Abstract
Glucagon-like peptide 1 (GLP-1) is a relatively recently discovered molecule originating in the so-called L-cells of the intestine. The peptide has insulinotrophic properties and it is this characteristic that has predominantly been investigated. This has led to the use of the GLP-1-like peptide exendin-4 (EX-4), which has a much longer plasma half-life than GLP-1 itself, being used in the treatment of type II diabetes. The mode of action of this effect appears to be a reduction in pancreatic apoptosis, an increase in beta cell proliferation or both. Thus, the effects of GLP-1 receptor stimulation are not based upon insulin replacement but an apparent repair of the pancreas. Similar data suggest that the same effects may occur in other peripheral tissues. More recently, the roles of GLP-1 and EX-4 have been studied in nervous tissue. As in the periphery, both peptides appear to promote cellular growth and reduce apoptosis. In models of Alzheimer's disease, Parkinson's disease and peripheral neuropathy, stimulation of the GLP-1 receptor has proved to be highly beneficial. In the case of Parkinson's disease this effect is evident after the neurotoxic lesion is established, suggesting real potential for therapeutic use. In the present review we examine the current status of the GLP-1 receptor and its potential as a therapeutic target.
Keywords: glucagon-like peptide 1, exendin-4, neuroprotection, anti-inflammatory, apoptosis, neurogenesis, Parkinson's disease
Introduction
Glucagon-like peptide 1 (GLP-1) was first described in 1985 (Schmidt et al., 1985) following cloning of the preproglucagon gene by the same authors. GLP-1 is classically associated with having insulinotropic actions and is linked with a substantial proportion of the so-called ‘incretin’ response to nutrient ingestion (Orskov et al., 1986). GLP-1 in the gastrointestinal tract is stored in enteroendocrine L cells in the intestine, but can also be found in the pancreas (Holst, 2007) and plasma levels rise by approximately threefold in response to a meal (Vilsboll et al., 2001). GLP-1 acts at a specific transmembrane G-protein linked receptor, the GLP-1 receptor (GLP-1R), stimulating adenylate cyclase and the formation of cyclic adenosine monophosphate (cAMP) (Yada et al., 1993) with downstream effects on gene expression (Figure 1). GLP-1Rs are found in the pancreas, adipose tissue, muscle, heart, the gastrointestinal tract and the liver where a predominant action is promotion of glucose uptake (De Leon et al., 2006, and see Holst, 2007 for review). As well as the periphery, GLP-1Rs are found throughout the central nervous system (CNS). Binding sites for GLP-1Rs have been found in the hypothalamus, striatum, brain stem, substantia nigra (SN) and subventricular zone (SVZ) among other structures (Campos et al., 1994; Merchenthaler et al., 1999). GLP-1Rs are present on glia as well as neuronal cell types (Chowen et al., 1999; Iwai et al., 2006).
Figure 1.
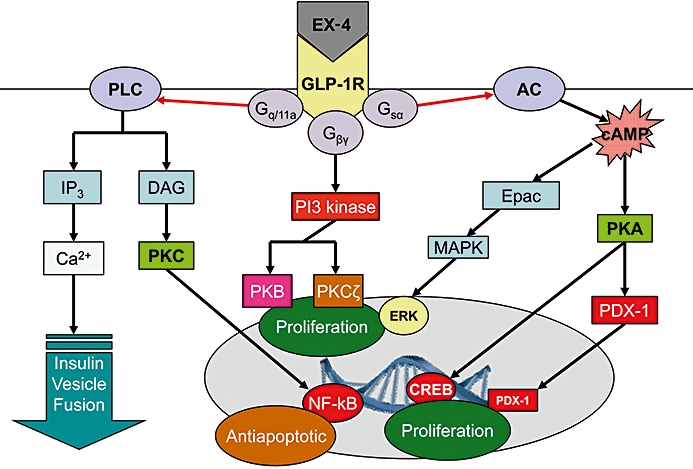
Intracellular events associated with stimulation of glucagon-like peptide 1 receptors (GLP-1Rs). The receptor is G-protein coupled with a consequent rise in cyclic adenosine monophosphate (cAMP). Further intracellular events lead to nuclear changes and alterations in transcription. EX-4, exendin-4; GLP-1R, GLP-1 receptor; PLC, phospholipase C; IP3, inositol triphosphate; DAG, diacylglycerol; PKC, protein kinase C; NF-kB, nuclear factor kappa B; PI3 kinase, phosphoinositide 3 kinase; PKB, protein kinase B; PKCζ, protein kinase C-zeta; AC, adenylate cylase; Epac, exchange proteins directly activated by cAMP; MAPK, mitogen-activated protein kinase; ERK, extracellular signal-regulated kinase; PKA, protein kinase A; PDX-1, pancreatic duodenal homeobox-1; CREB, cyclic AMP response element binding protein.
