Abstract
Background and purpose:
The current study was designed to: (i) examine whether functional interactions occur between receptors known to regulate alcohol self-administration; and (ii) characterize relapse to alcohol seeking following abstinence.
Experimental approach:
The selective cannabinoid CB1 receptor antagonist SR141716A (0.03–1.0 mg·kg−1 i.p.) resulted in a dose-dependent reduction in ethanol self-administration in ethanol-preferring Indiana-preferring rats. SR141716A was then co-administered with either the selective glutamate metabotropic glutamate 5 (mGlu5) receptor antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine (MTEP) or the selective adenosine A2A receptor antagonist SCH58261.
Key results:
When administered at individually sub-threshold doses, a combination of SR141716A (0.1 mg·kg−1) and SCH58261 (0.5 mg·kg−1 i.p.) produced a reduction (28%) in ethanol self-administration. Combinations of threshold doses of SR141716A (0.3 mg·kg−1) and SCH58261 (2.0 mg·kg−1, i.p.) caused an essentially additive reduction (68%) in alcohol self-administration. A combination of individually sub-threshold doses of CB1 and mGlu5 receptor antagonists did not affect alcohol self-administration; however, combined threshold doses of SR141716A (0.3 mg·kg−1) and MTEP (1.0 mg·kg−1 i.p.) did reduce ethanol self-administration markedly (80%). Cue-conditioned alcohol seeking was attenuated by pretreatment with MTEP (1.0 mg·kg−1) co-administered with SR141716A (0.3 mg·kg−1 i.p.). In contrast, SCH58261 (2.0 mg·kg−1) co-administered with SR141716A (0.3 mg·kg−1 i.p.) did not reduce cue-conditioned alcohol seeking.
Conclusions and implications:
Adenosine A2A and cannabinoid CB1 receptors regulated alcohol self-administration additively, but combined low-dose antagonism of these receptors did not prevent cue-conditioned alcohol seeking after abstinence. In contrast, combined low-dose antagonism of mGlu5 and CB1 receptors did prevent relapse-like alcohol seeking after abstinence, suggesting a prominent role for mGlu5 receptors in this paradigm.
Keywords: alcohol, relapse, glutamate mGlu5 receptor, cannabinoid CB1 receptor, adenosine A2A receptor
Introduction
Ethanol is the second most commonly abused psychotropic drug after caffeine (Nevo and Hamon, 1995). The consequence of alcohol abuse is far more serious, however, resulting in significant social and economic costs. The World Health Organization estimates that 2 billion people consume alcohol, and of those 76.3 million have diagnosable alcohol use disorders. In 1998, the estimated cost of alcohol abuse to the United States was $184.6 billion (World Health Organization, 2004). Due to the polymodal nature of alcohol addiction, relapse rates remain high under treatment (Graham et al., 2002; Heidbreder, 2005), and further investigation into the pathophysiology of the disease is required. Early theories on the mechanism of action of ethanol suggested that membrane lipids were a target, but more recent research refutes this simple view, with enzymes, receptors and various ion channels shown to be targets for ethanol (see Vengeliene et al., 2008).
The metabotropic glutamate 5 (mGlu5) receptor (nomenclature follows Alexander et al., 2008) is a member of the seven-transmembrane, G protein-coupled receptor family. High levels of mRNA encoding the mGlu5 receptor and associated protein are found within the olfactory tubercle and bulb, nucleus accumbens, caudate–putamen, lateral septum, hippocampus and cortex (Romano et al., 1995; Sahara et al., 2001). The mGlu5 receptor is positively coupled to adenylate cyclase through Gs/Golf proteins, and has been associated with phosphoinositide hydrolysis and activation of phospholipase C (Conn and Pin, 1997; Hermans and Challiss, 2001).
The mGlu5 receptor appears to be involved in the reinforcing properties of a number of drugs of abuse. Concerning ethanol, the mGlu5 receptor antagonist 2-methyl-6-(phenylethynyl)-pyridine (MPEP) decreased consumption in mice (Olive et al., 2005) and rats (McMillen et al., 2005), and operant self-administration in mice (Hodge et al., 2006) and rats (Schroeder et al., 2005). MPEP also prevented the reinstatement of ethanol-seeking behaviour induced by olfactory cues (Backstrom et al., 2004) or repeated deprivations (Schroeder et al., 2005) in rats. Administration of 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine (MTEP), a mGlu5 receptor antagonist with greater selectivity and bioavailability than MPEP (Anderson et al., 2002; Cosford et al., 2003), decreased ethanol self-administration in two strains of alcohol-preferring rats (Cowen et al., 2005b) and reduced both consummatory and appetitive responding for ethanol in C57/BL6J mice (Cowen et al., 2007). mGlu5 receptor-deficient mice consume less ethanol than their wild-type littermates, in a two-bottle free-choice paradigm, and are more susceptible to the hypnotic effects of ethanol (Bird et al., 2008).
The adenosine A2A receptor belongs to a family of four G protein-coupled adenosine receptors that are widely distributed throughout the body, with particularly strong expression in the basal ganglia (Fredholm et al., 2000; Yaar et al., 2005). Adenosine A2A receptors are localized pre-synaptically on glutamatergic afferents from the prefrontal cortex, as well as post-synaptically on enkephalin/dopamine D2 receptor expressing GABAergic medium spiny neurones, where they appear to modulate cortico-limbic-striatal glutamatergic neurotransmission (Schiffmann et al., 2007). The A2A receptor is positively coupled to adenylate cyclase via Gs or Golf proteins, and, upon activation, elevates cAMP and intracellular calcium levels (Ongini and Fredholm, 1996; Kull et al., 1999; Fredholm et al., 2000; Yaar et al., 2005).
Worldwide, the most commonly used psychoactive drug is caffeine, a non-selective adenosine receptor antagonist. The adenosine A2A receptor in particular appears to be involved in the reinforcing properties of ethanol and other drugs of abuse (Brown and Short, 2008; Castane et al., 2008; Brown et al., 2009). Adenosine A2A receptor-deficient mice are observed to be less sensitive to the acute intoxicating effects of ethanol (Naassila et al., 2002), and exhibit blunted ethanol withdrawal effects, a phenotype replicated in wild-type mice following treatment with A2A receptor antagonists (El Yacoubi et al., 2001). A2A receptor antagonism also attenuates operant self-administration of ethanol in rats (Arolfo et al., 2004; Thorsell et al., 2007; Adams et al., 2008).
Cannabinoid receptors are also seven-transmembrane-spanning, G protein-coupled receptors. There are two known subtypes, one of which, the CB1 receptor, is the most abundantly expressed G protein-coupled receptor within the CNS (Solinas et al., 2008). The CB1 receptor is negatively coupled through Gi/Go proteins to adenylate cyclase, and positively coupled to mitogen-activated protein kinases (Solinas et al., 2008). The CB1 receptor antagonist rimonabant (N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide hydrochloride; SR141716A) inhibits phasic dopamine release in the nucleus accumbens evoked by nicotine, ethanol and cocaine administration in freely moving Sprague-Dawley rats (Cheer et al., 2007) without altering basal dopamine release (Cheer et al., 2004). Considering the effect of many drugs of abuse upon phasic dopamine release, it is logical that the CB1 receptor is more widely implicated in the reinforcing properties of many drugs of abuse (Cheer et al., 2007).
CB1 receptor knockout mice display reduced consumption of ethanol in a two-bottle free-choice paradigm (Lallemand and de Witte, 2005; Thanos et al., 2005), a reduction in place preference for ethanol in a conditioned place preference paradigm (Thanos et al., 2005), and impaired neuroadaptation of NMDA and GABAA receptors following chronic ethanol exposure (Warnault et al., 2007). CB1 receptor knockout mice also show reduced cocaine self-administration, reduced drug-lever pairing discrimination and a lower progressive ratio breakpoint, results which mirror those obtained when the CB1 antagonist SR141716A is given to wild-type mice (Soria et al., 2005). Interestingly, however, when CB1 receptor-deficient mice are administered cocaine, they show similar dopamine release in the nucleus accumbens as their wild-type littermates (Soria et al., 2005), a finding perhaps suggestive of developmental adaptations within the nucleus accumbens.
