Abstract
Background and purpose:
Quercetin is a major flavonoid that contributes to the reduced risk of cardiovascular disease associated with dietary ingestion of fruits and vegetables. We have pharmacologically characterized the effect of quercetin, and its sulphate and glucuronide metabolites, on vasoconstrictor and vasodilator responses in the porcine isolated coronary artery.
Experimental approach:
Segments of the porcine coronary artery were prepared for either isometric tension recording or determination of cyclic GMP content. The effect of quercetin and metabolites on submaximal responses to U46619 was examined in the presence and absence of substance P, bradykinin, forskolin, sodium nitroprusside (SNP) and glyceryl trinitrate (GTN).
Key results:
Quercetin and quercetin 3′-sulphate inhibited endothelin and U46619-induced contractions with greater potency (three- to fivefold) against the former, while quercetin 3-glucoronide was inactive. Quercetin enhanced both the cyclic GMP content of the artery (threefold) and cyclic GMP-dependent relaxations to GTN and SNP (two to threefold), but forskolin-induced relaxations were unaffected. Although the effect of quercetin was qualitatively similar to that noted for UK-114,542, a selective inhibitor of phosphodiesterase 5, it was still evident against SNP-induced relaxations in the presence of 10 nM UK-114,542. Quercetin and quercetin 3′-sulphate significantly reduced the development of GTN-associated ‘tolerance’.
Conclusions and implications:
Quercetin and quercetin 3′-sulphate inhibited receptor-mediated contractions of the porcine isolated coronary artery by an endothelium-independent action. Quercetin selectively enhanced cyclic-GMP-dependent relaxations by a mechanism not involving phosphodiesterase 5 inhibition. In addition, quercetin and quercetin 3′-sulphate opposed GTN-induced tolerance in vitro, which may be beneficial for patients treated for angina pectoris.
Keywords: flavonoid, quercetin, cyclic GMP, porcine coronary artery, sodium nitroprusside, glyceryl trinitrate, vascular endothelium
Introduction
Numerous epidemiological studies have shown an inverse relationship between dietary flavonoid intake and the risk of cardiovascular diseases (Hertog et al., 1993; Knekt et al., 1996). Until recently, considerable attention has focused on the antioxidant properties of flavonoids, with the presumption that they scavenge free radicals and prevent deleterious changes to the vascular endothelium (Vita, 2005). However, the significance of this action has been called into question by reports that prolonged ingestion of non-flavonoid antioxidants failed to result in beneficial cardiovascular outcomes (see Brigelius-Flohe et al., 2005; Devaraj and Jialal, 2005). Thus, other potentially relevant actions of flavonoids, including anti-inflammatory, anti-thrombotic and direct vascular effects (Middleton et al., 2000), now need to be considered in greater detail.
Quercetin is the major dietary flavonol found in apples, onions, tea and red wine (Hertog et al., 1993). Although there are numerous studies demonstrating that quercetin can inhibit vasoconstrictor tone, the precise mechanism of action is unclear. In the rat isolated thoracic aorta, for example, quercetin has been reported to cause endothelium-dependent relaxation involving nitric oxide (Fitzpatrick et al., 1993; Chen and Pace-Asciak, 1996; Chan et al., 2000; Ajay et al., 2003), but in other reports, an endothelium-independent inhibitory effect has been noted (Duarte et al., 1993; Perez-Vizcaino et al., 2002). A combination of these actions has also been described; Fusi et al. (2003) reported both effects for the action of quercetin in the rat isolated aorta, while rutin (the rutoside derivative of quercetin) induced only endothelium-dependent relaxations. Although a free radical-scavenging effect of quercetin may contribute to endothelium-dependent relaxations (Lopez-Lopez et al., 2004), other mechanisms must account for the endothelium-independent inhibitory effects. Various studies have suggested this mode of action to be inhibition of either protein kinase C (Duarte et al., 1993), phosphodiesterase (Ko et al., 2004) or the influx of extracellular Ca2+ (Chan et al., 2000).
To date, the majority of these studies on quercetin have been conducted on blood vessels from the rat, which creates two major problems when assessing the significance of the observations. First, it is unclear whether the mechanisms described are applicable to other species and different vascular beds. Second, feeding studies with foods rich in quercetin have established that the flavonol undergoes extensive metabolism, with sulphate and glucuronide derivatives featuring prominently (Kroon et al., 2004; Manach et al., 2005; Wang and Morris, 2005). Both metabolites have been reported to exhibit biological effects in non-vascular preparations (Williamson et al., 2005; Loke et al., 2008), although we recently observed an anti-inflammatory activity in cultured human umbilical vein endothelial cells (Tribolo et al., 2008).
In the present study, we have undertaken a detailed pharmacological characterization of the effect of quercetin, and its principal human conjugates, quercetin 3′-sulphate and quercetin-3-glucuronide, on porcine isolated coronary arteries. The choice of this vessel was based on the similarity between human and porcine coronary arteries, as they share several characteristics in terms of the acute response of the vascular endothelium and underlying smooth muscle (Stork and Cocks, 1994; Hamilton et al., 2002) to vasoactive agents. Besides investigating the role of the endothelium in vascular responses, we have indirectly examined the contribution made by changes in cyclic nucleotides. Finally, the potential for quercetin and its conjugates to interact with a clinically used cardiovascular drug, glyceryl trinitrate, has been assessed.
Methods
Tissue preparation
Porcine hearts were obtained from a local abattoir and transported to the laboratory at 4°C in modified Krebs–Henseleit solution within 1 h. The anterior descending branch of the coronary artery was dissected from each heart and cleaned of connective tissues. The artery was then divided into 5 mm long segments and placed in 2 mL modified Krebs–Henseleit solution (composition given below) containing 2% Ficoll previously gassed with 95% O2 and 5% CO2 for 5 min, and stored at 4°C for 16–18 h. All dissection instruments were stored in 70% industrial methylated spirit.
Contractile studies
Following overnight storage, segments were taken out of the incubation solution and prepared for isometric tension recording. The segments were suspended between two stainless steel wire (0.4 mm diameter) supports and placed in a 20 mL isolated organ bath containing modified Krebs–Henseleit solution (pH 7.4) gassed with 95% O2 and 5% CO2, and maintained at 37°C. With the exception of preparations in which the endothelium was removed by gently rubbing the lumen with the edge of a fine forcep tip, care was taken to ensure that the integrity of the endothelium was maintained. The lower support was fixed to a glass holder, while the upper support was connected to a Grass FT03 isometric force transducer (Grass Technologies, Slough, UK) by cotton thread. Some laxity in the suspended segment was maintained for approximately 40 min before the application of resting tension. The force transducer was connected to a MacLab Bridge amplifier (AD Instruments Ltd, Hastings, UK) and linked via a four-channel MacLab unit to a Macintosh LC II computer running Chart 3.5.
