Abstract
The movement and dietary history of individuals can be studied using stable isotope records in archival keratinous tissues. Here, we present a chronology of temporally fine-scale data on the trophic niche of otariid seals by measuring the isotopic signature of serially sampled whiskers. Whiskers of male Antarctic fur seals breeding at the Crozet Islands showed synchronous and regular oscillations in both their δ13C and δ15N values that are likely to represent their annual migrations over the long term (mean 4.8 years). At the population level, male Antarctic fur seals showed substantial variation in both δ13C and δ15N values, occupying nearly all the ‘isotopic space’ created by the diversity of potential oceanic habitats (from high Antarctica to the subtropics) and prey (from Antarctic krill to subantarctic and subtropical mesopelagic fishes). At the individual level, whisker isotopic signatures depict a large diversity of foraging strategies. Some seals remained in either subantarctic or Antarctic waters, while the migratory cycle of most animals encompassed a wide latitudinal gradient where they fed on different prey. The isotopic signature of whiskers, therefore, revealed new multi-year foraging strategies of male Antarctic fur seals and is a powerful tool for investigating the ecological niche during cryptic stages of mammals' life.
Keywords: individual specialization, otariid, Southern Ocean, stable isotopes
1. Introduction
There is growing evidence that individuals within a population vary considerably in the way they use habitats and resources. Documenting the incidence and degree of niche variation and individual specialization is a first step towards understanding their basis and implications in various dimensions of ecology and evolutionary, and conservation biology (Bolnick et al. 2003). Stable isotopes are well suited for quantifying foraging strategies at both the population and the individual levels (Bearhop et al. 2004; Newsome et al. 2009). Stable carbon (δ13C) and nitrogen (δ15N) isotope ratios of consumers define the isotopic niche along two dimensions, with δ13C and δ15N values reflecting the consumers’ foraging habitat and trophic level, respectively. Correspondingly, diverse species-specific, sexual and individual foraging strategies have been described using stable isotopes on fur seals (Cherel et al. 2007). The temporal integration of blood, however, did not allow investigations of these strategies over the long term.
Here, we present a multi-year chronology of temporally fine-scale data on isotopic niche changes of male Antarctic fur seals (Arctocephalus gazella) breeding in the Crozet Islands, Southern Indian Ocean. Habitat and diet records were interpreted from the δ13C and δ15N values of whiskers that were serially sampled all along their length. The two basic underlying principles were first, that the isotopic composition of whiskers reflects diet at the time of their growth, because keratin is metabolically inert after synthesis (Rubenstein & Hobson 2004) and, second, that otariid whiskers grow continuously at a constant rate and are not shed (Hirons et al. 2001), thus retaining stable isotope records allowing the reconstruction of an individual's trophic history. Antarctic fur seals are arguably the best studied otariid. However, most work has focused on lactating females, with little information available on individual variations, the inter-breeding period and on adult males (Staniland 2005). The present work, therefore, documents the less well-known, cryptic life stage of the species, i.e. the individual at-sea migration patterns of adult males.
2. Material and methods
Field study was carried out on Possession Island (46°30′ S, 51°45′ E). One whisker was collected from 10 randomly chosen breeding male fur seals of unknown age during the summer season 2001–2002. Whiskers were cut as close to the face as possible. Prior to the isotopic analysis, they were hand-washed in 100 per cent ethanol and then cleaned in distilled water for 5 min in an ultrasonic bath. Whiskers were measured, dried and cut into 3 mm long consecutive sections starting from the proximal end. Sections (n = 710) were weighed with a micro-balance, packed in tin containers, and carbon and nitrogen isotope ratios were determined by a continuous flow mass spectrometer (Thermo Fisher, Delta V Advantage) coupled to an elemental analyser (Thermo Fisher, Flash EA 1112). Results are presented in the conventional notation relative to PeeDee belemnite marine fossil limestone and atmospheric N2 for δ13C and δ15N, respectively. Values are mean ± s.d.
Keratinous tissues (including whiskers) are approximately 3‰ 13C enriched over their diet in pinnipeds (Hobson et al. 1996). Taking into account both the keratinous effect and the latitudinal gradient in blood δ13C values of top predators in the Southern Ocean (Cherel & Hobson 2007; Cherel et al. 2007), the isotopic positions of the polar front (PF) and the subtropical front (STF) for fur seal whiskers were estimated at approximately −19 and −16‰, respectively (figures 1 and 2). The subtropical zone (STZ) is defined as the area north of the STF, the subantarctic zone (SAZ, where the Crozet Islands are located) as the area between the STF and the PF, and the Antarctic zone (AZ) as the area south of PF.