Another CB1 receptor antagonist, SR147778, attenuates ethanol acquisition, drinking and the deprivation effect in Sardinian alcohol-preferring (sP) rats in a two-bottle free-choice paradigm (Gessa et al., 2005). sP Rats exhibit greater CB1 receptor mRNA expression than Wistar rats within the mesocorticolimbic system (Cippitelli et al., 2005), suggesting that the differences observed may correlate with alcohol drinking behaviours. SR147778 significantly reduces ethanol preference and intake in a two-bottle free-choice paradigm (Lallemand and De Witte, 2006), and also self-administration under operant conditions (Economidou et al., 2007) in Wistar rats. Furthermore, SR141716A significantly decreases the motivation to self-administer ethanol, and blocks cue-induced reinstatement in both Wistar (Cippitelli et al., 2005; Economidou et al., 2006) and sP rats (Cippitelli et al., 2005), but does not affect stress-induced reinstatement in Wistar rats (Economidou et al., 2007).
We have previously reported that the selective adenosine A2A receptor antagonist SCH58261 and the selective glutamate mGlu5 receptor antagonist MTEP interact to produce an apparently synergistic reduction in ethanol self-administration, and in combination completely block cue-induced reinstatement in alcohol-preferring Indiana-preferring rat (iP) rats (Adams et al., 2008). The logic for investigating mGlu5 and A2A receptor interactions arose from the co-localization of the A2A and mGlu5 receptors within the mesocorticolimbic pathway, a circuit intimately involved in natural and drug-induced reward. The existence of heteromeric receptor complexes (Ferre et al., 2002), signal transduction commonalities (Agnati et al., 2003) and other functional evidence (Nishi et al., 2003; Kachroo et al., 2005; Rodrigues et al., 2005) also highlight the need to explore interactions between these receptors in relation to complex behavioural patterns.
Likewise, interactions between the cannabinoid CB1 receptor and other receptors have been reported. For example, µ-opioid and CB1 receptors have been found to interact synergistically via a common signal transduction pathway in cultured primary striatal and accumbal neurones, and this phenomenon was regulated by the adenosine A2A receptor (Yao et al., 2006). Reductions in tetrahydrocannabinol-induced rewarding or aversive effects have been found in mice lacking the A2A receptor, which display a normal distribution of the CB1 receptor (Soria et al., 2004).
Given the ability of the CB1 receptor to regulate alcohol self-administration, the similarities in the signal transduction pathways of CB1, mGlu5 and A2A receptors, and the co-localization of the receptors within the mesocorticolimbic pathway, here we seek to extend previous work and examine if interactions exist between A2A, mGlu5 and CB1 receptors in an operant, ethanol self-administration paradigm, utilizing alcohol-preferring (iP) rats. The choice of cue-conditioned alcohol seeking following abstinence, rather than typical extinction–reinstatement paradigms, is based on the premise that many human alcohol/drug users do not undergo the equivalent of extinction training via rehabilitation programmes. Indeed, it is common for humans with alcohol use disorders to relapse following a period of abstinence (40–60% within months, 70–80% by 1 year; Dawson et al. (2007). Consequently, we have established a model to address this often overlooked issue.
Methods
Animals
All experiments were performed in accordance with the Prevention of Cruelty to Animals Act 1986, under the guidelines of the National Health and Medical Research Council Code of Practice for the Care and Use of Animals for Experimental Purposes in Australia. Inbred alcohol-preferring (iP) rats were obtained from the breeding colony at the Howard Florey Institute (University of Melbourne). Parental stock had previously been obtained from Professor TK Li (while at Indiana University, Indianapolis, IN, USA). The animals were pair housed with ad libitum access to standard rat chow and water, with a 12 h light/dark cycle; lights on 0700 h.
Operant alcohol self-administration
Alcohol-preferring iP rats (n= 30) were trained to self-administer ethanol (10% v/v; Merck Pty Ltd, Victoria, Australia) under operant conditions using a fixed ratio of 3 (FR3) during 20 min sessions as previously described (Cowen et al., 2005a,b; Lawrence et al., 2006; Liang et al., 2006). Operant chambers supplied by Med Associates (St Albans, VT, USA) were employed. Each chamber was housed individually in sound attenuation cubicles, featuring a fan to provide airflow and mask external noise. The chambers were connected to a computer running Med-PC IV software (Med Associates) to record the activity. Availability of ethanol was conditioned by the presence of an olfactory cue (S+; 2 drops of vanilla essence, placed on the bedding of the operant chamber directly under the active lever), plus a 1 s light stimulus (CS+) occurred when FR3 was obtained. Ultimately, the rats were responding for a 10% ethanol solution under a fixed ratio requirement of 3 (FR3) with the presentation of alcohol and water randomized to minimize side preference. For each session, total ethanol and water responses were recorded with each delivery consisting of 100 µL of either water or ethanol solution, and the difference of fluid in the ethanol reservoir between the beginning and end of the session was also recorded to ensure the correct calibration of the delivery system. Following acquisition of lever-pressing behaviour and stable alcohol self-administration (<10% variation across sessions, 10% ethanol v/v), the rats were given the adenosine A2A receptor antagonist SCH58261 (5-amino-2-(2-furyl)-7-phenylethyl-pyrazolo[4,3-e]-1,2,4-triazolo[1,5c]pyrimidine, Sigma, St Louis, MO, USA; 1 and 2 mg·kg−1 i.p. and co-administered as detailed) suspended in methyl cellulose 30 min prior to the beginning of operant sessions. The mGlu5 receptor antagonist MTEP (Ascent Scientific, N. Somerset, UK; 0.25 and 1 mg·kg−1 i.p.) was dissolved in 1% dimethyl sulphoxide (DMSO) as previously described (Cowen et al., 2005b; 2007; Adams et al., 2008) and administered 20 min prior to the operant session. The CB1 receptor antagonist SR141716A (Sanofi Synthelabo Recherche, Montpelier, France; 0.03, 0.1, 0.3 and 1.0 mg·kg−1 i.p. and co-administered as detailed) was dissolved in Tween 80/saline, and administered 20 min prior to the operant session. Administration of MTEP with either SCH58261 or SR141716A involved two injections both at 0.5 mL·kg−1 at the time-points mentioned; vehicle controls were delivered in the same way. Drug administration weeks were structured so that Mondays and Fridays were no injection days; vehicle was injected either Tuesday or Wednesday (i.p.) followed by drug the following day (i.p.).
Cue-conditioned alcohol seeking
Following standard operant training as detailed earlier, iP rats (n= 30) were returned to their home cages for a period of 4 weeks. Alcohol seeking was then triggered by replacing S+ (i.e. the olfactory cue) under the ‘active’ lever and also reprogramming the software such that the stimulus light (CS+) was activated (for 1 s) after every FR3 response, although there was no delivery of ethanol into the receptacle. Prior to the session, the rats were treated with either vehicle or a combination of SCH58261 (0.5 mg·kg−1 i.p. 30 min prior) and SR141716A (0.1 mg·kg−1 i.p. 20 min prior) or MTEP (0.25 mg·kg−1 i.p. 20 min prior) and SR141716A (n= 10 per treatment).