An initial resting tension of approximately 100 mN was slowly applied to each segment at the end of the equilibration period, and the recorded tension declined to 40–60 mN over a further 40 min period. Each preparation was then exposed to 60 mM KCl for 20 min until a sustained response was obtained, followed by washout and further 20 min equilibration. Two to three further exposures to 60 mM KCl were performed until reproducible contractions were observed. The preparations were constricted with either a stable thromboxane mimetic, 9,11-dideoxy-11a, 9a-epoxymethanoprostaglandin F2α (U46619; 5–50 nM), endothelin-1 (3–10 nM) or KCl (24 mM) in order to produce a degree of tone equivalent to approximately 60% of the response to 60 mM KCl. Once a stable response was achieved, some preparations were exposed to cumulatively increasing half-log unit increments in concentrations of either quercetin or the metabolites, with a minimum of 30 min between each addition. Where necessary, the integrity of the endothelium was assessed by examining the effect of 10 nM substance P (in the presence of 3 µM indomethacin) against submaximal contractions to U46619.
The effect of cumulatively increasing concentrations of sodium nitroprusside, glyceryl trinitrate, bradykinin or forskolin was assessed against a submaximal contraction to U46619 (approximately 60% of the response to 60 mM KCl) in the presence and absence of 3–30 µM quercetin, 10 µM quercetin 3′-sulphate and 10 µM quercetin 3-glucuronide; the concentration of U46619 in the presence of quercetin was increased to obtain the appropriate degree of vasoconstrictor tone. Preparations that failed to maintain constrictor tone over 20 min were not used for further experiments (approximately 15% of preparations). The effect of a single concentration of substance P (10 nM) was also assessed in the presence and absence of 10 µM quercetin. Responses to submaximal concentrations of bradykinin and glyceryl trinitrate were not sustained, so further addition of the drugs was made following the attainment of the peak effect. For comparative purposes, the effect of sodium nitroprusside was also examined in the presence and absence of 10 nM UK-114,542, a selective inhibitor of PDE 5 (Kraus and Prast, 2002), while the effect of forskolin was examined in the presence and absence of 5 nM RP-73401, a selective inhibitor of PDE 4 (Souness et al., 1996). In a separate experiment, the vasodilator effects of UK-114,542 and quercetin against U46619-induced contractions were compared following endothelial denudation, exposure to 100 µM NG-nitro-l-arginine methyl ester (l-NAME), to inhibit nitric oxide synthase (Ignarro, 2002) and 3 µM 1H-[1,2,4]oxadiazolo[4,3-a]quinoxaline-1-one (ODQ), an inhibitor of soluble guanylyl cyclase (Garthwaite et al., 1995).
In a further set of experiments, some preparations were exposed to either 30 µM glyceryl trinitrate for 90 min, 10 µM quercetin for 120 min or a combination of 10 µM quercetin (120 min) and glyceryl trinitrate (90 min). Similar experiments were also conducted with the 3′-sulphate and 3-glucuronide metabolites of quercetin. The drugs were removed by exchanging the bathing medium on three occasions over 10 min, and submaximal contractions to U46619 were elicited; none of the conditions employed required a concentration of U46619 significantly different from that for control preparations. The effect of cumulatively increasing concentrations of glyceryl trinitrate was then examined against U46619-induced tone.
Cyclic GMP measurement
Segments of the coronary artery (approximately 30–45 mg wet weight) were cleaned of connective tissue, placed in pre-warmed, oxygenated, modified Krebs–Henseleit solution for 90 min and maintained at 37°C in a shaking water bath. The segments were then transferred to fresh tubes containing modified Krebs–Henseleit solution (final volume 1 mL) to which 10 µM quercetin or vehicle was added for 30 min. In some experiments, 10 µM sodium nitroprusside was added for a further 30 min. At the end of the incubation period, the tubes were immediately transferred to ice, and the supernatant was removed and stored at −80°C until the analysis was undertaken. The arterial segments were rapidly blotted with a paper towel using a 50 g weight for 10 s, and weighed. The cyclic GMP content was determined using an EIA kit (Caymen Chemical, Ann Arbor, MI, USA) as described by Liu et al. (2008).
Data analysis
Contractions produced by KCl and U46619 were measured in mN force. The effect of the vasodilator agents was determined as a percentage of U46619-induced tone. Where possible, the sensitivity of the preparation to the dilator agent has been determined as the negative logarithm of the concentration causing 50% of the maximum response (–log EC50) using the logistic equation (Kaleidagraph, Synergy Software, Reading, PA, USA). In the case of relaxations to substance P, the time for the peak response to decline by 25% (from the point of addition) was also estimated, and statistical comparisons were made using a Wilcoxon test for non-parametric data. Each of the observations recorded was made in tissue from different animals, and all responses have been reported as mean ± SEM. In most instances, differences between mean values were compared using a Student's paired t-test (two-tailed), and considered significant if P < 0.05.
Materials
The composition of the modified Krebs–Henseleit solution is (mM): NaCl, 118; KCl, 4.8; MgSO4·7H2O, 1.2; CaCl2·2H2O, 1.3; NaHCO3, 25.0; KH2PO4, 1.2. Chemicals in the Krebs–Henseleit solution, quercetin, bradykinin, l-NAME, sodium nitroprusside, indomethacin and Ficoll were all obtained from Sigma-Aldrich Company Ltd (Poole, Dorset, UK). Quercetin 3′-sulphate and quercetin 3′-glucuronide were synthesized as described by Needs and Kroon (2006). Substance P (Bachem UK Chemical Company, Delphe Court, Merseyside, UK), human endothelin-1 (Tocris, Bristol UK), ODQ (Tocris), U46619 (Alexis Corporation, Nottingham, UK), UK-114,542 (5-[2-ethoxy-5-(morpholinylacetyl)phenyl]-1,6-dihydro-1-methyl-3-propyl-7H-pyrazolo[4,3-d]pyrimidin-7-one methanesulphonate monohydrate; Pfizer, Sandwich, UK), RP-73401 (3-cyclopentyloxy-N-(3,5-dichloro-4-pyridl)-4-methoxybenzamide; Aventis, Dagenham Research Centre, UK) and glyceryl trinitrate (David Bull Laboratories, Warwick, UK) were also used. Quercetin was dissolved in DMSO (100 mM), while RP-73401 (1 mM), indomethacin (10 mM) and glyceryl trinitrate (10 mM) were dissolved in absolute alcohol. The volume of solvent used in each organ bath never exceeded 0.03% v/v.
Results
Flavonoid-induced vasorelaxation
Figure 1 shows that quercetin caused concentration-dependent inhibition of U46619- and endothelin-1-induced contractions that were slow in onset and required 30 min to achieve equilibrium. Quercetin was approximately fivefold more potent against endothelin-1 compared to contractions elicited by U46619. At the highest concentration employed, quercetin (30 µM) practically abolished contractions to both agonists, but significantly enhanced contractions (by 36 ± 13%, n= 8) elicited by KCl (Figure 2A). Quercetin 3′-sulphate did not affect KCl-induced contractions (Figure 2B), but at 30 µM caused a significant reduction (P < 0.05) in both U46619- (28 ± 8%, n= 8) and endothelin-1-induced (82 ± 5%, n= 6) contractions (Figures 1 and 2B). In marked contrast to quercetin and quercetin 3′-sulphate, 1–30 µM quercetin 3-glucuronide (n= 4) did not modify U46619-induced contractions.
Figure 1.