An important therapeutic substance with regard to manipulation of GLP-1Rs is the peptide exendin-4 (EX-4). EX-4 is a 39 amino acid peptide isolated from the saliva or venom of the lizard Heloderma suspectum, native to the southern desert regions of the USA. EX-4 shows an almost identical pharmacodynamic profile to GLP-1 but has a substantially longer plasma half-life since it is not metabolized by dipeptidyl peptidase IV, which metabolizes GLP-1 rapidly (Thum et al., 2002). It is now accepted that there is also a GLP-2 peptide and GLP-2 receptor (Drucker, 2001), but this will not be considered in the current review as there is little evidence for a cellular or neuroprotective role for agonism mediated via this site.
Protective effects of GLP-1R stimulation in the periphery
While the primary aim of this review is to consider the protective effects of GLP-1R stimulation in the CNS, the relative volume of work done in this respect on peripheral tissues, in particular the pancreas, necessitate some description. GLP-1 receptor stimulation exerts a number of effects on pancreatic cells, primarily β-cells. These effects include regulation of the differentiation of pancreatic progenitor cells (Urusova et al., 2004), stimulation of β-cell mass (Park et al., 2008) and a reduction in β-cell apoptosis (Egan et al., 2003; Li et al., 2003). In particular, the GLP-1R appears effective in protecting β-cells against cytokine-mediated apoptosis. Thus, EX-4 shows clear protection of β-cells incubated with either interleukin-1-beta (IL-1β), tumour necrosis factor alpha (TNF-α) or interferon gamma (IFN-γ) as well as combinations of these cytokines (Li et al., 2003). Subsequently these authors determined that the anti-apoptotic effect of GLP-1R stimulation against cytokines is mediated in a protein kinase B-dependent manner (Li et al., 2005).
While not directly protective at a cellular level the trophic effects of GLP-1R activation will be considered, as such effects may confer a greater integrity to an organ as a whole and potentially render its cells a greater facility to withstand cytotoxic and other stresses. Exogenous GLP-1 and EX-4 both increase β-cell mass by increasing both cellular differentiation as well as replication (Drucker, 2003; Stoffers, 2004). These effects are augmented by the anti-apoptotic actions of these agonists that preserve existing cells and probably help to protect young or otherwise vulnerable cells. Both chronic and even acute treatment with GLP-1R agonists increases β-cell mass in both normal and diabetic mice (Rolin et al., 2002; Kim et al., 2003). These effects can be also clearly demonstrated in models of type II diabetes such as the Goto–Kakizaki rat (Tourrel et al., 2002). Whether endogenous GLP-1R stimulation exerts an effect on β-cell mass is less clear. However, since GLP-1R knockout mice appear to have normal β-cell mass this would appear to not be the case (Ling et al., 2001). The fact that De Leon et al. (2006) reported a role for endogenous GLP-1 after a partial pancreatectomy in adult mice would concur with this.
GLP-1Rs have become well accepted as having anti-apoptotic properties. Both GLP-1 and EX-4 augment cellular integrity and overall cell survival following exposure to a range of pro-apoptotic agents. These include peroxides, cytokines and fatty acids (Hui et al., 2003; Li et al., 2003; Buteau et al., 2004). These observations appear to hold true in both rodents and humans (Delaney et al., 1997; Eizirik and Darville, 2001). Additionally, β-cell damage induced by exposure to reactive oxygen species (ROS), such as peroxynitrite, is reduced in the presence of GLP-1 (Tews et al., 2009). In addition GLP-1 appears to increase expression of anti-apoptotic genes Bc12 and Bclxl (Buteau et al., 2004). This may be the result of nuclear factor kappa B (NF-kB)-dependent transcription of Bc12 as well as lap2 (Li et al., 2005). Interestingly, GLP-1 also appears to reduce endoplasmic reticulum stress as indicated by the overproduction of misfolded protein aggregates (Yusta et al., 2006; Tsunekawa et al., 2007). Such an action in peripheral cells could have a clear relevance to neurodegenerative disorders such as Parkinson's disease (PD), where protein misfolding may be a significant aetiological factor. In addition to EX-4, other molecules that are GLP-1R agonists appear to exert a similar profile include liraglutide (NN2211) (Knudsen et al., 2000) and albugon (Baggio et al., 2004). The major difference between these molecules and GLP-1 appears to be pharmacokinetic rather than pharmacodynamic (De Leon et al., 2006). As well as having protective actions towards pancreatic β-cells, GLP-1R agonists appear to confer protection to other peripheral tissues. Thus, both EX-4 and GLP-1(9,36) amide, the primary endogenous metabolite of GLP-1, show protective effects against reperfusion injury in rat heart (Sonne et al., 2008), although possibly via a GLP-1R subtype as these effects were not reversed by the selective GLP-1R antagonist exendin-(9,39) [EX-(9,39)]. These actions were expressed as both a reduction in infarct size by EX-4 [but not GLP-1(9,36)], as well as an overall improvement in myocardial performance following both agonists. Additionally, a loss of GLP-1 signalling enhances hepatocyte susceptibility to experimental liver damage induced by FasL activation, or a methionine and choline deficient diet in mice (Sinclair et al., 2008). These effects appear to be cAMP-dependent with a secondary activation of cAMP response element binding protein. It may therefore be that GLP-1R signalling can mediate a relatively generic type of cellular protection in a range of peripheral cell types.