Statistics
Statistical analysis was performed with SigmaStat (version 3; SPSS Inc., Chicago, IL, USA). The data are presented as mean ± SEM. A significance level of P= 0.05 was used. In general, session totals and time-courses were analysed using a repeated-measures two-way analysis of variance (anova) with Student–Newman–Keuls post hoc tests. For every dose of drug, there is a corresponding vehicle injection, thus the factors for the session totals were treatment versus drug/vehicle; for the time-course analysis, the factors were drug/vehicle versus time-point. The effect of 0.5 mg·kg−1 i.p. SCH58261 was also examined using a paired t-test. Vehicle injections on each day were not significantly different for ethanol or water lever presses (as examined via a paired t-test), and were therefore pooled for graphical representation. The resultant data were analysed using a one-way anova with a Student–Newman–Keuls post hoc test.
Results
Effect of SR141716A on operant responding for ethanol
We have previously published dose–response curves demonstrating the effect of MTEP and SCH58261 upon ethanol self-administration in iP rats (Cowen et al., 2005b; Adams et al., 2008). Therefore, we first conducted a dose–response curve for the selective CB1 antagonist SR141716A. A cohort of 10 iP rats responding stably and preferentially for 10% v/v ethanol (38.7 ± 11.3 responses representing 0.6 ± 0.1 g·kg−1 ethanol per 20 min session) compared with water (3.8 ± 1.0 responses) were treated with either vehicle or SR141716A (Figure 1). A significant effect of treatment occurred [treatment: F(6,42)= 1.095, P= 0.381; drug vs. vehicle F(1,7)= 7.858, P= 0.026] indicating that SR141716A significantly reduced ethanol self-administration at 1.0 mg·kg−1 i.p. (−75%, P= 0.003) and 0.3 mg·kg−1 i.p. (−50%, P= 0.006). SR141716A at 1.0 mg·kg−1 i.p. also significantly attenuated water responding (P= 0.009), but 0.3 mg·kg−1 was without effect on water responding. No significant effects on self-administration of ethanol or water were noted when SR141716A was administered at 0.1 or 0.03 mg·kg−1 i.p.
Figure 1.
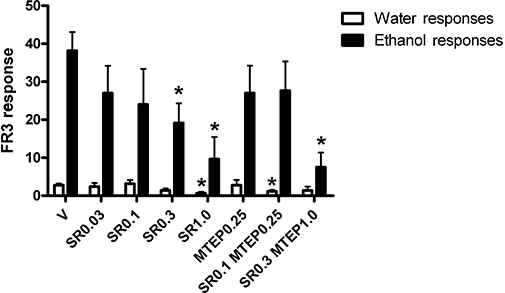
The effect of SR141716A and MTEP on operant ethanol self-administration in iP rats (n= 10). Ethanol self-administration was significantly reduced by SR141716A administration at 1.0 mg·kg−1 (P= 0.003) and 0.3 mg·kg−1 (P= 0.006) i.p. When co-administered at a low dose, SR141716A and MTEP had no effect on ethanol self-administration at 0.1 and 0.25 mg·kg−1, respectively, but did significantly reduce ethanol self-administration when co-administered at 0.3 and 1 mg·kg−1 i.p. (P= 0.001). Water responses were significantly reduced by SR141716A at 1 mg·kg−1 (P= 0.009) and SR141716A, and MTEP co-administered at 0.1 and 0.25 mg·kg−1 i.p. (P= 0.011). V, Vehicle; SR0.1 or 0.3, SR141716A at 0.1 or 0.3 mg·kg−1 i.p.; MTEP 0.25 or 1.0, MTEP at 0.25 or 1.0 mg·kg−1 i.p. *Significantly different to vehicle.
Effect of MTEP and SR141716A on operant responding for ethanol
From previously published experiments, the highest sub-threshold dose of MTEP in iP rats was 0.25 mg·kg−1 i.p. (Adams et al., 2008). This was repeated in the current cohort of iP rats to confirm no significant reduction in ethanol self-administration (Figure 1, P > 0.05). When co-administered at individually sub-threshold doses, SR141716A (0.1 mg·kg−1) and MTEP (0.25 mg·kg−1 i.p.) produced no reduction in ethanol self-administration; however, a small, but significant, reduction in water responding was observed (P= 0.011). When co-administered at individually threshold doses, SR141716A (0.3 mg·kg−1) and MTEP (1.0 mg·kg−1 i.p.) in combination produced a significant reduction in ethanol self-administration (−80%, P= 0.001). It should be noted that MTEP administered alone (1.0 mg·kg−1 i.p.) in a previous study with similar protocols and the same strain of rat significantly reduced ethanol (∼−55%), but not water self-administration (Cowen et al., 2005b).
Effect of SCH58261 and SR141716A on operant responding for ethanol
From previously published experiments, the highest sub-threshold dose of SCH58261 in iP rats was 0.5 mg·kg−1 i.p. (Adams et al., 2008). This was repeated in a cohort of iP rats with no significant reduction in ethanol self-administration (Figure 2). SR141716A (0.1 mg·kg−1 i.p.) was also administered to this cohort to reverify that this dose was sub-threshold for alcohol self-administration (Figure 2).
Figure 2.
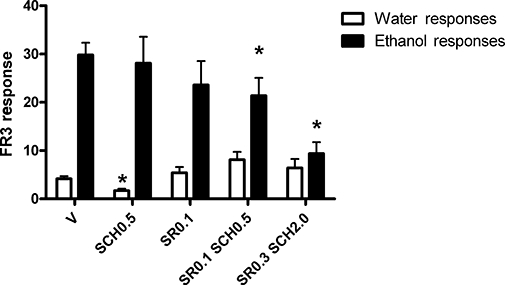
The effect of SCH58261 and SR141716A on operant ethanol self-administration in iP rats (n= 10). Ethanol self-administration was not significantly altered by SCH58261 at 0.5 mg·kg−1 i.p., although water responding was reduced (P= 0.035). SR141716A at 0.1 mg·kg−1 i.p. did not alter responding. When co-administered, SR141716A and SCH58261 significantly reduced responding for ethanol without affecting responding for water at 0.1 and 0.5 mg·kg−1 (P= 0.030), and 0.3 and 2 mg·kg−1 (P= 0.005) respectively. V, Vehicle; SCH0.5 or 2, SCH58261 at 0.5 or 2.0 mg·kg−1 i.p.; SR0.1 or 0.3, SR141716A at 0.1 or 0.3 mg·kg−1 i.p. *Significantly different to vehicle.
When co-administered at the highest sub-threshold doses, SR141716A (0.1 mg·kg−1) and SCH58261 (0.5 mg·kg−1 i.p.) produced a significant reduction in ethanol self-administration (−28%, P= 0.030, Figure 2). When co-administered at threshold doses, SR141716A (0.3 mg·kg−1) and SCH58261 (2.0 mg·kg−1 i.p.) together produced a significant reduction in ethanol self-administration (−68%, P= 0.005). We have previously shown that SCH58261 (2.0 mg·kg−1 i.p.) significantly reduces ethanol self-administration (−47%) in this rat strain using this paradigm (Adams et al., 2008).
Effect of SR141716A/SCH58261 and SR141716A/MTEP on cue-conditioned alcohol seeking
Following a period of withdrawal (4 weeks), the rats were tested for cue-conditioned alcohol seeking under extinction conditions (cues present, but no delivery of ethanol subsequent to lever pressing). Figure 3 shows that under these conditions, iP rats show robust alcohol seeking in this paradigm. MTEP (1.0 mg·kg−1) co-administered with SR141716A (0.3 mg·kg−1 i.p.) significantly reduced alcohol seeking (P < 0.001) compared to vehicle-treated iP rats. In contrast, SCH58261 (2 mg·kg−1) co-administered with SR141716A (0.3 mg·kg−1 i.p.) did not reduce active lever pressing during relapse-like behaviour (P= 1.0).
Figure 3.