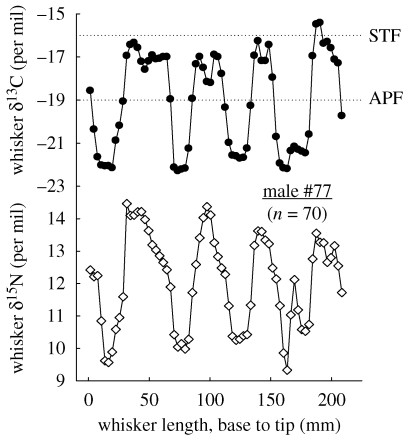
Figure 1.
δ13C (filled circles) and δ15N (open diamonds) values along the length of a whisker from a male Antarctic fur seal. Dotted lines illustrate the isotopic estimation of fronts and water masses. APF: Antarctic polar front; STF, subtropical front.
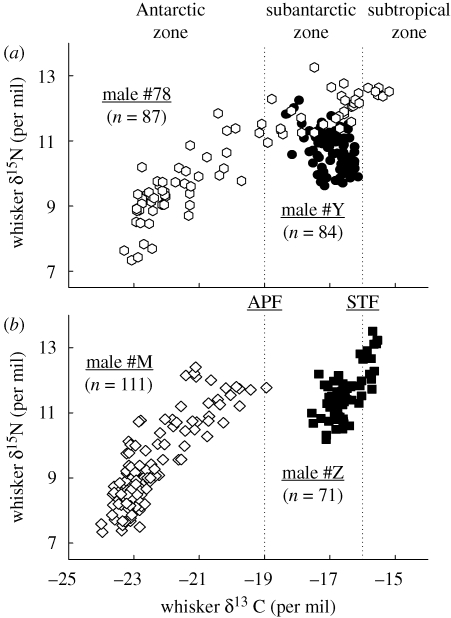
Figure 2.
Whisker δ15N versus δ13C values of four different male Antarctic fur seals. The numbers in parentheses refer to the number of isotopic measurements per whisker. Dotted lines illustrate the isotopic estimation of fronts and water masses. Abbreviations are the same as given in figure 1.
3. Results
The length of whiskers ranged from 84 to 333 mm, and the number of whisker sections analysed for each individual male Antarctic fur seal varied accordingly from 28 to 111 (see the electronic supplementary material). Whisker isotopic signatures were spread over a large range, with δ13C and δ15N values varying from −25.3 to −14.6‰ (a 10.7‰ difference) and from 7.2 to 14.5‰ (7.3‰), respectively. Overall, the lowest δ15N values (less than 9‰) were always associated with low δ13C values (less than −21‰).
Among the nine whiskers with greater than 60 isotopic measurements (see the electronic supplementary material), seven showed consistent isotopic oscillations (Fourier analysis) along their length and eight showed synchronous cycles (cross-autocorrelation analysis) for their δ13C and δ15N values (figure 1). This, therefore, clearly indicates the migration patterns. Whisker oscillation length varied from individual to individual from 39 to 60 mm (average 49 ± 8 mm) and the number of oscillations recorded per whisker ranged from 3.6 to 6.7 (4.8 ± 1.1). Assuming that the oscillations were annual, growth rates averaged 0.13 ± 0.02 mm d−1 and each 3 mm whisker section corresponded to 23.0 ± 3.9 d.
Male Antarctic fur seals showed large inter-individual variation in their isotopic niches, with means ranging from −22.4 ± 1.1 to −16.6 ± 0.5‰, and from 9.4 ± 1.3 to 12.6 ± 0.6‰ for δ13C and δ15N values, respectively (see the electronic supplementary material). Eight males showed large intra-individual variation in their δ13C values, indicating foraging along wide isotopic gradients (e.g. males #78 and #M shown in figure 2). Two individuals adopted a different strategy with a more restricted foraging range (i.e. males #Y and #Z in figure 2). Some males had no overlap in their δ13C values (figure 2b), and others (figure 2a), while overlapping in their carbon signatures (males #Y and #78 in SAZ, n = 84 and 23, −17.0 ± 0.5 and −16.9 ± 0.7‰, respectively; t-test, t = 0.53, p = 0.594), had different nitrogen signatures (10.7 ± 0.6 and 12.0 ± 0.5‰, respectively; t-test, t = 9.26, p < 0.0001).
