Abstract
Background and purpose:
Extracellular nucleotides produce vasodilatation through endothelial P2 receptor activation. As these autacoids are actively metabolized by the ecto-nucleotidase nucleoside triphosphate diphosphohydrolase-1 (NTPDase1), we studied the effects of this cell surface enzyme on nucleotide-dependent vasodilatation.
Experimental approach:
Vascular NTPDase expression and activity were evaluated by immunohistochemistry and histochemistry. The vascular effects of nucleotides were tested in vivo by monitoring mean arterial pressure, and in vitro comparing reactivity of aortic rings using wild-type and Entpd1−/− (lacking NTPDase1) mice.
Key results:
The absence of NTPDase1 in Entpd1−/− mice led to a dramatic drop in endothelial nucleotidase activity. This deficit was associated with an exacerbated decrease in blood pressure after nucleotide injection. Following ATP injection, mean arterial pressure was decreased in Entpd1+/+ and Entpd1−/− mice by 5.0 and 17%, respectively, and by 0.1 and 19% after UTP injection (10 nmole·kg−1 both). In vitro, the concentration-response curves of relaxation to ADP and ATP were shifted to the left, revealing a facilitation of endothelial P2Y1 and P2Y2 receptor activation in Entpd1−/− mice. EC50 values in Entpd1+/+ versus Entpd1−/− aortic rings were 14 µM versus 0.35 µM for ADP, and 29 µM versus 1 µM for ATP. In Entpd1−/− aortas, P2Y1 receptors were more extensively desensitized than P2Y2 receptors. Relaxations to the non-hydrolysable analogues ADPβS (P2Y1) and ATPγS (P2Y2) were equivalent in both genotypes confirming the normal functionality of these P2Y receptors in mutant mice.
Conclusions and implications:
NTPDase1 controls endothelial P2Y receptor-dependent relaxation, regulating both agonist level and P2 receptor reactivity.
Keywords: nucleotides, vasodilatation, P2 receptors, P2Y1, P2Y2, NTPDase1, desensitization, mouse
Introduction
Extracellular nucleotides take part to a wide range of physiological and pathological processes in virtually every tissue (Burnstock, 2006). In the circulation, red blood cells, endothelial cells, activated platelets and neutrophils release ATP and ADP in the lumen of the vessel in response to shear stress (Bodin et al., 1991), hyperoxia (Ahmad et al., 2004), hypoxia (Gordon, 1986) or agonist stimulation (Yang et al., 1994; Ostrom et al., 2000; Joseph et al., 2003). Nucleotides and nucleosides (adenosine) regulate cell function following autocrine and paracrine activation of purinergic (P2) receptors (nomenclature follows Alexander et al., 2008). To date, seven ligand-gated P2X (P2X1–7) (North, 2002) and eight G protein-coupled P2Y receptors (P2Y1, 2, 4, 6, 11–14) (Abbracchio et al., 2006) have been cloned in human. P2X receptors are activated by ATP, while P2Y receptors differ from each other according to the nucleotide(s) they respond to (ATP, ADP, UTP, UDP or UDP-glucose).
Nucleotides participate in local and systemic control of blood flow (Burnstock, 2002). The activation of P2 receptors on vascular smooth muscle cells (VSMCs) promotes vasoconstriction via either P2X (Gitterman and Evans, 2001) or pyrimidine-sensitive P2Y receptors. These processes take part in the neurogenic response of resistance arteries (von Kugelgen et al., 1987). In contrast, the activation of endothelial P2 receptors induces a local vasodilatation. This effect involves three major vasodilator factors depending on the species and the vascular territories considered: nitric oxide (NO), prostacyclin and endothelium-derived hyperpolarizing factor (EDHF) (Erlinge and Burnstock, 2008). P2Y1 and P2Y2 receptors are thought to be responsible for ADP and UTP/ATP-induced vasodilator responses in most tissues (Boarder and Hourani, 1998). More recently, P2X1 and P2X4 receptors were proposed to contribute to ATP-induced vasodilatation in mouse resistance arteries (Yamamoto et al., 2006; Harrington et al., 2007).