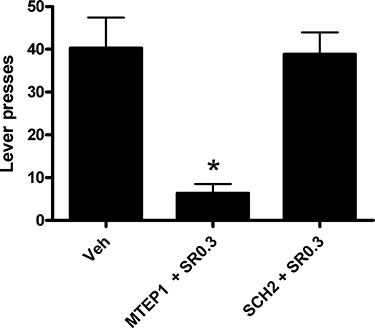
The effect of SCH58261 and MTEP, co-administered with SR141716A, on cue-conditioned alcohol seeking in iP rats (n= 10 per treatment). MTEP (1.0 mg·kg−1) and SR141716A (0.3 mg·kg−1) significantly reduced cue-conditioned alcohol seeking compared with vehicle (P < 0.001). SCH58261 (2.0 mg·kg−1) and SR141716A (0.3 mg·kg−1) co-administered i.p. had no effect on cue-conditioned alcohol seeking. Veh, Vehicle; SCH2.0, SCH58261 at 2.0 mg·kg−1 i.p.; SR0.3, SR141716A at 0.3 mg·kg−1 i.p.; MTEP1.0, MTEP at 1.0 mg·kg−1 i.p. *Significantly different to vehicle (P < 0.001).
Effects of combinations of SCH58261, MTEP and SR141716A on operant ethanol self-administration in iP rats
Figure 4A shows the normalized, synergistic effect of combining individually sub-threshold doses of SCH58261 and MTEP on ethanol self-administration in iP rats (primary data published in Adams et al., 2008). From the present data, it is clear that combining either individually sub-threshold doses of SR141716A (0.1 mg·kg−1 i.p.) and MTEP (0.25 mg·kg−1 i.p.; Figure 4B), or individually sub-threshold doses of SR141716A (0.1 mg·kg−1 i.p.) and SCH58261 (0.5 mg·kg−1 i.p.; Figure 4C), produces effects on alcohol self-administration that are essentially additive.
Figure 4.
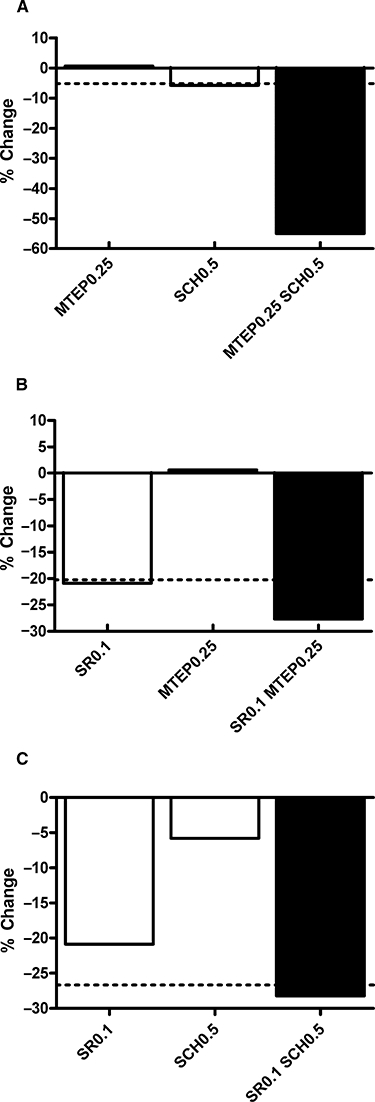
Summary figures describing the effects of SCH58261, MTEP and SR141716A on operant ethanol self-administration in iP rats. Open bars represent the percentage change in deliveries of 10% v/v ethanol on an FR3 schedule. The dotted, horizontal line represents the theoretical additive effect of each combination. The solid bar represents the actual percentage reduction in ethanol self-administration achieved with co-administration. (A) The combination of SCH58261 (0.5 mg·kg−1) and MTEP (0.25 mg·kg−1) injected i.p. produces a synergistic reduction in ethanol self-administration in iP rats. Primary data for Figure 4a have been reported previously (Adams et al., 2008). (B) The combination of SR141716A (0.1 mg·kg−1) and MTEP (0.25 mg·kg−1 both i.p.) and (C) the combination of SR141716A (0.1 mg·kg−1) and SCH58261 (0.5 mg·kg−1 i.p.) produced essentially additive effects on ethanol self-administration. SCH: SCH58261, SR: SR141716A.
Discussion
Here, we report that combinations of antagonists that target glutamate mGlu5, adenosine A2A and cannabinoid CB1 receptors can regulate alcohol self-administration and/or seeking following a period of abstinence. We have previously noted that co-administration of low doses of A2A and mGlu5 receptor antagonists results in a dramatic reduction of alcohol self-administration and blocks cue-induced reinstatement following extinction (Adams et al., 2008). We now provide more evidence for the specificity of this A2A/mGlu5 interaction, because essentially additive effects were observed for combinations of A2A/CB1 or mGlu5/CB1 receptor antagonists in relation to self-administration of alcohol. Moreover, combined low-dose CB1/A2A receptor antagonists had no effect on alcohol seeking following a period of abstinence. In contrast, while an mGlu5/CB1 receptor antagonist combination again suggested a simple additive effect in terms of reducing alcohol self-administration, this combination was able to prevent alcohol seeking following abstinence. Consequently, this study demonstrates that in iP rats, following a period of withdrawal the re-exposure to cues previously associated with the availability of alcohol precipitates a relapse-like response that apparently involves mGlu5-mediated signalling.
At sub-threshold doses, the effect of SR141716A and MTEP in combination produced essentially an additive effect (Figure 4B), suggesting that CB1 and mGlu5 receptors may not functionally interact in a co-operative or facilitatory manner within this paradigm to reduce operant ethanol self-administration. Although sub-threshold combinations of SR141716A co-administered with SCH58261 i.p. reduced ethanol self-administration, the effect was no greater than additive (Figure 4C), suggesting that CB1 and adenosine A2A receptors probably work independently within this paradigm.
Striatal cannabinoid CB1 receptors are localized pre-synaptically on GABAergic terminals of interneurones, collaterals from GABAergic efferent neurones and also on glutamatergic but not dopaminergic terminals (Kofalvi et al., 2005). If CB1 receptors are found post-synaptically, their population is sparse (for review, see Ferre et al., 2009). Adenosine A2A receptors are localized within the striatum post-synaptically on dopamine D2 receptor/enkephalin expressing GABAergic medium spiny neurones receiving glutamatergic input, and a smaller population pre-synaptically (Hettinger et al., 2001; Rosin et al., 2003). In the striatum, CB1 and A2A receptors are co-localized on glutamatergic nerve terminals and in the dendritic spines of GABAergic/enkephalinergic neurons (Shindou et al., 2008). As a heteromer in cell culture, cannabinoid CB1 receptor function is co-dependent on adenosine A2A receptor function, with activation of the heteromer resulting in Gi protein signalling (Carriba et al., 2007).
mGlu5 receptors are mainly located within the post-synaptic density of glutamatergic synapses (Pin et al., 2003). The anatomical co-localization of the A2A and mGlu5 receptors, and their existence as a heteromer in striatal extracts (Ferre et al., 2002) suggest opportunities for interaction. Receptor heteromer interactions are proposed to occur via various mechanisms. These include direct, protein–protein interactions and intra-membrane lipid and scaffolding protein interactions resulting in allosteric modulation of receptors and subsequent alterations in ligand affinity, and phosphorylation and signal transduction interactions (Franco et al., 2003). One interesting finding from Yao et al. (2008) reveals a substantial cross-talk between PKA (mGlu5 and dopamine D2 receptors) and PKC (cannabinoid CB1 and adenosine A2A receptors) signalling occurs whereby PKC activation leads to potentiation of Gs receptor signalling. Additionally, the activator of G protein signalling 3 (AGS3) levels within the nucleus accumbens core are significantly elevated following abstinence, knockdown of which normalizes heightened alcohol-seeking responses in rats (Bowers et al., 2008). Further work exploring these interactions within signalling transduction pathways would seem essential.