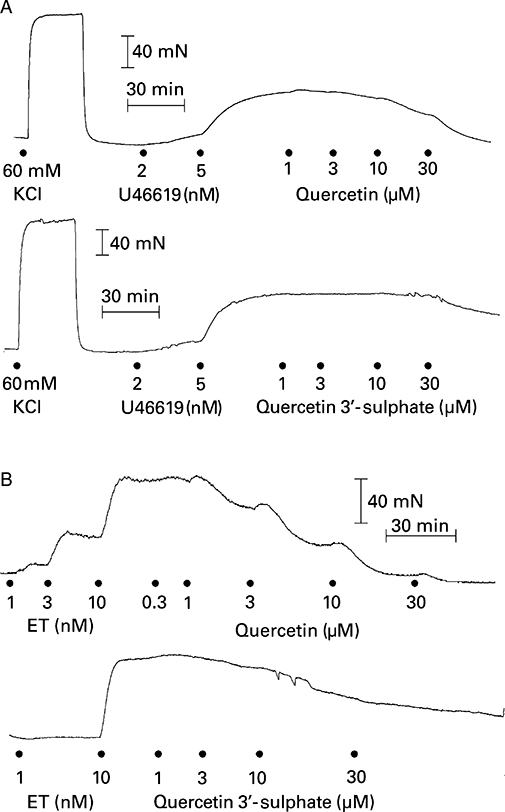
Representative digitized recording of the effect of quercetin and quercetin 3′-sulphate on U46619-induced and endothelin-1 (ET) induced contractions of the porcine isolated coronary artery.
Figure 2.
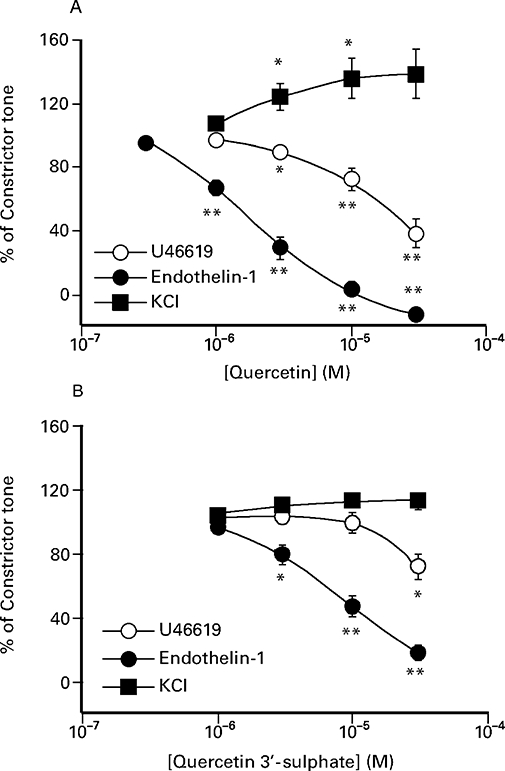
Graphical representation of the effect of (A) quercetin and (B) quercetin 3′-sulphate on U46619-, endothelin-1 and KCl-induced contractions. The values shown are the mean ± SEM of 8–13 observations for quercetin, and 4–8 observations for quercetin 3′-sulphate. *(P < 0.05) and **(P < 0.01) denote a statistically significant difference from the pre-drug control value (Student's t-test).
The effect of quercetin on endothelium-dependent relaxations
Figure 3 shows that bradykinin caused concentration-dependent relaxations of U46619-induced contractions that were unaffected by the presence of 10 µM quercetin. In the presence of 3 µM indomethacin, relaxations to 10 nM substance P were abolished by 100 µM l-NAME (n= 4). The magnitude of relaxations induced by 10 nM substance P (79 ± 7%, n= 8) was not affected by the presence of either 10 µM quercetin (80 ± 6%, n= 8), 30 µM quercetin 3′-sulphate (79 ± 5%, n= 6) or 30 µM quercetin 3-glucuronide (82 ± 4%, n= 6). However, in the case of the 10 µM quercetin, the time course of the transient relaxation (based on the time to recover 25% of the U46619-induced tone) was significantly (P < 0.05, Wilcoxon test) reduced from 8.3 ± 1.6 min (n= 8) to 5.2 ± 0.5 min (n= 8).
Figure 3.
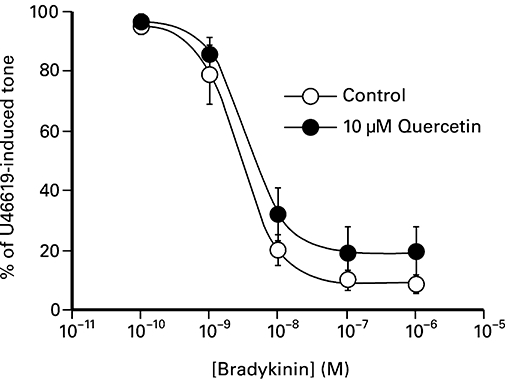
The effect of bradykinin on U46619-induced contractions of the porcine isolated coronary artery in the presence and absence of 10 µM quercetin. The values shown represent the mean ± SEM of eight observations.
Effects of quercetin on endothelium-independent relaxations
Forskolin (Figure 4A) and sodium nitroprusside (Figure 4B) caused sustained, concentration-dependent relaxation of U46619-induced contractions of the porcine isolated coronary artery. The presence of 5 nM RP-43701, a selective inhibitor of PDE4, and 10 nM UK-114,542, a selective inhibitor of PDE5, significantly enhanced the potency of forskolin and sodium nitroprusside, respectively, by 2- to 10-fold (Figure 4 and Table 1).
Figure 4.
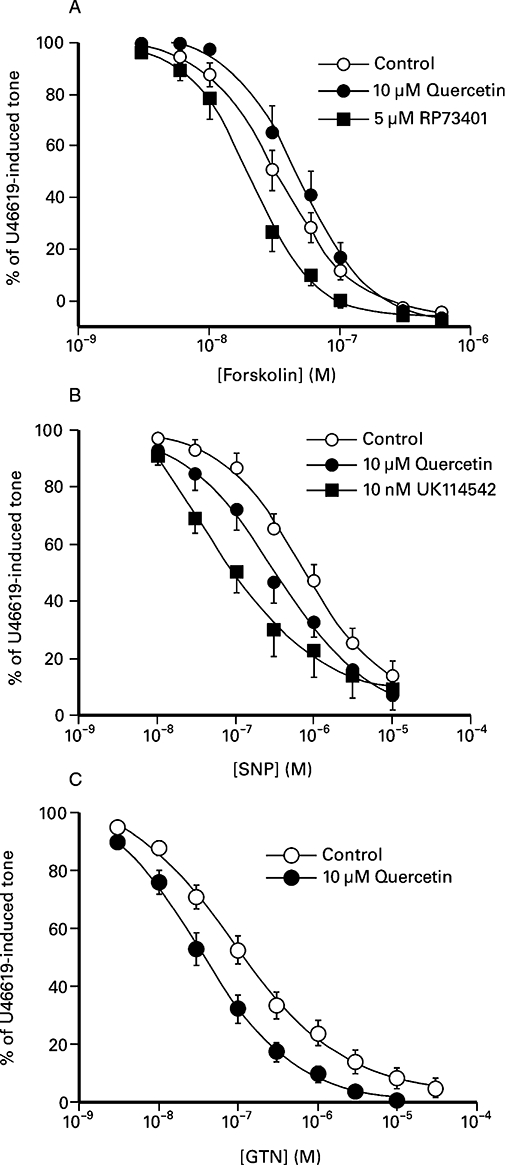
Comparison of the effect of quercetin on non-endothelium-dependent relaxants of the porcine isolated coronary artery. (A) Forskolin-induced inhibition of U46619 contractions in the presence and absence of either 10 µM quercetin and 5 nM RP-73401. The values shown represent the mean ± SEM of between four and eight observations. (B) Sodium nitroprusside (SNP)-induced inhibition of U46619-induced contractions in the presence and absence of 10 µM quercetin and 10 nM UK 114542. The values shown represent the mean ± SEM of between four and eight observations. (C) Glyceryl trinitrate (GTN)-induced inhibition of contractions to U46619 in the presence and absence of 10 µM quercetin. The values shown represent the mean ± SEM of 12 observations.