Once released, the biological effect of extracellular nucleotides is tightly regulated by ecto-nucleotidases (Zimmermann, 2000). Among these enzymes, members of the ecto-nucleoside triphosphate diphosphohydrolase (E-NTPDase) family are dominant (Robson et al., 2006). Two NTPDases are expressed in the vasculature: NTPDase1 is expressed on endothelial cells and VSMCs, while NTPDase2 is expressed in the adventitia of blood vessels, probably at the surface of fibroblasts (Sévigny et al., 2002). NTPDase1 regulates local concentration of nucleotides in the blood stream, and, as a consequence, affects the activation of P2 receptors expressed in blood cells and endothelial cells (Enjyoji et al., 1999). NTPDase1 contributes to the anti-thrombotic property of endothelial cells by actively hydrolysing ADP, which promotes platelet aggregation (Marcus et al., 1991; Cote et al., 1992). The key role of this enzyme in the prevention of thrombosis was confirmed in knockout mice (Entpd1−/−) (Enjyoji et al., 1999; Pinsky et al., 2002). Further work with Entpd1−/− mice highlighted a role of NTPDase1 in regulating other endothelium-related functions, such as angiogenesis (Goepfert et al., 2001) and vascular permeability (Guckelberger et al., 2004).
We recently observed that the absence of NTPDase1 was associated with an enhanced vasoconstrictor effect of nucleotides through the activation of P2 receptors on VSMCs (Kauffenstein et al., 2009). Considering the importance of NTPDase1 as the major ecto-nucleotidase in the vasculature, as well as the central role of nucleotides in vasomotor responses, in the present study, we explored the role of NTPDase1 on endothelial P2 receptor-dependent relaxation. Our results show that NTPDase1 tightly regulated nucleotide-dependent vascular relaxation in vivo and in vitro. Moreover, our in vitro experiments revealed that NTPDase1 exerted a complex role, limiting endothelial P2 receptor activation, but also protecting P2Y1 receptors from homologous desensitization.
Methods
Animals
All animal care and experimental procedures were carried out in accordance with the guidelines of the Institutional Ethical Committee for Experimental Animals. NTPDase1-deficient (Entpd1−/−) mice were provided by Dr SC Robson (BIDMC, HMS, Boston, MA, USA). Entpd1−/− mice, originally from the background 129 SVJ × C57 BL/6, were backcrossed for seven generations onto a C57 BL/6 background. Male mice aged of 16–25 weeks were used in all experiments. A confirmatory experiment was also performed with P2ry2−/− mice, backcrossed seven generations onto a C57 BL/6 background, provided by Dr. B. Robaye (ULB, Belgium).
Enzyme histochemistry and immunolocalization
Nucleotide hydrolysis in situ was evaluated as previously described (Braun et al., 2000). Briefly, 6 µm cryosections of mouse aorta and liver were fixed in cold acetone mixed with 10% phosphate-buffered formalin and then preincubated for 45 min at room temperature in 0.25 mM sucrose, 2 mM CaCl2 and 50 mM Tris-maleate, pH 7.4. Enzymatic assays were carried out at 37°C for 60 min in the same buffer, complemented with 2 mM Pb(NO3)2, 5 mM MnCl2, 3% dextran T-250 and 200 µM nucleotides (ATP, ADP) as substrate. In control experiments, divalent cations were replaced by 10 mM EDTA. The lead orthophosphate precipitate (brown deposit) resulting from nucleotide hydrolysis was visualized by incubating the sections in an aqueous solution of 1% v/v (NH4)2S. Sections were counterstained with haematoxylin, mounted with 20% mowiol 4–88 in 50% glycerol, 0.2 M Tris HCl, pH 8.5. For immunohistochemistry, the endothelial cell marker anti-mouse CD31/PECAM (clone MEC13.3, BD Pharmingen, San Diego, CA, USA) and the guinea pig anti-mouse NTPDase1 anti-sera (mN1-2c) were used (Martín-Satuéet al., 2009). Staining was performed with Vectastain ABC elite kit (Vector Laboratories, Burlingame, CA, USA), with 3,30-diaminobenzidine as chromogen.