There is evidence to support the existence of adenosine A2A/mGlu5/dopamine D2 receptor mosaics. Indeed, A2A or mGlu5 receptor agonists reduce the affinity of dopamine D2 binding sites, with concurrent stimulation resulting in synergistic interactions for c-fos expression, ERK phosphorylation and DARPP-32 (Ferre et al., 2002; Nishi et al., 2003). Functional interactions also occur between A2A and mGlu5 receptor antagonists, which synergistically increase locomotion in reserpinized mice (Coccurello et al., 2004; Kachroo et al., 2005).
The CB1 receptor, generally located across the synapse from the A2A/mGlu5 receptor complex, has no such opportunity for ‘direct’ interaction as a mosaic, although the cannabinoid system has been demonstrated to signal via retrograde messaging (Matyas et al., 2006), and so the possibility exists for A2A and/or mGlu5 receptors to modulate the synthesis and/or release of endocannabinoids from medium spiny neurones. Excited cortico-striatal glutamatergic inputs induce retrograde endocannabinoid signalling, which is involved in dopamine D2 receptor-mediated long-term synaptic plasticity in this region (Giuffrida et al., 1999; Centonze et al., 2004). Ethanol self-administration dose-dependently increases dialysate levels of 2-arachidonoylglycerol within the nucleus accumbens shell of rats (Caille et al., 2007). This plasticity subsequently results in the reduced probability of glutamate release (Choi and Lovinger, 1997), and as previously alluded to, adenosine A2A or mGlu5 receptor antagonists effectively function as positive allosteric modulators of dopamine D2 receptors within the receptor mosaic. Further work to extend the current experiment into a chronic setting is required to examine striatal plasticity within the context of alcohol studies.
The association of instrumental actions (lever pressing) followed by reward is mediated within the dorsal striatum, as lesions to, or dopamine antagonists infused into this area, abolish this association (Faure et al., 2005; Vanderschuren et al., 2005). Drug-primed reinstatement is associated with glutamatergic inputs into the basal ganglia (Kalivas and McFarland, 2003), and reversible inactivation of the anterior cingulate prevents cue-, foot shock stress- and cocaine-primed reinstatement in rats (McFarland and Kalivas, 2001; See, 2002). In fact, glutamatergic innervation of the accumbens core via the anterior cingulate is critical for cue-induced reinstatement (Di Ciano and Everitt, 2001). As mGlu5 receptors (Homayoun et al., 2004) can modulate glutamatergic transmission onto medium spiny neurones, it is possible that striatal mGlu5 receptors may play a role in relapse to alcohol seeking following abstinence.
Threshold doses of SCH58261 (2.0 mg·kg−1) and SR141716A (0.3 mg·kg−1) did not block relapse-like alcohol seeking, although SR141716A (0.3 mg·kg−1) and MTEP (1.0 mg·kg−1 i.p.) did (Figure 3), suggesting that attenuation of relapse in the current paradigm is mediated primarily by the mGlu5 receptor. Importantly, MPEP alone (3.0 mg·kg−1 i.p.) reduces cue-induced reinstatement of ethanol-seeking (Backstrom et al., 2004), and MTEP alone (1.0 mg·kg−1 i.p.) reduces operant ethanol self-administration (Cowen et al., 2005b). MPEP blocks nicotine-induced drug-seeking behaviour and reinstatement in Wistar rats (Tessari et al., 2004; Bespalov et al., 2005), and reduces incentive motivational properties of nicotine, cocaine and food (Paterson and Markou, 2005). While SR141716A can prevent reinstatement of alcohol seeking, this typically occurs at doses of 1 mg·kg−1 or greater, depending on rat strain (Cippitelli et al., 2005). In the present study, we used SR141716A at a dose of 0.3 mg·kg−1 during drug combination trials, which in combination with MTEP (1.0 mg·kg−1) did prevent relapse, although the same dose of SR141716A in combination with SCH58261 (2.0 mg·kg−1) had no impact on alcohol seeking. Higher doses of SR141716A were confounded by altered water responding, and therefore not pursued further. Therefore, while cannabinoid CB1 receptors and adenosine A2A receptors can regulate drug seeking, it is likely that under the conditions employed in the present study, the mGlu5 receptor plays a more relevant role in alcohol seeking following abstinence. It would also appear that mGlu5 receptors do not synergistically interact with CB1 receptors in this context, perhaps due to the subcellular localization of the receptor types.
One explanation as to why CB1–mGlu5 receptor antagonists attenuate cue-conditioned alcohol seeking while CB1–A2A antagonists do not is the mGlu5–NMDA receptor heterodimer, found in medium spiny neurons on the post-synaptic membrane of striatal glutamate terminals (Fuxe et al., 2007). The attenuation of function of this receptor complex would reduce glutamatergic function from the prefrontal cortex, a structure known to modulate reinstatement (Weitlauf and Woodward, 2008), but less involved in self-administration.
There is debate as to the validity of relapse and reinstatement models in animal research. Issues of volition, for example, are difficult to model. A valid model of relapse is significant considering up to 85% of abstinent alcoholics displaying no withdrawal symptoms relapse (Boothby and Doering, 2005). Extinction–reinstatement and cue-conditioned relapse appear to be mediated via differing neural circuitry (Fuchs et al., 2006). Extinction training, perhaps the rodent correlate of human rehabilitation clinic attendance, has questionable construct validity as the vast majority of drug users do not seek help in achieving abstinence (Cunningham, 1999), but rather become ‘spontaneously abstinent’. Thus, forced abstinence may be a more accurate model of the typical human experience. Abstinence also induces incubation of craving, suggested to be an important component of the persisting susceptibility for relapse in humans (Grimm et al., 2001). Unlike many studies using a brief extinction protocol, the current study used a 1 month abstinence period as human relapse is an enduring phenomenon (Epstein et al., 2006).
We found evidence for an apparently additive effect between antagonists of cannabinoid CB1 and either adenosine A2A or glutamate mGlu5 receptors in relation to alcohol self-administration. Combination treatment approaches may potentially reduce doses of individual drugs, and thus minimize off-target effects. We also demonstrate that relapse to alcohol seeking can be precipitated following a period of abstinence, and this appears to be mediated in part by mGlu5 receptors.
Acknowledgments
This study was funded by the NHMRC Australia, of which A.J.L. is a senior fellow. We thank Sanofi Synthelabo Recherche for the gift of SR141716A.