Neuroprotective effects of GLP-1Rs
Evidence for CNS effects of GLP-1Rs was shown by reports that intracerebroventricular (I.C.V.) injection of GLP-1 reduces food intake (Gunn et al., 1996) and that this effect is GLP-1R-mediated since it is blocked by the GLP-1R antagonist EX-(9,39) (Turton et al., 1996). Similarly, I.C.V. injection of GLP-1 or EX-4 leads to a reduction in body weight in rats (Donahey et al., 1998; Meeran et al., 1999). Perry et al. (2002a) observed that GLP-1 and EX-4 stimulated neurite outgrowth in rat phaechromocytoma (PC12) cultured cells in a manner similar to nerve growth factor (NGF). This gave a clear indication of the potential that GLP-1Rs could be stimulants for neuronal growth. These authors also reported that EX-4 was able to augment NGF-induced neuronal differentiation and apparently attenuate neural degeneration following NGF withdrawal, giving further support to this possibility (Perry et al., 2002a) and indicating a potential neuroprotective role for GLP-1Rs. These findings led to the suggestion that stimulation of GLP-1Rs could be of value in neurodegenerative disorders such as Alzheimer's or PD as well as other neuropathies. Interestingly, at least with regard to PD, many neurons in the area postrema have been found to express tyrosine hydroxylase (TH), the rate-limiting enzyme in dopamine (DA) synthesis, as well as surface receptors for GLP-1 (Yamamoto et al., 2003). It was subsequently reported that both GLP-1 and EX-4 protected cultured hippocampal neurons from glutamate-induced excitotoxic cell death in a concentration and cAMP-dependent manner (Perry et al., 2002b). Additionally, it was observed that both GLP-1R agonists reduced choline acetyl transferase loss following an ibotenic acid lesion in the rat in vivo (Perry et al., 2002b). The seemingly general ability of GLP-1 and EX-4 to provide neuroprotection to different neuronal phenotypes led these authors to suggest that this may be generic to any neurons expressing the GLP-1R. Thus, GLP-1R deficient mice have been observed to have both a lower seizure threshold and increased susceptibility to kainate-induced neuronal damage (During et al., 2003). Conversely, rats over-expressing GLP-1Rs show enhancement of learning and memory in behavioural tests of these parameters (During et al., 2003). Overall these findings strengthened the belief that positive modulation of the GLP-1R could have therapeutic utility in neurodegenerative disorders such as Alzheimer's disease (Perry and Greig, 2004). This will be considered more fully below. More recently, Perry et al. (2007) reported that GLP-1 mediates neuroprotection in a rat model of peripheral sensory neuropathy induced by pyridoxine. Both GLP-1 and EX-4, when delivered via osmotic minipumps, were able to arrest or prevent axonal degeneration of the sciatic nerve. The functional consequence of this was a clear improvement by rats in the rotarod test, although animals were still significantly impaired compared with sham-treated rats (Perry et al., 2007). The mechanism by which these effects occurred was not described but the effects were reversed by EX-(9,39), and importantly, Nakagawa et al. (2004) detected GLP-1 gene expression in a peripheral nerve structure (the nodose ganglion). These observations are collectively likely to be relevant to diabetic patients, in which peripheral neuropathy naturally progresses, particularly if the condition is poorly controlled. Moreover, the findings of Perry et al. (2007) may have wider implications since these observations, to our knowledge, are the first description of a neuroprotective effect of GLP-1R activation in a condition simulating a human neuropathology. Relatively soon after this, evidence was reported to support the contention that GLP1-Rs may be potential therapeutic targets in PD by the findings of Bertilsson et al. (2008) and Harkavyi et al. (2008), who both described beneficial effects of EX-4 in preclinical models of PD. Bertilsson et al. (2008) examined the effects of the drug in 6-hydroxydopamine (6-OHDA)-lesioned rats. These authors allowed the lesion to develop for 5 weeks prior to administering EX-4 at a dose of 0.1 µg·kg−1 twice daily and within 2 weeks observed clear decreases in amphetamine-induced circling behaviour, which continued to decline until the end of the experiment. In vitro these authors found that EX-4 stimulates neurogenesis using adenosine triphosphate generation and BrdU incorporation assays in cultured stem/progenitor cells. Ex vivo tissue analysis indicated that GLP-1Rs can mediate an increase in neurogenesis in the SVZ, where stem and progenitor cells are found. This was causally linked to an EX-4-mediated increase in neuronal precursor cells seen in the medial striatum (Bertilsson et al., 2008) and thus associated with the improvement seen in the PD-like features of 6-OHDA-treated rats. These also included