Table 1.
Comparison of the effect of PDE inhibitors and quercetin on the potency (–log EC50) of non-endothelium-dependent relaxants in the porcine isolated coronary artery
| Forskolin | Sodium nitroprusside | Glyceryl trinitrate | |
|---|---|---|---|
| Control | 7.39 ± 0.07 (n= 4) | 6.22 ± 0.13 (n= 4) | 6.47 ± 0.16 (n= 8) |
| PDE inhibitor | 7.70 ± 0.08 (n= 4)* | 7.30 ± 0.30 (n= 4)* | 7.22 ± 0.16 (n= 8)* |
| (5 nM RP-73401) | (10 nM UK114542) | (10 nM UK114542) | |
| Control | 7.50 ± 0.08 (n= 8) | 6.04 ± 0.13 (n= 8) | 7.06 ± 0.12 (n= 12) |
| 10 µM Quercetin | 7.35 ± 0.08 (n= 8) | 6.62 ± 0.21 (n= 8)* | 7.45 ± 0.11 (n= 12)* |
| Control | ND | 6.11 ± 0.12 (n= 8) | 6.87 ± 0.15 (n= 12) |
| 30 µM Quercetin | 6.37 ± 0.17 (n= 8) | 7.27 ± 0.14 (n= 12)* |
A statistically significant difference between control and treatment (P < 0.05, Student's paired t-test).
The values shown represent the mean ± SEM of 4–12 observations.
ND, not done.
The presence of quercetin (10 µM) enhanced sodium nitroprusside-induced relaxations (Figure 4B), and increased the potency threefold, but did not significantly alter forskolin-induced relaxations (Table 1). Glyceryl trinitrate also caused concentration-dependent relaxations of U46619-induced contractions (Figure 4C), and quercetin (10 µM) increased the potency of glyceryl trinitrate 2.5-fold (Figure 4C, Table 1). Similar experiments conducted in the presence of 30 µM quercetin also resulted in a 2.5-fold increase in the potency of glyceryl trinitrate (Table 1), but the effect of 3 µM quercetin (log shift of 0.30 ± 0.14, n= 12) did not reach statistical significance (paired Student's t-test, P > 0.05). In contrast to quercetin, concentration-dependent relaxations to glyceryl trinitrate (–log EC50− 7.22 ± 0.10, n= 6) were not affected by the presence of either 10 µM quercetin 3′-sulphate (–log EC50− 7.07 ± 0.09, n= 6) or 10 µM quercetin 3-glucuronide (–log EC50− 7.10 ± 0.11, n= 4).
The qualitative similarity in the effect of quercetin and the PDE 5 inhibitor, UK-114,542, on sodium nitroprusside and glyceryl trinitrate-induced relaxations prompted a more detailed comparison. UK-114,542 caused concentration-dependent relaxations of U46619-induced contractions; the maximum effect, (produced by 100 nM) reduced responses by approximately 60%. As shown in Table 2, UK-114,542-induced relaxations of U46619-induced tone were significantly reduced by the removal of the endothelium and exposure to 3 µM ODQ, a selective inhibitor of soluble guanylyl cyclase. Furthermore, the relaxations were abolished by exposure to 100 µM l-NAME. In contrast, quercetin-induced relaxations were not affected by either endothelial denudation or exposure to 3 µM ODQ (Table 2). As shown in Figure 5, the potency of sodium nitroprusside in the presence of a combination of 10 µM quercetin and 10 nM UK-114/542 (–log EC50, 7.19 ± 0.10, n= 8) was significantly greater (P < 0.05, paired Student's t-test) than that seen in the presence of 10 nM UK114542 alone (–log EC50, 6.91 ± 0.03, n= 8).
Table 2.
Comparison of the vasodilator effect of UK114,542 and quercetin against U46619-induced contractions of the porcine isolated coronary artery under various conditions
| 100 nM UK114,542 | 30 µM Quercetin | |
|---|---|---|
| Control (E+) | 50.3 ± 4.3 (n= 7) | 59.5 ± 11.1 (n= 6) |
| Denuded (E–) | 29.2 ± 6.2 (n= 7)* | 69.5 ± 13.0 (n= 6) |
| Control (E+) | 65.6 ± 5.5 (n= 7) | 61.6 ± 8.8 (n= 7) |
| 3 µM ODQ (E+) | 28.3 ± 9.0 (n= 7)* | 56.2 ± 10.2 (n= 7) |
| Control (E+) | 58.2 ± 12.0 (n= 8) | 80.3 ± 6.3 (n= 15) |
| 100 µM l-NAME (E+) | 4.0 ± 2.0 (n= 8)* | 52.3 ± 8.7 (n= 15)* |
A statistically significant difference between control and treatment conditions (P < 0.05, Student's paired t-test).
The responses shown are the percentage relaxations ± SEM (n= 6–15).
Figure 5.
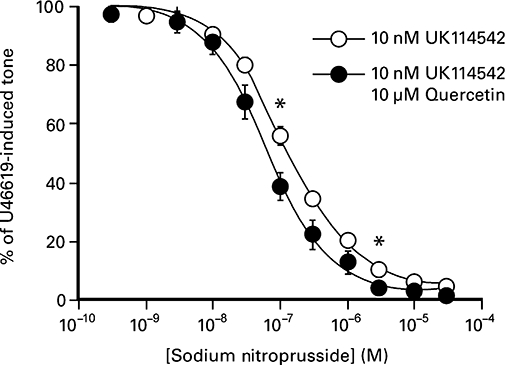
The effect of sodium nitroprusside on U46619-induced contractions of the porcine isolated coronary artery in the presence of either 10 nM UK114542 or a combination of 10 nM UK114542 and 10 µM quercetin. The values shown represent the mean ± SEM of eight observations, and significant difference between responses is denoted by *(P < 0.05) using a paired Student's t-test.
Quercetin (10 µM) caused a significant twofold increase in cyclic GMP production in coronary artery segments (control 6.32 ± 1.0 fmol·mg−1 wet weight, n= 5; quercetin 14.8 ± 3.9, fmol·mg−1 wet weight, n= 5). Co-incubation of arterial segments with 10 µM quercetin and 10 µM sodium nitroprusside caused a further increase in cyclic GMP content (32.4 ± 13.3 fmol·mg−1 wet weight, n= 5), but this did not reach statistical significance (compared to quercetin alone).