Glossary
Abbreviations:
- AGS3
activator of G protein signalling 3
- CS+
conditioned stimulus
- DARPP-32
dopamine and cAMP-regulated phosphoprotein of 32 kDa
- DMSO
dimethyl sulphoxide
- ERK
extracellular signal-regulated kinase
- iP
Indiana-preferring rat
- MPEP
2-methyl-6-(phenylethynyl)-pyridine
- MTEP
3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine
- NAcc
nucleus accumbens
- NMDA
N-methyl-d-aspartic acid
- PKA
protein kinase A
- PKC
protein kinase C
- S+
unconditioned stimulus
- SCH58261
5-amino-2-(2-furyl)-7-phenylethyl-pyrazolo[4,3-e]-1,2,4-triazolo[1,5c]pyrimidine
- sP
Sardinian alcohol-preferring rat
- SR141716A
N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide hydrochloride
- SR147778
5-(4-bromophenyl)-1-(2,4-dichlorophenyl)-4-ethyl-N-(1-piperidinyl)-1H-pyrazole-3-carboxamide
- THC
tetrahydrocannabinol
References
- Adams CL, Cowen MS, Short JL, Lawrence AJ. Combined antagonism of glutamate mGlu5 and adenosine A2A receptors interact to regulate alcohol-seeking in rats. Int J Neuropsychopharmacol. 2008;11:229–241. doi: 10.1017/S1461145707007845. [DOI] [PubMed] [Google Scholar]
- Agnati LF, Ferre S, Lluis C, Franco R, Fuxe K. Molecular mechanisms and therapeutical implications of intramembrane receptor/receptor interactions among heptahelical receptors with examples from the striatopallidal GABA neurons. Pharmacol Rev. 2003;55:509–550. doi: 10.1124/pr.55.3.2. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd. Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JJ, Rao SP, Rowe B, Giracello DR, Holtz G, Chapman DF, et al. [3H]Methoxymethyl-3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine binding to metabotropic glutamate receptor subtype 5 in rodent brain: in vitro and in vivo characterization. J Pharmacol Exp Ther. 2002;303:1044–1051. doi: 10.1124/jpet.102.040618. [DOI] [PubMed] [Google Scholar]
- Arolfo MP, Yao L, Gordon AS, Diamond I, Janak PH. Ethanol operant self-administration in rats is regulated by adenosine A2 receptors. Alcohol Clin Exp Res. 2004;28:1308–1316. doi: 10.1097/01.alc.0000139821.38167.20. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Bachteler D, Koch S, Hyytia P, Spanagel R. mGluR5 Antagonist MPEP reduces ethanol-seeking and relapse behavior. Neuropsychopharmacology. 2004;29:921–928. doi: 10.1038/sj.npp.1300381. [DOI] [PubMed] [Google Scholar]
- Bespalov AY, Dravolina OA, Sukhanov I, Zakharova E, Blokhina E, Zvartau E, et al. Metabotropic glutamate receptor (mGluR5) antagonist MPEP attenuated cue- and schedule-induced reinstatement of nicotine self-administration behavior in rats. Neuropharmacology. 2005;49:167–178. doi: 10.1016/j.neuropharm.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Bird MK, Kirchhoff J, Djouma E, Lawrence AJ. Metabotropic glutamate 5 receptors regulate sensitivity to ethanol in mice. Int J Neuropsychopharm. 2008;11:1–10. doi: 10.1017/S1461145708008572. [DOI] [PubMed] [Google Scholar]
- Boothby LA, Doering PL. Acamprosate for the treatment of alcohol dependence. Clin Ther. 2005;27:695–714. doi: 10.1016/j.clinthera.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Bowers MS, Hopf FW, Chou JK, Guillory AM, Chang SJ, Janak PH, et al. Nucleus accumbens AGS3 expression drives ethanol seeking through Gβγ. Proc Natl Acad Sci USA. 2008;105:12533–12538. doi: 10.1073/pnas.0706999105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RM, Short JL. Adenosine A2A receptors and their role in drug addiction. J Pharm Pharmacol. 2008;60:1409–1430. doi: 10.1211/jpp/60.11.0001. [DOI] [PubMed] [Google Scholar]
- Brown RM, Short JL, Cowen MS, Ledent C, Lawrence AJ. A differential role for the adenosine A2A receptor in opiate reinforcement vs. opiate-seeking behavior. Neuropsychopharmacology. 2009;34:844–856. doi: 10.1038/npp.2008.72. [DOI] [PubMed] [Google Scholar]
- Caille S, Alvarez-Jaimes L, Polis I, Stouffer D, Parsons L. Specific alterations of extracellular endocannabinoid levels in the nucleus accumbens by ethanol, heroin, and cocaine self-administration. J Neurosci. 2007;27:3695–3702. doi: 10.1523/JNEUROSCI.4403-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriba P, Ortiz O, Patkar K, Justinova Z, Stroik J, Themann A, et al. Striatal adenosine A2A and cannabinoid CB1 receptors form functional heteromeric complexes that mediate the motor effects of cannabinoids. Neuropsychopharmacology. 2007;32:2249–2259. doi: 10.1038/sj.npp.1301375. [DOI] [PubMed] [Google Scholar]
- Castane A, Wells L, Soria G, Hourani S, Ledent C, Kitchen I, et al. Behavioural and biochemical responses to morphine associated with its motivational properties are altered in adenosine A2A receptor knockout mice. Br J Pharmacol. 2008;155:757–766. doi: 10.1038/bjp.2008.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centonze D, Battista N, Rossi S, Mercuri NB, Finazzi-Agro A, Bernardi G, et al. A critical interaction between dopamine D2 receptors and endocannabinoids mediates the effects of cocaine on striatal GABAergic transmission. Neuropsychopharmacology. 2004;29:1488–1497. doi: 10.1038/sj.npp.1300458. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Heien ML, Phillips PE, Wightman RM. Cannabinoids enhance sub second dopamine release in the nucleus accumbens of awake rats. J Neurosci. 2004;24:4393–4400. doi: 10.1523/JNEUROSCI.0529-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Sombers LA, Heien ML, Ariansen JL, Aragona BJ, et al. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J Neurosci. 2007;27:791–795. doi: 10.1523/JNEUROSCI.4152-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Lovinger DM. Decreased probability of neurotransmitter release underlies striatal long-term depression and postnatal development of corticostriatal synapses. Proc Natl Acad Sci USA. 1997;94:2665–2670. doi: 10.1073/pnas.94.6.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A, Bilbao A, Hansson AC, del Arco I, Sommer W, Heilig M, et al. Cannabinoid CB1 receptor antagonism reduces conditioned reinstatement of ethanol-seeking behavior in rats. Eur J Neurosci. 2005;21:2243–2251. doi: 10.1111/j.1460-9568.2005.04056.x. [DOI] [PubMed] [Google Scholar]
- Coccurello R, Breysse N, Amalric M. Simultaneous blockade of adenosine A2A and metabotropic glutamate mGlu5 receptors increase their efficacy in reversing Parkinsonian deficits in rats. Neuropsychopharmacology. 2004;29:1451–1461. doi: 10.1038/sj.npp.1300444. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Cosford ND, Tehrani L, Roppe J, Schweiger E, Smith ND, Anderson J, et al. 3-[(2-Methyl-1,3-thiazol-4-yl)ethynyl]-pyridine: a potent and highly selective metabotropic glutamate subtype 5 receptor antagonist with anxiolytic activity. J Med Chem. 2003;46:204–206. doi: 10.1021/jm025570j. [DOI] [PubMed] [Google Scholar]
- Cowen MS, Adams C, Kraehenbuehl T, Vengeliene V, Lawrence AJ. The acute anti-craving effect of acamprosate in alcohol-preferring rats is associated with modulation of the mesolimbic dopamine system. Addict Biol. 2005a;10:233–242. doi: 10.1080/13556210500223132. [DOI] [PubMed] [Google Scholar]
- Cowen MS, Djouma E, Lawrence AJ. The mGlu5 antagonist MTEP reduces ethanol self-administration in multiple strains of alcohol-preferring rats and regulates olfactory glutamatergic systems. J Pharmacol Exp Ther. 2005b;310:590–600. doi: 10.1124/jpet.105.090449. [DOI] [PubMed] [Google Scholar]
- Cowen MS, Krstew E, Lawrence AJ. Assessing appetitive and consummatory phases of ethanol self-administration in C57BL/6J mice under operant conditions: regulation by mGlu5 receptor antagonism. Psychopharmacology. 2007;190:21–29. doi: 10.1007/s00213-006-0583-0. [DOI] [PubMed] [Google Scholar]