increased TH+ staining neurons in the SN as well as an increase in numbers of vesicular monoamine transporter 2 (VMAT2)-positive cells in the same region. VMAT2 is the vesilcular transporter for monoamines and is generally indicative of the presence of monoaminergic neurons, which in the case of the SN are likely to be dopaminergic. These findings therefore suggest that there is an increase in monoaminergic neuronal numbers in the SN, which are logically likely to be dopaminergic. We have also observed clear and dose-dependent effects of EX-4 in reducing PD-like pathology in rodent preclinical models of the illness (Harkavyi et al., 2008). EX-4 (0.1 or 0.5 µg·kg−1 twice daily for 7 days) was given 7 days after either 6-OHDA or Lipopolysaccharide (LPS)-induced hemiparkinsonian lesions. This time point for commencing EX-4 treatment was chosen since we have observed the lesion to be established but still progressing (Abuirmeileh et al., 2007), in theory much as is the case in a PD patient. EX-4 reversed numerous behavioural, neurochemical and histological (Figure 2) indices of PD-like pathology in both rodent models used (Harkavyi et al., 2008). These observations were very recently given strong support by the observations of Li et al. (2009). These authors reported that EX-4 protected ventral mesencephalic (dopaminergic) cells in culture exposed to 6-OHDA. This effect was also seen in SH-SY5Y cells, a cultured human neuroblastoma cell line, which can be differentiated into neurons with a dopaminergic phenotype. GLP-1 itself was also effective in protecting both cells types against 6-OHDA toxicity but these effects of GLP-1R activation were not seen in cells grown from GLP-1R knockout mice (Li et al., 2009). These authors also found that 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice were protected by EX-4 against nigrostriatal damage as indicated by TH+ staining and a decrease in tissue DA loss. We have also observed, by injecting the marker fluorogold into the striatum at the end of the EX-4 treatment period, clear evidence of neuronal regrowth or restoration of function in the SN (Figure 3, our unpublished data). It is logical that these cell bodies are dopaminergic, since the dopamine pathway is the only one of significance linking these two brain regions. Whether this represents de novo neurogenesis and the creation of new connections is as yet unclear but would be remarkable. It may be more likely that EX-4 is ‘rescuing’ neurons unable to take fluorogold into their striatal terminals. A possible explanation for this may lie in the anti-inflammatory properties of EX-4. GLP-1 has been found to inhibit LPS-induced production of IL-1β by cultured rat astrocytes, an effect which was cAMP-dependent (Iwai et al., 2006). GLP-1 also decreased expression of interleukin-6 and inducible nitric oxide synthase, but not interleukin-10 mRNA (Iwai et al., 2006). Inhibition of IL-1β activity as observed by Li et al. (2005) with EX-4, dramatically reduces cell death (Rothwell et al., 1996). These findings therefore are in general agreement with those of Li et al. (2005), who reported a protective effect of EX-4 against cytokine-induced apoptosis, probably by reducing cytokine-evoked inhibition of protein kinase B phosphorylation. Thus, functionally, EX-4 appears to have ‘anti-inflammatory’ properties.
Figure 2.

Neuroprotection by exendin-4 (EX-4) in the 6-hydroxydopamine (6-OHDA) rat model of Parkinson's disease. (A) EX-4 shows clear restoration of motor behaviour; (B) tissue dopamine (DA) content; (C) striatal tyrosine hydroxylase (TH) activity; (D) nigral TH immunoreactivity shown as a qualitative index of TH staining. In all groups, EX-4 or vehicle was given twice daily for 7 days after the initial 6-OHDA insult. In each case groups are made up of six rats per group and were subjected to one-way analysis of variance (anova) followed by a post hoc Bonferonni's Multiple Comparison Test (A–C). One-way anova revealed significant differences between treatments in for all of the parameters studied (A–C; P < 0.001). Bonferonni's Multiple Comparison Test revealed significant differences (*) from all other groups (A) and from sham or EX-4 treated groups (B and C). Data adapted from (Harkavyi et al., 2008). L-DOPA, L-3,4-dihydroxyphenylalanine; EX-9-39, exendin-(9,39).
Figure 3.

Photomicrographs of selected nigral sections from rats treated with fluorogold and visualized under UV light. Rats were lesioned with 6-hydroxydopamine (6-OHDA) as indicated in the text and either treated with vehicle (upper panel; twice daily for 7 days) starting 7 days post toxin or 0.5 µg·kg−1 exendin-4 (EX-4) (lower panel). Massive loss of retrograde staining by 6-OHDA and retention or reversal of this is apparent. The extent of cell loss in the 6-OHDA only group suggests that treatment with EX-4 effects a restoration of nigrostriatal neurons. Bar is 100 µm.