Effect of quercetin on GTN-induced tolerance in the coronary artery
Exposure of the coronary artery to 30 µM glyceryl trinitrate for 90 min, followed by washout, did not alter the concentration of U46619 required to produce submaximal vasoconstrictor tone (approximately 60% of maximum to KCl). However, this treatment was associated with a significant reduction in the response to both submaximal and maximally effective concentrations of glyceryl trinitrate. Based on the −log EC50 values, the potency of glyceryl trinitrate was significantly reduced 10-fold from 6.95 ± 0.08 (control, n= 24) to 5.95 ± 0.09 (glyceryl trinitrate treatment, n= 24). This phenomenon was taken as equivalent to the development of ‘tolerance’ to the glyceryl trinitrate.
Figure 6 shows that the presence of either 10 µM quercetin or 10 µM quercetin 3′-sulphate, during the incubation period (and subsequently washed out), significantly reduced the ability of 30 µM glyceryl trinitrate to induce ‘tolerance’. Thus, there was a 10-fold (log shift 1.00 ± 0.19, n= 8) difference in the potency of glyceryl trinitrate in segments pretreated with 30 µM glyceryl trinitrate and those pretreated with a combination of 30 µM glyceryl trinitrate and 10 µM quercetin (Figure 6A; Table 3). There was also a fourfold difference (log shift 0.64 ± 0.21, n= 8) in the potency of glyceryl trinitrate following pretreatment with either 30 µM glyceryl trinitrate or 30 µM glyceryl trinitrate with 10 µM quercetin 3′-sulphate (Figure 6C). However, the effect of prolonged exposure to 30 µM glyceryl trinitrate on the subsequent responses to glyceryl trinitrate was not affected by the presence of 10 µM quercetin 3-glucuronide (Figure 6B; Table 3). In a separate experiment, exposure to 10 µM quercetin alone for 90 min, followed by washout and establishment of a submaximal response to U46619, was not associated with a significant alteration the potency (leftward log shift 0.19 ± 0.11, n= 7) of glyceryl trinitrate.
Figure 6.
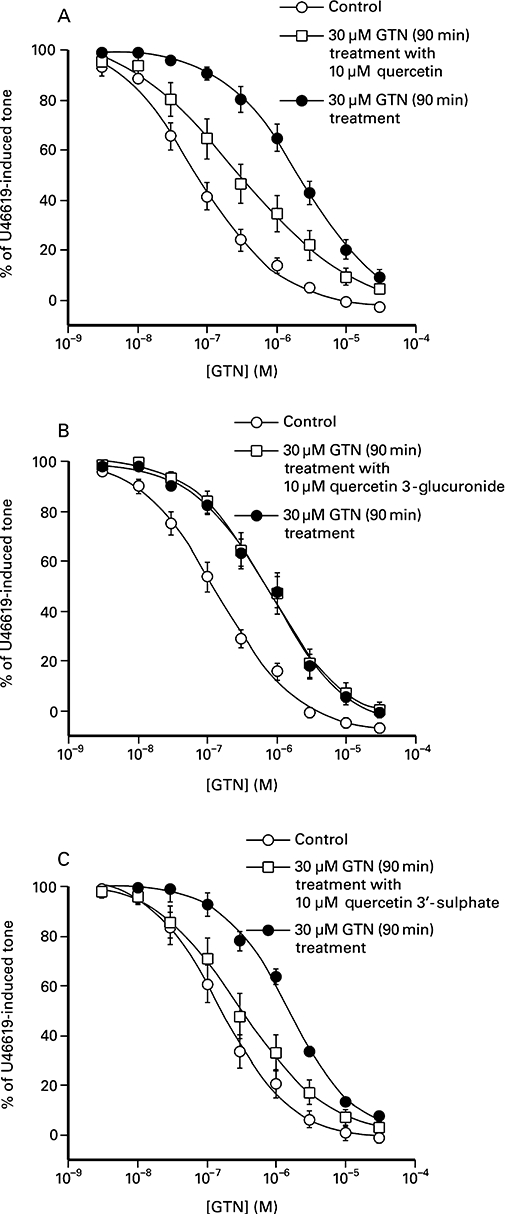
Comparison of the effect of quercetin and quercetin metabolites on glyceryl trinitrate (GTN)-induced tolerance in the porcine isolated coronary artery. Segments were exposed for 90 min to either vehicle (control), 30 µM glyceryl trinitrate or a combination of 30 µM glyceryl trinitrate and (A) 10 µM quercetin, (B) quercetin 3-glucuronide and (C) quercetin 3′-sulphate. The glyceryl trinitrate was then removed by washing before the induction of vasoconstrictor tone with U46619 prior to assessment of the effect of glyceryl trinitrate. The values shown are the mean ± SEM of eight observations.
Table 3.
Comparison of the potency of glyceryl trinitrate (–log EC50) against U46619-induced contractions following exposure to 30 µM glyceryl trinitrate (GTN) with or without quercetin or quercetin metabolites (n= 8)
| Control | 30 µM GTN 90 min | 30 µM GTN 90 min 10 µM Quercetin |
| 7.13 ± 0.09 | 5.58 ± 0.07 | 6.59 ± 0.22* |
| Control | 30 µM GTN 90 min | 30 µM GTN 90 min 10 µM Quercetin glucuronide |
| 6.81 ± 0.13 | 6.09 ± 0.09 | 6.13 ± 0.12 |
| Control | 30 µM GTN 90 min | 30 µM GTN 90 min 10 µM Quercetin sulphate |
| 6.83 ± 11 | 5.92 ± 0.15 | 6.57 ± 0.15* |
Significant difference (P < 0.05, paired Student's t-test) in potency compared to tissue exposed to 30 µM glyceryl trinitrate alone.
Discussion
The principal observation of this study is that quercetin causes endothelium-independent relaxations of the porcine coronary artery that are associated with a selective enhancement of responses involving elevation of cyclic GMP. These actions of quercetin were not shared by one of its principal metabolites, quercetin 3-glucuronide, but quercetin 3′-sulphate inhibited vasoconstrictor tone at high concentrations and, like quercetin, reduced the propensity of glyceryl trinitrate to induce tolerance in the coronary artery.
Evidence for endothelium-independent inhibition of constrictor tone
Quercetin and quercetin 3′-sulphate inhibited vasoconstrictor tone produced by the thromboxane-mimetic U46619 and endothelin, with the latter being more sensitive to the flavonoids. The finding that quercetin 3′-sulphate exerted a vasorelaxant effect in the porcine coronary artery contrasts with recent observations on the rat isolated aorta (Lodi et al., 2009). In the case of quercetin, similar-sized contractions elicited by KCl were significantly enhanced rather than reduced. As KCl contractions involve the opening of voltage-sensitive calcium channels (Yanagisawa and Okada, 1994), the latter observation lends support to the suggestions that quercetin can increase the opening of voltage-sensitive calcium channels in pituitary tumour (GH3) cells (Wu et al., 2003), and that other flavonoids exert similar effects on vascular smooth muscle (Saponara et al., 2002; Fusi et al., 2003). As this pro-constrictor effect opposes any inhibitory action of flavonoids on vascular smooth muscle, the greater sensitivity of U46619- and endothelin-induced contractions to quercetin and quercetin 3′-sulphate may be related to a less significant role for voltage-sensitive calcium channels in these responses (Yasutsune et al., 1999). It is noteworthy that a preferential inhibitory effect on endothelin-1-induced responses, by virtue of inhibiting endogenous synthesis, has been reported as conferring health benefits for polyphenols found in red wine (Corder et al., 2001).