4. Discussion
The results of the present study report the migration patterns and long-term intra- and inter-individual variations in the isotopic niche of an otariid species, using whiskers as an archival tissue of the individuals' foraging history. Consistent isotopic oscillations along whiskers were previously noted in Steller sea lions. Each oscillation was interpreted as reflecting a complete annual migratory cycle, because the estimated whisker growth rate for each oscillation in wild animals was similar to the range of growth rates in captive individuals (Hirons et al. 2001). Estimated whisker growth rates are identical in wild Steller sea lions and Antarctic fur seals (ranges 0.10–0.14 and 0.11–0.16 mm d−1, respectively). Hence, isotopic cyclicity in whiskers of male Antarctic fur seals is likely to record their annual migratory pattern. As whiskers showed on average 4.8 oscillations, and the age of territorial male fur seals varies from 7 to 13 years (Wickens & York 1997), a single whisker recorded a substantial part (37–69%) of the animal's lifespan.
In the Southern Ocean, δ13C values vary with latitudes and along an inshore/offshore gradient (Cherel & Hobson 2007). While we cannot preclude an inshore/offshore influence, the large oscillations in whisker δ13C values are likely to represent regular latitudinal movements of the fur seals. At the population level, the range of δ13C values and our isotopic estimation of the position of oceanic fronts show that male seals foraged preferentially in SAZ (46% of the 710 whisker sections) and AZ (45%), and marginally in STZ (9%). The synchronous patterns of δ15N with δ13C values indicate periodic changes in the animals' diet associated with migration, because variation in the δ15N baseline level in the Southern Ocean cannot alone account for the large range of seal δ15N signatures (Best & Schell 1996). Indeed, whisker δ15N values decreased with latitudes from approximately 12.8‰ in STZ to 10.0‰ in AZ, which is in agreement with both a higher δ15N baseline level in the subtropics (Best & Schell 1996) and fur seals feeding on different species of mesopelagic fishes in subtropical and subantarctic waters (Beauplet et al. 2004; Cherel et al. 2007). However, the further drop of δ15N values from approximately 10.0‰ associated with low δ13C signatures indicates feeding on lower trophic level prey. The fur seal nitrogen signature is close to that of krill-eating Adélie penguins (Cherel 2008), indicating that, in high-Antarctic waters, adult males from Crozet preyed upon krill, which forms the staple food of the species elsewhere (Staniland 2005).
The isotopic patterns observed show large intra- and inter-individual trophic niche variations in male Antarctic fur seals. Whisker δ13C values indicate that the migratory cycle of individuals encompassed either one (n = 3), two (n = 6) or three (n = 1) oceanographic zones, with no seal foraging exclusively in the subtropics. Among the six individuals foraging in the AZ, four seals reached high-Antarctic waters where their δ15N values showed that they all fed on krill. The isotopic niches of individual males either overlapped or showed some levels of habitat and resource partitioning. Two examples illustrate the inter-individual segregating mechanisms (figure 2). First (figure 2a), the foraging ranges of males #78 and #Y were overall different but overlapped in SAZ, where their δ15N signatures indicate dietary segregation. Second (figure 2b), their whisker δ13C values show that males #M and #Z never foraged in the same habitats for over more than four years. How can we explain the fact that males breeding in the same subantarctic colony did not show any overlap in their foraging habitats? Territorial males fast on land at the beginning of the reproductive cycle (Staniland 2005). They, therefore, retain the isotopic signature of their foraging areas and diets where they build up energy reserves. Thus, the two seals reproduced at Crozet, but one individual did not feed significantly in subantarctic waters when travelling back and forth to its preferred Antarctic foraging grounds.
In summary, whisker isotopic signatures reveal new multi-year migratory patterns of male Antarctic fur seals at both the population and the individual levels. The subsequent step will be to use the method to depict species-, sex- and individual-related foraging strategies allowing the coexistence of two sympatric fur seal species at the Crozet Islands (Cherel et al. 2007). Together with other keratinous tissues (Best & Schell 1996; Cerling et al. 2009), whiskers represent an archive of foraging ecology of wild mammals, thus providing unique opportunities to reconstruct the habitats, diets and environmental conditions experienced by those animals. The method is at its most powerful when used on challenging cryptic species and cryptic life stages.
Acknowledgements
The ethics committee of IPEV approved the field procedure.