We have previously found the corticotrophin releasing factor (CRF)-like peptide urocortin to display very similar properties to EX-4 in the 6-OHDA and lipopolysaccharide models of PD (Abuirmeileh et al., 2007), although unfortunately, unlike EX-4, urocortin does not cross the blood brain barrier. This effect is almost certainly mediated by CRF1receptors (Abuirmeileh et al., 2008). Interestingly, therefore, the selective CRF1 antagonist NBI 27914 attenuates the neuroprotective effects of EX-4 (our unpublished data) suggesting a link between the two systems. This idea is supported by observations that the anorexic effect of GLP-1 in chicks requires the involvement of CRF receptor activation (Tachibana et al., 2006).
As well as PD there is evidence that activation of GLP-1Rs could be beneficial in Alzheimer's disease also. GLP-1 has been found to protect SH-SY5Y cells from apoptosis induced by amyloid-β peptide (1-42) (Qin et al., 2008). This effect was apparently mediated by preventing a deleterious rise in cytosolic calcium levels. These authors also observed that GLP-1 decreased the expression of Bax, a molecule primarily found in the cytosol of normal tissues but which translocates to the mitochondria to regulate apoptosis and cell death (Qin et al., 2008). Amyloid-β peptide (1-42) mediated upregulation of Bax mRNA was potently reduced by GLP-1 treatment which in turn promoted cell growth. It would be of considerable interest to see if these effects could translate into a reduction in cognitive deficits in rodent models of Alzheimer's disease in vivo. Finally, the apparently generic nature of the GLP-1R as a mediator of neuroprotection is emphasized by the finding that EX-4 reduces infarct size as well as behavioural deficits in the middle cerebral artery occlusion model of stroke in the rat where the initial insult (hypoxia) is quite non-specific (Li et al., 2009).
Conclusions
The potential utility of stimulating the GLP-1R in a number of neuropathologies is beginning to emerge. While the native peptide would have very limited value, having a half-life of only minutes, EX-4 opens the way to therapeutic treatment with a half-life of several hours. This is apparent from its use in type II diabetes, where it is effective at remarkably low doses at helping regulate plasma glucose. Its effectiveness at reducing nerve damage in a model of sensory neuropathy would dovetail well with its use in diabetes if this is reproduced in humans. Of the serious neurodegenerative disorders the potential of EX-4 in Alzheimer's disease is far from the clinic. In contrast, in the case of PD the preclinical data are robust and demonstrable at doses near identical to those currently used in diabetic patients. Indeed, plans to put EX-4 into clinical trials in the PD patient population are currently underway (R. Wyse, pers. comm.). It may be that EX-4 will prove to deliver the promise of glial cell-derived neurotrophic factor but without the issues of delivery that have dogged that peptide. The range of peripheral tissues in which GLP-1 shows a protective effect is highly encouraging. If this indicates some form of generic cellular repair system, and should this extend to the nervous system then the future for individuals currently suffering from incurable neurological disorders may be considerably brighter.
Glossary
Abbreviations:
- 6-OHDA
6-hydroxydopamine
- cAMP
cyclic adenosine monophosphate
- CNS
central nervous system
- CRF
corticotrophin releasing factor
- EX-4
exendin-4
- GLP-1
glucagon-like peptide 1
- GLP-1R
glucagon-like peptide 1 receptor
- GLP-2
glucagon-like peptide 2
- I.C.V.
intracerebroventricular
- IFN-γ
interferon gamma
- IL-1β
interleukin-1-beta
- NGF
nerve growth factor
- PD
Parkinson's disease
- ROS
reactive oxygen species
- SVZ
subventricular zone
- TH
tyrosine hydroxylase
- TNF-α
tumour necrosis factor alpha
References