Despite the numerous reports attesting to a role for the vascular endothelium in the inhibitory effect of quercetin in the rat aorta (Fitzpatrick et al., 1993; Chen and Pace-Asciak, 1996; Chan et al., 2000; Ajay et al., 2003; Fusi et al., 2003), neither endothelium denudation nor inhibition of nitric oxide synthase with l-NAME was associated with a pronounced alteration of the vasodilator action in the porcine coronary artery. The possibility of indirect involvement of the vascular endothelium, arising from the free radical-scavenging action of quercetin (Lopez-Lopez et al., 2004), was also excluded. Neither the time course of endothelium-dependent relaxations to substance P (entirely mediated by nitric oxide) nor the potency of bradykinin (a mixture of nitric oxide and EDHF; Graier et al. 1996) was enhanced by the presence of quercetin. Taken together, these observations indicate that the vasodilator action of quercetin in the porcine coronary artery is mediated by a direct effect on the smooth muscle, as has been suggested for mesenteric resistance vessels in the rat (Perez-Vizcaino et al., 2002).
Selective enhancement of cyclic GMP-dependent relaxations
The level of cyclic AMP and cyclic GMP in vascular smooth muscle is tightly regulated by the activity of biosynthetic and metabolizing enzymes (Polson and Strada, 1996), as both cyclic nucleotides are potent modulators of vascular tone. Interestingly, Flesch et al. (1998) noted that, despite the endothelium-independent nature of relaxations to quercetin in the rat isolated thoracic aorta, the response was associated with an elevation of cellular cyclic GMP. Thus, we examined the effect of quercetin on relaxations mediated by elevation of cyclic AMP and cyclic GMP in the porcine coronary artery. Forskolin and sodium nitroprusside inhibit vascular smooth muscle tone by elevating cyclic AMP (Shafiq et al., 1992) and cyclic GMP (Ignarro, 2002) respectively. Forskolin-induced relaxations were enhanced by the selective PDE 4 inhibitor RP 73401 (Souness et al., 1996), but not by quercetin. Thus, in contrast to the action of genistein (Lee et al., 2004) and kaempferol (Xu et al., 2006), there is no evidence to implicate cyclic AMP in the vasodilator action of quercetin in the porcine isolated coronary artery.
Both UK114,542, a selective inhibitor of PDE 5 (Kraus and Prast, 2002), and quercetin enhanced sodium nitroprusside-induced and glyceryl trinitrate-induced relaxations. The finding that quercetin also elevated coronary artery cyclic GMP content threefold, as has been reported for PDE 5 inhibitors in this vessel (Sakuma et al., 2002), raises the possibility that both compounds act by a similar mechanism. However, two observations argue against inhibition of PDE 5 as a target for quercetin. First, endothelial denudation and inhibition of soluble guanylyl cyclase by ODQ (Garthwaite et al., 1995) revealed major differences between the vasorelaxant effect of UK114542 and quercetin (see Table 2). Second, the combination of UK-114,542 and quercetin produced a greater enhancement of sodium nitroprusside-induced relaxations than UK-114,542 alone; the concentration of UK-114,542 (10 nM) used in this experiment was fivefold greater than the reported Ki value for PDE 5 (Kraus and Prast, 2002). Thus, while the precise mechanism underlying the action of quercetin on cyclic GMP-dependent relaxations has not been revealed by these studies, it does not appear to involve inhibition of PDE 5.
Modulation of glyceryl trinitrate-associated tolerance
Glyceryl trinitrate is widely used as an effective treatment for angina, but its effectiveness is limited by the propensity to develop tolerance with prolonged use (Ahlner et al., 1991; Gori and Parker, 2002). At a cellular level, this effect in vascular smooth muscle is associated with a reduction in both the elevation of cyclic GMP and activation cyclic GMP-dependent kinase to nitrovasodilators (Zhang et al., 1993; Dou et al., 2008). On the basis of the above observations, we examined whether quercetin could reduce the ability of glyceryl trinitrate to induce tolerance in vitro. While prolonged exposure to glyceryl trinitrate caused a 10- to 20-fold reduction in potency to the nitrovasodilator, similar to that reported by Dou et al. (2008), prior incubation with 10 µM quercetin significantly reduced the development of tolerance to glyceryl trinitrate. Crucially, this effect of quercetin was shared by its major metabolite quercetin 3′-sulphate, but not by quercetin 3-glucuronide. As these changes were evident after removal of quercetin from the bathing medium, the direct vasodilator action of quercetin is not the primary mechanism underlying the prevention of glyceryl trinitrate-induced tolerance. In this respect, the action of quercetin is qualitatively similar to that of another polyphenol, resveratrol, which has been reported to reduce glyceryl trinitrate-induced tolerance in human internal mammary arteries without exerting a direct dilator effect (Coskun et al., 2006). It remains to be determined whether exposure to quercetin prevents the development of glyceryl trinitrate-induced tolerance by altering the associated changes in cyclic GMP-dependent protein kinase (Dou et al., 2008).
Feeding studies have revealed that the consumption of onions and apples, for example, achieves peak plasma concentrations of ‘total quercetin’ equivalent to 3–10 µM (Manach et al., 2005). However, much of this is due to the presence of the principal metabolites rather than ‘free’ quercetin, which is normally present in nanomolar concentrations (Kroon et al., 2004; Wang and Morris, 2005). While the vasodilator activity of quercetin 3′-sulphate is clearly less pronounced than for quercetin, it may still be possible for metabolites to modify vascular responses in vivo. For example, Kawai et al. (2008) demonstrated that quercetin 3-glucuronide is accumulated in cells and that quercetin can then be generated by cells possessing the appropriate metabolizing enzyme. Thus, dietary consumption of quercetin-rich foods could yield general cardiovascular benefits in man.
Research into the cardiovascular effects of dietary flavonoids has generally focused on their presumed ability to modify disease processes (Vita, 2005). However, the finding that quercetin selectively enhances cyclic GMP-dependent relaxations to exogenous nitrovasodilators, and prevents the development of tolerance to glyceryl trinitrate, may be of importance. Glyceryl trinitrate is widely used for treating angina associated with ischaemic heart disease, so a quercetin-rich diet could benefit patients by reducing the dose required for pain relief and/or the frequency of administration. Clearly, it would be useful to establish whether similar effects are observed in either human resistance arteries or veins. The latter vessels are considered central to the development of tolerance to glyceryl trinitrate (Ahlner et al., 1991; MacPherson et al., 2006).
In summary, the major dietary flavonoid quercetin inhibited receptor-mediated contractions of the porcine isolated coronary arteries by an action independent of the endothelium and its free radical-scavenging activity. As quercetin also selectively enhanced the cyclic-GMP-dependent vasodilator glyceryl trinitrate by two different mechanisms, one of which is mimicked by quercetin 3′-sulphate, these effects may be of significance to patients with angina pectoris.