- Cunningham JA. Untreated remissions from drug use: the predominant pathway. Addict Behav. 1999;24:267–270. doi: 10.1016/s0306-4603(98)00045-8. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Grant BF. Rates and correlates of relapse among individuals in remission from DSM-IV alcohol dependence: a 3-year follow-up. Alcohol Clin Exp Res. 2007;31:2036–2045. doi: 10.1111/j.1530-0277.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Dissociable effects of antagonism of NMDA and AMPA/KA receptors in the nucleus accumbens core and shell on cocaine-seeking behavior. Neuropsychopharmacology. 2001;25:341–360. doi: 10.1016/S0893-133X(01)00235-4. [DOI] [PubMed] [Google Scholar]
- Economidou D, Mattioli L, Cifani C, Perfumi M, Massi M, Cuomo V, et al. Effect of the cannabinoid CB1 receptor antagonist SR141716A on ethanol self-administration and ethanol-seeking behaviour in rats. Psychopharmacology. 2006;183:394–403. doi: 10.1007/s00213-005-0199-9. [DOI] [PubMed] [Google Scholar]
- Economidou D, Mattioli L, Ubaldi M, Lourdusamy A, Soverchia L, Hardiman G, et al. Role of cannabinoidergic mechanisms in ethanol self-administration and ethanol seeking in rat adult offspring following perinatal exposure to Δ9-tetrahydrocannabinol. Toxicol Appl Pharmacol. 2007;223:73–85. doi: 10.1016/j.taap.2007.05.008. [DOI] [PubMed] [Google Scholar]
- El Yacoubi M, Ledent C, Parmentier M, Daoust M, Costentin J, Vaugeois J. Absence of the adenosine A2A receptor or its chronic blockade decrease ethanol withdrawal-induced seizures in mice. Neuropharmacology. 2001;40:424–432. doi: 10.1016/s0028-3908(00)00173-8. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure A, Haberland U, Conde F, El Massioui N. Lesion to the nigrostriatal dopamine system disrupts stimulus–response habit formation. J Neurosci. 2005;25:2771–2780. doi: 10.1523/JNEUROSCI.3894-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre S, Karcz-Kubicha M, Hope BT, Popoli P, Burgueno J, Gutierrez MA, et al. Synergistic interaction between adenosine A2A and glutamate mGlu5 receptors: implications for striatal neuronal function. Proc Natl Acad Sci USA. 2002;99(18):11940–11945. doi: 10.1073/pnas.172393799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre S, Goldberg SR, Lluis C, Franco R. Looking for the role of cannabinoid receptor heteromers in striatal function. Neuropharmacology. 2009;56:226–234. doi: 10.1016/j.neuropharm.2008.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco R, Canals M, Marcellino D, Ferre S, Agnati L, Mallol J, et al. Regulation of heptaspanning-membrane-receptor function by dimerization and clustering. Trends Biochem Sci. 2003;28:238–243. doi: 10.1016/S0968-0004(03)00065-3. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Arslan G, Halldner L, Kull B, Schulte G, Wasserman W. Structure and function of adenosine receptors and their genes. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:364–374. doi: 10.1007/s002100000313. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Feltenstein MW, See RE. The role of the basolateral amygdala in stimulus-reward memory and extinction memory consolidation and in subsequent conditioned cued reinstatement of cocaine seeking. Eur J Neurosci. 2006;23:2809–2813. doi: 10.1111/j.1460-9568.2006.04806.x. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Canals M, Torvinen M, Marcellino D, Terasmaa A, Genedani S, et al. Intramembrane receptor–receptor interactions: a novel principle in molecular medicine. J Neural Transm. 2007;114:49–75. doi: 10.1007/s00702-006-0589-0. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Serra S, Vacca G, Carai MA, Colombo G. Suppressing effect of the cannabinoid CB1 receptor antagonist, SR147778, on alcohol intake and motivational properties of alcohol in alcohol-preferring sP rats. Alcohol Alcohol. 2005;40:46–53. doi: 10.1093/alcalc/agh114. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Parsons LH, Kerr TM, Rodriguez de Fonseca F, Navarro M, Piomelli D. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci. 1999;2:358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- Graham R, Wodak AD, Whelan G. New pharmacotherapies for alcohol dependence. Med J Aust. 2002;177:103–107. doi: 10.5694/j.1326-5377.2002.tb04683.x. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder C. Novel pharmacotherapeutic targets for the management of drug addiction. Eur J Pharmacol. 2005;526:101–112. doi: 10.1016/j.ejphar.2005.09.038. [DOI] [PubMed] [Google Scholar]
- Hermans E, Challiss RA. Structural, signalling and regulatory properties of the group I metabotropic glutamate receptors: prototypic family C G-protein-coupled receptors. Biochem J. 2001;359:465–484. doi: 10.1042/0264-6021:3590465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettinger BD, Lee A, Linden J, Rosin DL. Ultrastructural localization of adenosine A2A receptors suggests multiple cellular sites for modulation of GABAergic neurons in rat striatum. J Comp Neurol. 2001;431:331–346. doi: 10.1002/1096-9861(20010312)431:3<331::aid-cne1074>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Miles MF, Sharko AC, Stevenson RA, Hillmann JR, Lepoutre V, et al. The mGluR5 antagonist MPEP selectively inhibits the onset and maintenance of ethanol self-administration in C57BL/6J mice. Psychopharmacology. 2006;183:429–438. doi: 10.1007/s00213-005-0217-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayoun H, Stefani MR, Adams BW, Tamagan GD, Moghaddam B. Functional interaction between NMDA and mGlu5 receptors: effects on working memory, instrumental learning, motor behaviors, and dopamine release. Neuropsychopharmacology. 2004;29:1259–1269. doi: 10.1038/sj.npp.1300417. [DOI] [PubMed] [Google Scholar]
- Kachroo A, Orlando LR, Grandy DK, Chen J-F, Young AB, Schwarzschild MA. Interactions between metabotropic glutamate 5 and adenosine A2A receptors in normal and Parkinsonian mice. J Neurosci. 2005;25:10414–10419. doi: 10.1523/JNEUROSCI.3660-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology. 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kofalvi A, Rodrigues RJ, Ledent C, Mackie K, Vizi ES, Cunha RA, et al. Involvement of cannabinoid receptors in the regulation of neurotransmitter release in the rodent striatum: a combined immunochemical and pharmacological analysis. J Neurosci. 2005;25:2874–2884. doi: 10.1523/JNEUROSCI.4232-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kull B, Ferre S, Arslan G, Svenningsson P, Fuxe K, Owman C, et al. Reciprocal interactions between adenosine A2A and dopamine D2 receptors in Chinese hamster ovary cells co-transfected with the two receptors. Biochem Pharmacol. 1999;58:1035–1045. doi: 10.1016/s0006-2952(99)00184-7. [DOI] [PubMed] [Google Scholar]
- Lallemand F, de Witte P. Ethanol induces higher BEC in CB1 cannabinoid receptor knockout mice while decreasing ethanol preference. Alcohol Alcohol. 2005;40:54–62. doi: 10.1093/alcalc/agh115. [DOI] [PubMed] [Google Scholar]
- Lallemand F, De Witte P. SR147778, a CB1 cannabinoid receptor antagonist, suppresses ethanol preference in chronically alcoholized Wistar rats. Alcohol. 2006;39:125–134. doi: 10.1016/j.alcohol.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. Br J Pharmacol. 2006;148:752–759. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang JH, Chen F, Krstew E, Cowen MS, Carroll FY, Crawford D, et al. The GABAB receptor allosteric modulator CGP7930, like baclofen, reduces operant self-administration of ethanol in alcohol-preferring rats. Neuropharmacology. 2006;50:632–639. doi: 10.1016/j.neuropharm.2005.11.011. [DOI] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen BA, Crawford MS, Kulers CM, Williams HL. Effects of a metabotropic, mGlu5, glutamate receptor antagonist on ethanol consumption by genetic drinking rats. Alcohol Alcohol. 2005;40:494–497. doi: 10.1093/alcalc/agh200. [DOI] [PubMed] [Google Scholar]