- Abuirmeileh A, Harkavyi A, Kingsbury A, Lever R, Whitton PS. The CRF-like peptide urocortin greatly attenuates loss of extracellular striatal dopamine in rat models of Parkinson's disease by activating CRF1 receptors. Eur J Pharmacol. 2008;604:45–50. doi: 10.1016/j.ejphar.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Abuirmeileh A, Harkavyi A, Lever R, Biggs CS, Whitton PS. Urocortin, a CRF-like peptide, restores key indicators of damage in the substantia nigra in a neuroinflammatory model of Parkinson's disease. J Neuroinflammation. 2007;4:19. doi: 10.1186/1742-2094-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggio LL, Huang Q, Brown TJ, Drucker DJ. A recombinant human glucagon-like peptide (GLP)-1-albumin protein (albugon) mimics peptidergic activation of GLP-1 receptor-dependent pathways coupled with satiety, gastrointestinal motility, and glucose homeostasis. Diabetes. 2004;53:2492–2500. doi: 10.2337/diabetes.53.9.2492. [DOI] [PubMed] [Google Scholar]
- Bertilsson G, Patrone C, Zachrisson O, Andersson A, Dannaeus K, Heidrich J, et al. Peptide hormone exendin-4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson's disease. J Neurosci Res. 2008;86:326–338. doi: 10.1002/jnr.21483. [DOI] [PubMed] [Google Scholar]
- Buteau J, El-Assaad W, Rhodes CJ, Rosenberg L, Joly E, Prentki M. Glucagon-like peptide-1 prevents beta cell glucolipotoxicity. Diabetologia. 2004;47:806–815. doi: 10.1007/s00125-004-1379-6. [DOI] [PubMed] [Google Scholar]
- Campos RV, Lee YC, Drucker DJ. Divergent tissue-specific and developmental expression of receptors for glucagon and glucagon-like peptide-1 in the mouse. Endocrinology. 1994;134:2156–2164. doi: 10.1210/endo.134.5.8156917. [DOI] [PubMed] [Google Scholar]
- Chowen JA, de Fonseca FR, Alvarez E, Navarro M, Garcia-Segura LM, Blazquez E. Increased glucagon-like peptide-1 receptor expression in glia after mechanical lesion of the rat brain. Neuropeptides. 1999;33:212–215. doi: 10.1054/npep.1999.0757. [DOI] [PubMed] [Google Scholar]
- De Leon DD, Crutchlow MF, Ham JY, Stoffers DA. Role of glucagon-like peptide-1 in the pathogenesis and treatment of diabetes mellitus. Int J Biochem Cell Biol. 2006;38:845–859. doi: 10.1016/j.biocel.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Delaney CA, Pavlovic D, Hoorens A, Pipeleers DG, Eizirik DL. Cytokines induce deoxyribonucleic acid strand breaks and apoptosis in human pancreatic islet cells. Endocrinology. 1997;138:2610–2614. doi: 10.1210/endo.138.6.5204. [DOI] [PubMed] [Google Scholar]
- Donahey JC, van Dijk G, Woods SC, Seeley RJ. Intraventricular GLP-1 reduces short- but not long-term food intake or body weight in lean and obese rats. Brain Res. 1998;779:75–83. doi: 10.1016/s0006-8993(97)01057-3. [DOI] [PubMed] [Google Scholar]
- Drucker DJ. Minireview: the glucagon-like peptides. Endocrinology. 2001;142:521–527. doi: 10.1210/endo.142.2.7983. [DOI] [PubMed] [Google Scholar]
- Drucker DJ. Glucagon-like peptide-1 and the islet beta-cell: augmentation of cell proliferation and inhibition of apoptosis. Endocrinology. 2003;144:5145–5148. doi: 10.1210/en.2003-1147. [DOI] [PubMed] [Google Scholar]
- During MJ, Cao L, Zuzga DS, Francis JS, Fitzsimons HL, Jiao X, et al. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat Med. 2003;9:1173–1179. doi: 10.1038/nm919. [DOI] [PubMed] [Google Scholar]
- Egan JM, Bulotta A, Hui H, Perfetti R. GLP-1 receptor agonists are growth and differentiation factors for pancreatic islet beta cells. Diabetes Metab Res Rev. 2003;19:115–123. doi: 10.1002/dmrr.357. [DOI] [PubMed] [Google Scholar]
- Eizirik DL, Darville MI. Beta-cell apoptosis and defense mechanisms: lessons from type 1 diabetes. Diabetes. 2001;50(Suppl. 1):S64–9. doi: 10.2337/diabetes.50.2007.s64. [DOI] [PubMed] [Google Scholar]
- Gunn I, O'Shea D, Turton MD, Beak SA, Bloom SR. Central glucagon-like peptide-I in the control of feeding. Biochem Soc Trans. 1996;24:581–584. doi: 10.1042/bst0240581. [DOI] [PubMed] [Google Scholar]
- Harkavyi A, Abuirmeileh A, Lever R, Kingsbury AE, Biggs CS, Whitton PS. Glucagon-like peptide 1 receptor stimulation reverses key deficits in distinct rodent models of Parkinson's disease. J Neuroinflammation. 2008;5:19. doi: 10.1186/1742-2094-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- Hui H, Nourparvar A, Zhao X, Perfetti R. Glucagon-like peptide-1 inhibits apoptosis of insulin-secreting cells via a cyclic 5′-adenosine monophosphate-dependent protein kinase A- and a phosphatidylinositol 3-kinase-dependent pathway. Endocrinology. 2003;144:1444–1455. doi: 10.1210/en.2002-220897. [DOI] [PubMed] [Google Scholar]