Acknowledgments
This work was supported by a grant (BB/C508418/1) from the Biotechnology and Biological Sciences Research Council, UK. We are grateful to G. Woods and Sons (Clipstone, Nottinghamshire) for the supply of pig hearts, and to Lyndon Cochrane for assistance with the preparation of the figures.
Glossary
Abbreviations:
- ET
endothelin-1
- GTN
glyceryl trinitrate
- l-NAME
NG-nitro-l-arginine methyl ester
- PDE
phosphodiesterase
References
- Ahlner J, Andersson RG, Torfgard K, Axelsson KL. Organic nitrate esters: clinical use and mechanisms of actions. Pharmacol Rev. 1991;43:351–423. [PubMed] [Google Scholar]
- Ajay M, Gilani AU, Mustafa MR. Effects of flavonoids on vascular smooth muscle of the isolated rat thoracic aorta. Life Sci. 2003;74:603–612. doi: 10.1016/j.lfs.2003.06.039. [DOI] [PubMed] [Google Scholar]
- Brigelius-Flohe R, Kluth D, Banning D. Is there a future for antioxidants in atherogenesis? Mol Nutr Food Res. 2005;49:1083–1089. doi: 10.1002/mnfr.200500094. [DOI] [PubMed] [Google Scholar]
- Chan EC, Pannangpetch P, Woodman OL. Relaxation to flavones and flavonols in rat isolated thoracic aorta: mechanism of action and structure–activity relationships. J Cardiovasc Pharmacol. 2000;35:326–333. doi: 10.1097/00005344-200002000-00023. [DOI] [PubMed] [Google Scholar]
- Chen CK, Pace-Asciak CR. Vasorelaxing activity of resveratrol and quercetin in isolated rat aorta. Gen Pharmacol. 1996;27:363–366. doi: 10.1016/0306-3623(95)02001-2. [DOI] [PubMed] [Google Scholar]
- Corder R, Douthwaite JA, Lees DM, Khan NQ, Viseau dos Santos AC, Wood EG, et al. Endothelin-1 synthesis reduced by red wine. Nature. 2001;414:863–864. doi: 10.1038/414863a. [DOI] [PubMed] [Google Scholar]
- Coskun B, Soylemez S, Parlar AH, Ulus AT, Katircioglu SF, Akar F. Effects of resveratrol on nitrate tolerance in isolated human internal mammary artery. J Cardiovasc Pharmacol. 2006;47:437–445. doi: 10.1097/01.fjc.0000211798.91023.14. [DOI] [PubMed] [Google Scholar]
- Devaraj S, Jialal I. Failure of vitamin E in clinical trials: is alpha-tocopherol the answer. Nut Rev. 2005;63:290–293. doi: 10.1111/j.1753-4887.2005.tb00143.x. [DOI] [PubMed] [Google Scholar]
- Dou D, Zheng X, Qin X, Liu L, Raj JU, Gao Y. Role of cGMP-dependent protein kinase in development of tolerance to nitroglycerine in the porcine coronary artery. Br J Pharmacol. 2008;153:497–507. doi: 10.1038/sj.bjp.0707600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte J, Perez-Vizcaino F, Zarzuelo A, Jimenez J, Tamargo J. Vasodilator effects of quercetin in isolated rat vascular smooth muscle. Eur J Pharmacol. 1993;239:1–7. doi: 10.1016/0014-2999(93)90968-n. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick DF, Hirschfield SL, Coffey RG. Endothelium-dependent vasorelaxing activity of wine and other grape products. Am J Physiol. 1993;265:H774–H778. doi: 10.1152/ajpheart.1993.265.2.H774. [DOI] [PubMed] [Google Scholar]
- Flesch M, Schwarz A, Bohm M. Effects of red and white wine on endothelium-dependent vasorelaxation of rat aorta and human coronary arteries. Am J Physiol. 1998;275:H1183–H1190. doi: 10.1152/ajpheart.1998.275.4.H1183. [DOI] [PubMed] [Google Scholar]
- Fusi F, Saponara S, Pessina F, Gorelli B, Sgaragli G. Effects of quercetin and rutin on vascular preparations: a comparison between mechanical and electrophysiological phenomena. Eur J Nutr. 2003;42:10–17. doi: 10.1007/s00394-003-0395-5. [DOI] [PubMed] [Google Scholar]
- Garthwaite J, Southham E, Boulton CL, Nielsen EB, Schmidt K, Mayer B. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxaline-1-one. Mol Pharmacol. 1995;48:184–188. [PubMed] [Google Scholar]
- Gori T, Parker JD. Nitrate tolerance: a unifying hypothesis. Circulation. 2002;106:2510–2513. doi: 10.1161/01.cir.0000036743.07406.53. [DOI] [PubMed] [Google Scholar]
- Graier WF, Holzmann S, Hoebel BG, Kukovetz WR, Kostner GM. Mechanism of l-NG-nitroarginine/indomethacin resistant relaxations in bovine and porcine coronary arteries. Br J Pharmacol. 1996;119:1177–1186. doi: 10.1111/j.1476-5381.1996.tb16020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JR, Moffatt JD, Tatoulis J, Cocks TM. Enzymatic activation of endothelial protease-activated receptors is dependent on artery diameter in human and porcine isolated coronary arteries. Br J Pharmacol. 2002;136:492–501. doi: 10.1038/sj.bjp.0704714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen elderly study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- Ignarro LJ. Nitric oxide as a unique signaling molecule in the vascular system: a historical overview. J Physiol Pharmacol. 2002;53:503–514. [PubMed] [Google Scholar]
- Kawai Y, Nishikawa T, Shiba Y, Saito S, Murota K, Shibata N, et al. Macrophage as a target of quercetin glucuronides in human atherosclerotic arteries: implications in the anti-atherosclerosis mechanism of dietary flavonoids. J Biol Chem. 2008;283:9424–9234. doi: 10.1074/jbc.M706571200. [DOI] [PubMed] [Google Scholar]
- Knekt P, Jarvinen R, Reunanen A, Maatela J. Flavonoid intake and coronary mortality in Finland: a cohort study. BMJ. 1996;312:478–481. doi: 10.1136/bmj.312.7029.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko W-C, Shih C-M, Lai Y-H, Chen J-H, Huang H-L. Inhibitory effect of flavonoids on phosphodiesterase isozymes from guinea-pig and their structure–activity relationship. Biochem Pharmacol. 2004;68:2087–2094. doi: 10.1016/j.bcp.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Kraus MM, Prast H. Involvement of nitric oxide, cyclic GMP and phosphodiesterase 5 in excitatory amino acid and GABA release in the nucleus accumbens evoked by activation of the hippocampal fimbra. Neuroscience. 2002;112:331–343. doi: 10.1016/s0306-4522(02)00079-9. [DOI] [PubMed] [Google Scholar]
- Kroon PA, Clifford MN, Crozier A, Day AJ, Donovan JL, Manch C, et al. How should we assess the effects of exposure to dietary polyphenols in vitro? Am J Clin Nutr. 2004;80:15–21. doi: 10.1093/ajcn/80.1.15. [DOI] [PubMed] [Google Scholar]
