D. Ponton (DRN Province Sud, IRD) gave logistical support. Two anonymous referees provided insightful comments on previous versions of this manuscript. We thank the Center National de la Recherche Scientifique, the Australian Research Council and the Australian Government (Endeavour Awards) for funding.
References
- Ancel A., Visser H., Handrich Y., Masman D., Le Maho Y.1997Energy saving in huddling penguins. Nature 385, 304–305 (doi:10.1038/385304a0) [Google Scholar]
- Arends A., Bonaccorso F. J., Genoud M.1995Basal rates of metabolism of nectarivorous bats (Phyllostomidae) from a semiarid thorn forest in Venezuela. J. Mammal. 76, 947–956 (doi:10.2307/1382765) [Google Scholar]
- Aubret F., Shine R.2009Causes and consequences of aggregation by neonatal tiger snakes (Notechis scutatus, Elapidae). Aust. Ecol. 34, 210–217 (doi:10.1111/j.1442-9993.2008.01923.x) [Google Scholar]
- Bennett A. F.1990Thermal dependence of locomotor capacity. Am. J. Physiol. 259, R253–R258 [DOI] [PubMed] [Google Scholar]
- Bonnet X., Brischoux F., Pearson D., Rivalan P.2009Beach rock as a keystone habitat for amphibious sea snakes. Environ. Conserv. 36, 62–70 (doi:10.1017/S0376892909005451) [Google Scholar]
- Brischoux F., Bonnet X., Cook T. R., Shine R.2007Snakes at sea: diving performances of free-ranging sea kraits. In Proc. 11th Annual Meeting on Health, Science and Technology, Tours University, France [Google Scholar]
- Brown C. R., Foster G. G.1992The thermal and energetic significance of clustering in the speckled mousebird, Colius striatus. J. Comp. Physiol. B 162, 658–664 (doi:10.1007/BF00296648) [Google Scholar]
- Canals M., Rosenmann M., Bozinovic F.1989Energetics and geometry of huddling in small mammals. J. Theor. Biol. 141, 181–189 (doi:10.1016/S0022-5193(89)80016-5) [DOI] [PubMed] [Google Scholar]
- Canals M., Rosenmann M., Novoa F. F., Bozinovic F.1998Modulating factors of the energetic effectiveness of huddling in small mammals. Acta Theriol. 43, 337–348 [Google Scholar]
- Dawson W. R.1975On the physiological significance of preferred body temperature of reptiles. In Perspectives of biophysical ecology (eds Gates D. M., Schmeri R. B.), pp. 443–474 New York, NY: Springer-Verlag [Google Scholar]
- Dorcas M. E., Peterson C. R., Flint M. E. T.1997The thermal biology of digestion in rubber boas (Charina bottea): physiology, behaviour, and environmental constraints. Physiol. Zool. 70, 292–300 [DOI] [PubMed] [Google Scholar]
- Ehmann H., Swan G., Swan G., Smith B.1991Nesting, egg incubation and hatching by the heath monitor Varanus rosenbergi in a termite mound. Herpetofauna 21, 17–24 [Google Scholar]
- Finlayson H. H.1941On central Australian mammals. Part II. The Muridae. Trans. R. Soc. South Australia 65, 215–232 [Google Scholar]
- Leloup P.1984Various aspects of venomous snake breeding in large scale. Acta Zool. Pathol. Antverp. 78, 177–198 [Google Scholar]
- Magnusson W. E., Lima A. P., Sampaio R. M.1985Source of heat for nests of Paleosuchus trigonatus and a review of crocodilian nest temperatures. J. Herpetol. 19, 199–207 (doi:10.2307/1564173) [Google Scholar]
- Myres B. C., Eells M. M.1968Thermal aggregation in Boa constrictor. Herpetologica 24, 61–66 [Google Scholar]
- Newman D. G.1987Burrow use and population densities of Tuatara (Sphenodon punctatus) and how they are influenced by fairy prions (Pachyptila turtur) on Stephens Island, New Zealand. Herpetologica 43, 336–344 [Google Scholar]
- Shah B., Shine R., Hudson S., Kearney M.2003Sociality in lizards: why do thick-tailed geckos (Nephrurus milii) aggregate? Behaviour 140, 1039–1052 (doi:10.1163/156853903322589632) [Google Scholar]
- Shine R., Phillips B., Waye H., LeMaster M., Mason R. T.2001Benefits of female mimicry to snakes. Nature 414, 267 (doi:10.1038/35104687) [DOI] [PubMed] [Google Scholar]
- Vanzolini P. E.1948Notas sobre os ofidios e lagartos da Cachoeira de Emas, no municipio de Pirassununga, Estado de São Paulo. Rev. Brasil. Biol. 8, 377–400 [PubMed] [Google Scholar]
- Worrell E.1958Song of the snake Sydney, Australia: Angus and Robertson [Google Scholar]