The authors thank F. Bailleul, S. Luque and J. Sinclair for their help in the field, D. Pinaud for the statistical analysis and G. Guillou for the stable isotope analysis. The present work was supported financially and logistically by the ANR-VMC IPSOS-SEAL and the Institut Polaire Français Paul Emile Victor (Programme no. 109, H. Weimerskirch).
References
- Bearhop S., Adams C. E., Waldron S., Fuller R. A., MacLeod H.2004Determining trophic niche width: a novel approach using stable isotope analysis. J. Anim. Ecol. 73, 1007–1012 (doi:10.1111/j.0021-8790.2004.00861.x) [Google Scholar]
- Beauplet G., Dubroca L., Guinet C., Cherel Y., Dabin W., Gagne C., Hindell M.2004Foraging ecology of subantarctic fur seals Arctocephalus tropicalis breeding on Amsterdam Island: seasonal changes in relation to maternal characteristics and pup growth. Mar. Ecol. Prog. Ser. 273, 211–225 (doi:10.3354/meps273211) [Google Scholar]
- Best P. B., Schell D. M.1996Stable isotopes in southern right whale (Eubalaena australis) baleen as indicators of seasonal movements, feeding and growth. Mar. Biol. 124, 483–494 (doi:10.1007/BF00351030) [Google Scholar]
- Bolnick D. I., Svanback R., Fordyce J. A., Yang L. H., Davis J. M., Hulsey C. D., Forister M. L.2003The ecology of individuals: incidence and implications of individual specialization. Am. Nat. 161, 1–28 (doi:10.1086/343878) [DOI] [PubMed] [Google Scholar]
- Cerling T. E., Wittemyer G., Ehleringer J. R., Remien C. H., Douglas-Hamilton I.2009History of animals using isotope records (HAIR): a 6-year dietary history of one family of African elephants. Proc. Natl Acad. Sci. 106, 8093–8100 (doi:10.1073/pnas.0902192106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherel Y.2008Isotopic niches of emperor and Adélie penguins in Adélie Land, Antarctica. Mar. Biol. 154, 813–821 (doi:10.1007/s00227-008-0974-3) [Google Scholar]
- Cherel Y., Hobson K. A.2007Geographical variation in carbon stable isotope signatures of marine predators: a tool to investigate their foraging areas in the Southern Ocean. Mar. Ecol. Prog. Ser. 329, 281–287 (doi:10.3354/meps329281) [Google Scholar]
- Cherel Y., Hobson K. A., Guinet C., Vanpé C.2007Stable isotopes document seasonal changes in trophic niches and winter foraging individual specialisation in diving predators from the Southern Ocean. J. Anim. Ecol. 76, 826–836 (doi:10.1111/j.1365-2656.2007.01238.x) [DOI] [PubMed] [Google Scholar]
- Hirons A. C., Schell D. M., St. Aubin D. J.2001Growth rates of vibrissae of harbor seals (Phoca vitulina) and Steller sea lions (Eumetobias jubatus). Can. J. Zool. 79, 1053–1061 (doi:10.1139/cjz-79-6-1053) [Google Scholar]
- Hobson K. A., Schell D. M., Renouf D., Noseworthy E.1996Stable carbon and nitrogen isotopic fractionation between diet and tissues of captive seals: implications for dietary reconstructions involving marine mammals. Can. J. Fish. Aquat. Sci. 53, 528–533 (doi:10.1139/cjfas-53-3-528) [Google Scholar]
- Newsome S. D., Tinker M. T., Monson D. H., Oftedal O. T., Ralls K., Staedler M. M., Fogel M. L., Estes J. E.2009Using stable isotopes to investigate individual diet specialization in California sea otters (Euhydra lutris nereis). Ecology 90, 961–974 (doi:10.1890/07-1812.1) [DOI] [PubMed] [Google Scholar]
- Rubenstein D. R., Hobson K. A.2004From birds to butterflies: animal movement patterns and stable isotopes. Trends Ecol. Evol. 19, 256–263 (doi:10.1016/j.tree.2004.03.017) [DOI] [PubMed] [Google Scholar]
- Staniland I.2005Sexual segregation in seals. In Sexual segregation in vertebrates: ecology of the two sexes (eds Ruckstuhl K. E., Neuhaus P.), pp. 53–73 Cambridge, UK: Cambridge University Press [Google Scholar]
- Wickens P., York A. E.1997Comparative population dynamics of fur seals. Mar. Mamm. Sci. 13, 241–292 (doi:10.1111/j.1748-7692.1997.tb00631.x) [Google Scholar]