- Lee MYK, Leung SWS, Vanhoutte PM, Man RYK. Genistein reduces agonist-induced contractions of porcine coronary arterial smooth muscle in a cyclic AMP-dependent manner. Eur J Pharmacol. 2004;503:165–172. doi: 10.1016/j.ejphar.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Liu CQ, Leung FP, Lee VW, Lau CW, Yao X, Lu L, et al. Prevention of nitroglycerin tolerance in vitro by T0156, a selective phosphodiesterase type 5 inhibitor. Eur J Pharmacol. 2008;590:250–254. doi: 10.1016/j.ejphar.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Lodi F, Jimenez R, Moreno L, Kroon PA, Needs PW, Hughes DA, et al. Glucuronidated and sulfated metabolites prevent endothelial dysfuntion but lack direct vasorelaxant effect in the rat aorta. Atherosclerosis. 2009;204:34–39. doi: 10.1016/j.atherosclerosis.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Loke WM, Proudfoot JM, Stewart S, McKinley AJ, Needs PW, Kroon P, et al. Metabolic transformation has a profound effect on anti-inflammatory activity of flavonoids such as quercetin: lack of association between antioxidant and lipoxygenase inhibitory activity. Biochem Pharmacol. 2008;75:1045–1053. doi: 10.1016/j.bcp.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Lopez-Lopez G, Moreno L, Cogolludo A, Galisteo M, Ibarra M, Duarte J, et al. Nitric oxide (NO) scavenging and NO protecting effects of quercetin and their biological significance in vascular smooth muscle. Mol Pharmacol. 2004;65:851–859. doi: 10.1124/mol.65.4.851. [DOI] [PubMed] [Google Scholar]
- MacPherson JD, Gillespie TD, Dunkerley HA, Maurice DH, Bennett BM. Inhibition of phosphodiesterase selectively reverses nitrate tolerance in the venous circulation. J Pharmacol Exp Ther. 2006;317:188–195. doi: 10.1124/jpet.105.094763. [DOI] [PubMed] [Google Scholar]
- Manach C, Williams G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in human. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:S230S–S242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- Middleton E, Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- Needs PW, Kroon PA. Convenient syntheses of metabolically important glucuronides and sulfates. Tetrahedron. 2006;62:6862–6868. [Google Scholar]
- Polson JB, Strada SJ. Cyclic nucleotide phosphodiesterase and vascular smooth muscle. Ann Rev Pharmacol Toxicol. 1996;36:403–427. doi: 10.1146/annurev.pa.36.040196.002155. [DOI] [PubMed] [Google Scholar]
- Perez-Vizcaino F, Ibarra M, Cogolludo AL, Duarte J, Zaragoza-Arnaez F, Moreno L, et al. Endothelium-independent vasodilator effects of the flavonoid quercetin and its methylated metabolites in rat conductance and resistance arteries. J Pharmacol Exp Ther. 2002;302:66–72. doi: 10.1124/jpet.302.1.66. [DOI] [PubMed] [Google Scholar]
- Sakuma I, Akaishi Y, Tomioka H, Sato A, Kitabatake A, Hattori Y. Interaction of sildenafil with various coronary vasodilators in isolated porcine coronary artery. Eur J Pharmacol. 2002;437:155–163. doi: 10.1016/s0014-2999(01)01622-3. [DOI] [PubMed] [Google Scholar]
- Saponara S, Sgaragli G, Fusi F. Quercetin as a novel activator of l-type Ca2+ channels in rat tail artery smooth muscle cells. Br J Pharmacol. 2002;135:1819–1827. doi: 10.1038/sj.bjp.0704631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafiq J, Suzuki S, Itoh T, Kuriyama H. Mechanism of vasodilatation induced by NKH744, a water soluble derivative, in smooth muscle of the porcine coronary artery. Circ Res. 1992;71:70–81. doi: 10.1161/01.res.71.1.70. [DOI] [PubMed] [Google Scholar]
- Souness JE, Griffiths M, Maslen C, Ebsworth K, Scott LC, Pollock K, et al. Evidence that cyclic AMP phosphodiesterase inhibitors suppress TNFα generation from human monocytes by interacting with a ‘low affinity’ phosphodiesterase 4 conformer. Br J Pharmacol. 1996;118:649–658. doi: 10.1111/j.1476-5381.1996.tb15450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork AP, Cocks TM. Pharmacological reactivity of human epicardial coronary arteries: characterization of relaxation responses to endothelium-derived relaxing factor. Br J Pharmacol. 1994;113:1099–1104. doi: 10.1111/j.1476-5381.1994.tb17109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribolo S, Lodi F, Connor C, Suri S, Wilson VG, Taylor MA, et al. Human metabolites of quercetin are less effective than quercetin inhibiting molecules expression on activated vascular endothelial cells. Atherosclerosis. 2008;197:50–56. doi: 10.1016/j.atherosclerosis.2007.07.040. [DOI] [PubMed] [Google Scholar]
- Vita JA. Polyphenols and cardiovascular disease: effects on endothelial and platelet function. Am J Clin Nutr. 2005;81:292S–297. doi: 10.1093/ajcn/81.1.292S. [DOI] [PubMed] [Google Scholar]
- Wang L, Morris ME. Liquid chromatography–tandem mass spectroscopy assay for quercetin and conjugated quercetin metabolites in human plasma and urine. J Chromatogr B. 2005;821:194–201. doi: 10.1016/j.jchromb.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Williamson G, Barron D, Shimoi K, Terao J. In vitro biological properties of flavonoid conjugates found in vivo. Free Radic Res. 2005;39:457–469. doi: 10.1080/10715760500053610. [DOI] [PubMed] [Google Scholar]
- Wu S-N, Chiang H-T, Shen A-Y, Lo Y-K. Differential effect of quercetin, a natural polyphenolic flavonoid, on L-type calcium current in pituitary tumour (GH3) cells and neuronal NG108-15 cells. J Cell Physiol. 2003;195:298–308. doi: 10.1002/jcp.10244. [DOI] [PubMed] [Google Scholar]
- Xu YC, Yeung DK, Man RY, Leung SW. Kaempferol enhances endothelium-independent and dependent relaxation in the porcine coronary artery. Mol Cell Biochem. 2006;287:61–67. doi: 10.1007/s11010-005-9061-y. [DOI] [PubMed] [Google Scholar]
- Yanagisawa T, Okada Y. KCl depolarization increases Ca2+ sensitivity of contractile elements in coronary arterial smooth muscle. Am J Physiol. 1994;267:H614–H621. doi: 10.1152/ajpheart.1994.267.2.H614. [DOI] [PubMed] [Google Scholar]
- Yasutsune T, Kawakami N, Hirano K, Nishimura J, Yasui H, Kitamura K, et al. Vasorelaxation and inhibition of the voltage-operated Ca2+ channels by FK506 in the porcine coronary artery. Br J Pharmacol. 1999;126:717–729. doi: 10.1038/sj.bjp.0702339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LM, Castresana MR, Stefansson S, Newman WH. Tolerance to sodium nitroprusside: studies in cultured porcine vascular smooth muscle cells. Anaesthesiology. 1993;79:1094–1103. [PubMed] [Google Scholar]