- Iwai T, Ito S, Tanimitsu K, Udagawa S, Oka J. Glucagon-like peptide-1 inhibits LPS-induced IL-1beta production in cultured rat astrocytes. Neurosci Res. 2006;55:352–360. doi: 10.1016/j.neures.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Kim JG, Baggio LL, Bridon DP, Castaigne JP, Robitaille MF, Jette L, et al. Development and characterization of a glucagon-like peptide 1-albumin conjugate: the ability to activate the glucagon-like peptide 1 receptor in vivo. Diabetes. 2003;52:751–759. doi: 10.2337/diabetes.52.3.751. [DOI] [PubMed] [Google Scholar]
- Knudsen LB, Nielsen PF, Huusfeldt PO, Johansen NL, Madsen K, Pedersen FZ, et al. Potent derivatives of glucagon-like peptide-1 with pharmacokinetic properties suitable for once daily administration. J Med Chem. 2000;43:1664–1669. doi: 10.1021/jm9909645. [DOI] [PubMed] [Google Scholar]
- Li Y, Hansotia T, Yusta B, Ris F, Halban PA, Drucker DJ. Glucagon-like peptide-1 receptor signaling modulates beta cell apoptosis. J Biol Chem. 2003;278:471–478. doi: 10.1074/jbc.M209423200. [DOI] [PubMed] [Google Scholar]
- Li Y, Cao X, Li LX, Brubaker PL, Edlund H, Drucker DJ. Beta-Cell Pdx1 expression is essential for the glucoregulatory, proliferative, and cytoprotective actions of glucagon-like peptide-1. Diabetes. 2005;54:482–491. doi: 10.2337/diabetes.54.2.482. [DOI] [PubMed] [Google Scholar]
- Li Y, Perry T, Kindy MS, Harvey BK, Tweedie D, Holloway HW, et al. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Natl Acad Sci U S A. 2009;106:1285–1290. doi: 10.1073/pnas.0806720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Z, Wu D, Zambre Y, Flamez D, Drucker DJ, Pipeleers DG, et al. Glucagon-like peptide 1 receptor signaling influences topography of islet cells in mice. Virchows Arch. 2001;438:382–387. doi: 10.1007/s004280000374. [DOI] [PubMed] [Google Scholar]
- Meeran K, O'Shea D, Edwards CM, Turton MD, Heath MM, Gunn I, et al. Repeated intracerebroventricular administration of glucagon-like peptide-1-(7-36) amide or exendin-(9-39) alters body weight in the rat. Endocrinology. 1999;140:244–250. doi: 10.1210/endo.140.1.6421. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403:261–280. doi: 10.1002/(sici)1096-9861(19990111)403:2<261::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Nakagawa A, Satake H, Nakabayashi H, Nishizawa M, Furuya K, Nakano S, et al. Receptor gene expression of glucagon-like peptide-1, but not glucose-dependent insulinotropic polypeptide, in rat nodose ganglion cells. Auton Neurosci. 2004;110:36–43. doi: 10.1016/j.autneu.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Orskov C, Holst JJ, Knuhtsen S, Baldissera FG, Poulsen SS, Nielsen OV. Glucagon-like peptides GLP-1 and GLP-2, predicted products of the glucagon gene, are secreted separately from pig small intestine but not pancreas. Endocrinology. 1986;119:1467–1475. doi: 10.1210/endo-119-4-1467. [DOI] [PubMed] [Google Scholar]
- Park S, Hong SM, Sung SR. Exendin-4 and exercise promotes beta-cell function and mass through IRS2 induction in islets of diabetic rats. Life Sci. 2008;82:503–511. doi: 10.1016/j.lfs.2007.12.018. [DOI] [PubMed] [Google Scholar]
- Perry T, Lahiri DK, Chen D, Zhou J, Shaw KT, Egan JM, et al. A novel neurotrophic property of glucagon-like peptide 1: a promoter of nerve growth factor-mediated differentiation in PC12 cells. J Pharmacol Exp Ther. 2002a;300:958–966. doi: 10.1124/jpet.300.3.958. [DOI] [PubMed] [Google Scholar]
- Perry T, Haughey NJ, Mattson MP, Egan JM, Greig NH. Protection and reversal of excitotoxic neuronal damage by glucagon-like peptide-1 and exendin-4. J Pharmacol Exp Ther. 2002b;302:881–888. doi: 10.1124/jpet.102.037481. [DOI] [PubMed] [Google Scholar]
- Perry T, Holloway HW, Weerasuriya A, Mouton PR, Duffy K, Mattison JA, et al. Evidence of GLP-1-mediated neuroprotection in an animal model of pyridoxine-induced peripheral sensory neuropathy. Exp Neurol. 2007;203:293–301. doi: 10.1016/j.expneurol.2006.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry TA, Greig NH. A new Alzheimer's disease interventive strategy: GLP-1. Curr Drug Targets. 2004;5:565–571. doi: 10.2174/1389450043345245. [DOI] [PubMed] [Google Scholar]
- Qin Z, Sun Z, Huang J, Hu Y, Wu Z, Mei B. Mutated recombinant human glucagon-like peptide-1 protects SH-SY5Y cells from apoptosis induced by amyloid-beta peptide (1-42) Neurosci Lett. 2008;444:217–221. doi: 10.1016/j.neulet.2008.08.047. [DOI] [PubMed] [Google Scholar]