- Matyas F, Yanovsky Y, Mackie K, Kelsch W, Misgeld U, Freund TF. Subcellular localization of type 1 cannabinoid receptors in the rat basal ganglia. Neuroscience. 2006;137:337–361. doi: 10.1016/j.neuroscience.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Naassila M, Ledent C, Daoust M. Low ethanol sensitivity and increased ethanol consumption in mice lacking adenosine A2A receptors. J Neurosci. 2002;22:10487–10493. doi: 10.1523/JNEUROSCI.22-23-10487.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevo I, Hamon M. Neurotransmitter and neuromodulatory mechanisms involved in alcohol abuse and alcoholism. Neurochem Int. 1995;26:305–336. doi: 10.1016/0197-0186(94)00139-l. [DOI] [PubMed] [Google Scholar]
- Nishi A, Liu F, Matsuyama S, Hamada M, Higashi H, Nairn AC, et al. Metabotropic mGlu5 receptors regulate adenosine A2A receptor signaling. Proc Natl Acad Sci USA. 2003;100:1322–1327. doi: 10.1073/pnas.0237126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF, McGeehan AJ, Kinder JR, McMahon T, Hodge CW, Janak PH, et al. The mGluR5 antagonist 6-methyl-2-(phenylethynyl)pyridine decreases ethanol consumption via a protein kinase C ε-dependent mechanism. Mol Pharmacol. 2005;67:349–355. doi: 10.1124/mol.104.003319. [DOI] [PubMed] [Google Scholar]
- Ongini E, Fredholm BB. Pharmacology of adenosine A2A receptors. Trends Pharmacol Sci. 1996;17:364–372. [PubMed] [Google Scholar]
- Paterson NE, Markou A. The metabotropic glutamate receptor 5 antagonist MPEP decreased break points for nicotine, cocaine and food in rats. Psychopharmacology. 2005;179:255–261. doi: 10.1007/s00213-004-2070-9. [DOI] [PubMed] [Google Scholar]
- Pin JP, Galvez T, Prezeau L. Evolution, structure, and activation mechanism of family 3/C G-protein-coupled receptors. Pharmacol Ther. 2003;98:325–354. doi: 10.1016/s0163-7258(03)00038-x. [DOI] [PubMed] [Google Scholar]
- Rodrigues RJ, Alfaro TM, Rebola N, Oliveira CR, Cunha RA. Co-localization and functional interaction between adenosine A2A and metabotropic group 5 receptors in glutamatergic nerve terminals of the rat striatum. J Neurochem. 2005;92:433–441. doi: 10.1111/j.1471-4159.2004.02887.x. [DOI] [PubMed] [Google Scholar]
- Romano C, Sesma MA, McDonald CT, O'Malley K, Van den Pol AN, Olney JW. Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J Comp Neurol. 1995;355:455–469. doi: 10.1002/cne.903550310. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Hettinger BD, Lee A, Linden J. Anatomy of adenosine A2A receptors in brain: morphological substrates for integration of striatal function. Neurology. 2003;61:S12–S18. doi: 10.1212/01.wnl.0000095205.33940.99. [DOI] [PubMed] [Google Scholar]
- Sahara Y, Kubota T, Ichikawa M. Cellular localization of metabotropic glutamate receptors mGluR 1, 2/3, 5 and 7 in the main and accessory olfactory bulb of the rat. Neurosci Lett. 2001;312:59–62. doi: 10.1016/s0304-3940(01)02184-x. [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Fisone G, Moresco R, Cunha RA, Ferre S. Adenosine A2A receptors and basal ganglia physiology. Prog Neurobiol. 2007;83:277–292. doi: 10.1016/j.pneurobio.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JP, Overstreet DH, Hodge CW. The mGluR5 antagonist MPEP decreases operant ethanol self-administration during maintenance and after repeated alcohol deprivations in alcohol-preferring (P) rats. Psychopharmacology. 2005;179:262–270. doi: 10.1007/s00213-005-2175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav. 2002;71:517–529. doi: 10.1016/s0091-3057(01)00682-7. [DOI] [PubMed] [Google Scholar]
- Shindou T, Arbuthnott GW, Wickens JR. Actions of adenosine A2A receptors on synaptic connections of spiny projection neurons in the neostriatal inhibitory network. J Neurophysiol. 2008;99:1884–1889. doi: 10.1152/jn.01259.2007. [DOI] [PubMed] [Google Scholar]
- Solinas M, Goldberg SR, Piomelli D. The endocannabinoid system in brain reward processes. Br J Pharmacol. 2008;154:369–383. doi: 10.1038/bjp.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria G, Castane A, Berrendero F, Ledent C, Parmentier M, Maldonado R, et al. Adenosine A2A receptors are involved in physical dependence and place conditioning induced by THC. Eur J Neurosci. 2004;20:2203–2213. doi: 10.1111/j.1460-9568.2004.03682.x. [DOI] [PubMed] [Google Scholar]
- Soria G, Mendizabal V, Tourino C, Robledo P, Ledent C, Parmentier M, et al. Lack of CB1 cannabinoid receptor impairs cocaine self-administration. Neuropsychopharmacology. 2005;30:1670–1680. doi: 10.1038/sj.npp.1300707. [DOI] [PubMed] [Google Scholar]
- Tessari M, Pilla M, Andreoli M, Hutcheson DM, Heidbreder CA. Antagonism at metabotropic glutamate 5 receptors inhibits nicotine- and cocaine-taking behaviours and prevents nicotine-triggered relapse to nicotine-seeking. Eur J Pharmacol. 2004;499:121–133. doi: 10.1016/j.ejphar.2004.07.056. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Dimitrakakis ES, Rice O, Gifford A, Volkow ND. Ethanol self-administration and ethanol conditioned place preference are reduced in mice lacking cannabinoid CB1 receptors. Behav Brain Res. 2005;164:206–213. doi: 10.1016/j.bbr.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Johnson J, Heilig M. Effect of the adenosine A2A receptor antagonist 3,7-dimethyl-propargylxanthine on anxiety-like and depression-like behavior and alcohol consumption in Wistar Rats. Alcohol Clin Exp Res. 2007;31:1302–1307. doi: 10.1111/j.1530-0277.2007.00425.x. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Di Ciano P, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. J Neurosci. 2005;25:8665–8670. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, Molander A, Spanagel R. Neuropharmacology of alcohol addiction. Br J Pharmacol. 2008;154:299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnault V, Houchi H, Barbier E, Pierrefiche O, Vilpoux C, Ledent C, et al. The lack of CB1 receptors prevents neuroadapatations of both NMDA and GABAA receptors after chronic ethanol exposure. J Neurochem. 2007;102:741–752. doi: 10.1111/j.1471-4159.2007.04577.x. [DOI] [PubMed] [Google Scholar]
- Weitlauf C, Woodward JJ. Ethanol selectively attenuates NMDAR-mediated synaptic transmission in the prefrontal cortex. Alcohol Clin Exp Res. 2008;32:690–698. doi: 10.1111/j.1530-0277.2008.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Global Status Report on Alcohol. Geneva: World Health Organization; 2004. [Google Scholar]
- Yaar R, Jones MR, Chen JF, Ravid K. Animal models for the study of adenosine receptor function. J Cell Physiol. 2005;202:9–20. doi: 10.1002/jcp.20138. [DOI] [PubMed] [Google Scholar]
- Yao L, McFarland K, Fan P, Jiang Z, Ueda T, Diamond I. Adenosine A2A blockade prevents synergy between µ-opiate and cannabinoid CB1 receptors and eliminates heroin-seeking behavior in addicted rats. Proc Natl Acad Sci USA. 2006;103:7877–7882. doi: 10.1073/pnas.0602661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Fan P, Jiang Z, Gordon A, Mochly-Rosen D, Diamond I. Dopamine and ethanol cause translocation of εPKC associated with εRACK: cross-talk between cAMP-dependent protein kinase A and protein kinase C signaling pathways. Mol Pharmacol. 2008;73:1105–1112. doi: 10.1124/mol.107.042580. [DOI] [PMC free article] [PubMed] [Google Scholar]