- Rolin B, Larsen MO, Gotfredsen CF, Deacon CF, Carr RD, Wilken M, et al. The long-acting GLP-1 derivative NN2211 ameliorates glycemia and increases beta-cell mass in diabetic mice. Am J Physiol Endocrinol Metab. 2002;283:E745–E752. doi: 10.1152/ajpendo.00030.2002. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ, Luheshi G, Toulmond S. Cytokines and their receptors in the central nervous system: physiology, pharmacology, and pathology. Pharmacol Ther. 1996;69:85–95. doi: 10.1016/0163-7258(95)02033-0. [DOI] [PubMed] [Google Scholar]
- Schmidt WE, Siegel EG, Creutzfeldt W. Glucagon-like peptide-1 but not glucagon-like peptide-2 stimulates insulin release from isolated rat pancreatic islets. Diabetologia. 1985;28:704–707. doi: 10.1007/BF00291980. [DOI] [PubMed] [Google Scholar]
- Sinclair EM, Yusta B, Streutker C, Baggio LL, Koehler J, Charron MJ, et al. Glucagon receptor signaling is essential for control of murine hepatocyte survival. Gastroenterology. 2008;135:2096–2106. doi: 10.1053/j.gastro.2008.07.075. [DOI] [PubMed] [Google Scholar]
- Sonne DP, Engstrom T, Treiman M. Protective effects of GLP-1 analogues exendin-4 and GLP-1(9-36) amide against ischemia-reperfusion injury in rat heart. Regul Pept. 2008;146:243–249. doi: 10.1016/j.regpep.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Stoffers DA. The development of beta-cell mass: recent progress and potential role of GLP-1. Horm Metab Res. 2004;36:811–821. doi: 10.1055/s-2004-826168. [DOI] [PubMed] [Google Scholar]
- Tachibana T, Sato M, Oikawa D, Furuse M. Involvement of CRF on the anorexic effect of GLP-1 in layer chicks. Comp Biochem Physiol A Mol Integr Physiol. 2006;143:112–117. doi: 10.1016/j.cbpa.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Tews D, Lehr S, Hartwig S, Osmers A, Paslack W, Eckel J. Anti-apoptotic action of exendin-4 in INS-1 beta cells: comparative protein pattern analysis of isolated mitochondria. Horm Metab Res. 2009;41:294–301. doi: 10.1055/s-0028-1105911. [DOI] [PubMed] [Google Scholar]
- Thum A, Hupe-Sodmann K, Goke R, Voigt K, Goke B, McGregor GP. Endoproteolysis by isolated membrane peptidases reveal metabolic stability of glucagon-like peptide-1 analogs, exendins-3 and -4. Exp Clin Endocrinol Diabetes. 2002;110:113–118. doi: 10.1055/s-2002-29087. [DOI] [PubMed] [Google Scholar]
- Tourrel C, Bailbe D, Lacorne M, Meile MJ, Kergoat M, Portha B. Persistent improvement of type 2 diabetes in the Goto-Kakizaki rat model by expansion of the beta-cell mass during the prediabetic period with glucagon-like peptide-1 or exendin-4. Diabetes. 2002;51:1443–1452. doi: 10.2337/diabetes.51.5.1443. [DOI] [PubMed] [Google Scholar]
- Tsunekawa S, Yamamoto N, Tsukamoto K, Itoh Y, Kaneko Y, Kimura T, et al. Protection of pancreatic beta-cells by exendin-4 may involve the reduction of endoplasmic reticulum stress; in vivo and in vitro studies. J Endocrinol. 2007;193:65–74. doi: 10.1677/JOE-06-0148. [DOI] [PubMed] [Google Scholar]
- Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- Urusova IA, Farilla L, Hui H, D'Amico E, Perfetti R. GLP-1 inhibition of pancreatic islet cell apoptosis. Trends Endocrinol Metab. 2004;15:27–33. doi: 10.1016/j.tem.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Vilsboll T, Krarup T, Madsbad S, Holst JJ. No reactive hypoglycaemia in Type 2 diabetic patients after subcutaneous administration of GLP-1 and intravenous glucose. Diabet Med. 2001;18:144–149. doi: 10.1046/j.1464-5491.2001.00424.x. [DOI] [PubMed] [Google Scholar]
- Yada T, Itoh K, Nakata M. Glucagon-like peptide-1-(7-36)amide and a rise in cyclic adenosine 3′,5′-monophosphate increase cytosolic free Ca2+ in rat pancreatic beta-cells by enhancing Ca2+ channel activity. Endocrinology. 1993;133:1685–1692. doi: 10.1210/endo.133.4.8404610. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Kishi T, Lee CE, Choi BJ, Fang H, Hollenberg AN, et al. Glucagon-like peptide-1-responsive catecholamine neurons in the area postrema link peripheral glucagon-like peptide-1 with central autonomic control sites. J Neurosci. 2003;23:2939–2946. doi: 10.1523/JNEUROSCI.23-07-02939.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusta B, Baggio LL, Estall JL, Koehler JA, Holland DP, Li H, et al. GLP-1 receptor activation improves beta cell function and survival following induction of endoplasmic reticulum stress. Cell Metab. 2006;4:391–406. doi: 10.1016/j.cmet.2006.10.001. [DOI] [PubMed] [Google Scholar]