In vivo blood pressure measurement
The carotid artery of anaesthetized mice was cannulated with PE-10 polyethylene tubing (Becton Dickinson, Oakville, ON, Canada) containing 50 U·mL−1 heparin in saline. A second cannula, inserted in the contralateral jugular vein, was used for intravenous infusions of agonists. Changes in mean arterial pressure (ΔMAP) were detected through the carotid with a pressure transducer connected to a Blood Pressure Analyzer-200A (Micro-Med, Tustin, CA, USA).
Isometric contraction of aortic rings
Mice were anaesthetized by intraperitoneal injection of a mixture of ketamine hydrochloride (100 mg·kg−1) and xylazine (20 mg·kg−1). Thoracic aortas were carefully removed and washed in saline/heparin (20 U·mL−1) at room temperature. Extra adventitial fat was removed with microscissors, and vessels were kept on ice until used in Krebs solution [composition (in mM): 118 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 KH2PO4, 1.2 MgSO4, 25 NaHCO3, and 5.5 glucose]. Aortas were divided into 2 mm segments and mounted between two tungsten triangles in 5 mL organ baths containing Krebs solution (37°C, aerated with 95% O2/5% CO2, pH 7.4). Aortic rings were gradually stretched during 1 h to reach a stable loading tension of 0.75 g. Overall, the procedure from death until the first measurement took approximately 2.5 h. Isometric changes in vascular tone were measured by force transducers (model 52–9545, Harvard Apparatus, South Natick, MA, USA), coupled to LKB chart recorders (model 2210 or REC 102, LKB, Rockville, MD, USA). After two equilibrating contractions with depolarizing KCl (50 mM), the relaxing effect of nucleotides was measured on U46619- (a thromboxane mimetic, 30 nM) precontracted aortic rings. In desensitization experiments, the stable nucleotides ADPβS and ATPγS were used to induce P2Y1 and P2Y2 receptor-dependent relaxation, respectively. ATPγS relaxation was performed in the presence of the P2Y1-receptor antagonist MRS2500 (1 µM) to avoid responses from P2Y1 receptors, due to contaminating ADP present in ATPγS. Desensitization of P2Y1 receptors was achieved by ADP (10 µM–10 mM), while desensitization of P2Y2 receptors was achieved by ATP (10 µM–10 mM) for 30 min followed by seven washes with 5 mL Krebs solution.
Statistical analyses
The data were compared by two-way anova, followed by a Bonferroni post-hoc test for multi-group comparisons.
Materials
U46619 was purchased from Cayman Chemical (Ann Arbor, MI, USA), ARL67156 and MRS2500 from Tocris Bioscience (Ellisville, MO, USA). Apyrase (grade VII), ADP, ATP, ADPβS, and ATPγS were from Sigma-Aldrich (Oakville, ON, Canada).
Results
Functional expression of NTPDase1 in endothelial cells
The contribution of NTPDase1 in the hydrolysis of nucleotides was evaluated in situ by a lead precipitation method (Braun et al., 2000). Entpd1−/− vessels exhibit an important deficit in ATPase and ADPase activity in the vascular wall. Figure 1 illustrates the nucleotidase activity of an aorta and a hepatic central vein. The ATPase activity localized in the adventitial layer of Entpd1−/− aorta is due to NTPDase2, which is a nucleoside triphosphatase with low diphosphatase activity (Heine et al., 1999; Sévigny et al., 2002). The endothelial activity of NTPDase1 was more evident in veins, such as the hepatic central vein (Figure 1B), of wild-type animals because of the near absence of SMC. This activity was virtually absent in Entpd1−/− vessels (Figure 1). The same observations were made in the Entpd1−/− vasculature of other tissues analysed, namely heart, lungs, bowel, bladder and vas deferens (data not shown). In accordance with its activity, NTPDase1 immunoreactivity was localized in the vascular wall at the level of the intima and the media layers (Figure 1: Entpd1+/+). It is noteworthy that prolonged exposure of Entpd1−/− aortas to ADP, but not ATP, led to a slight lead precipitation at the endothelial surface (data not shown), suggesting the presence of an ADPase activity. This activity may be attributable to NTPDase6 that has previously been shown to be expressed by endothelial cells in the heart (Yeung et al., 2000).
Figure 1.
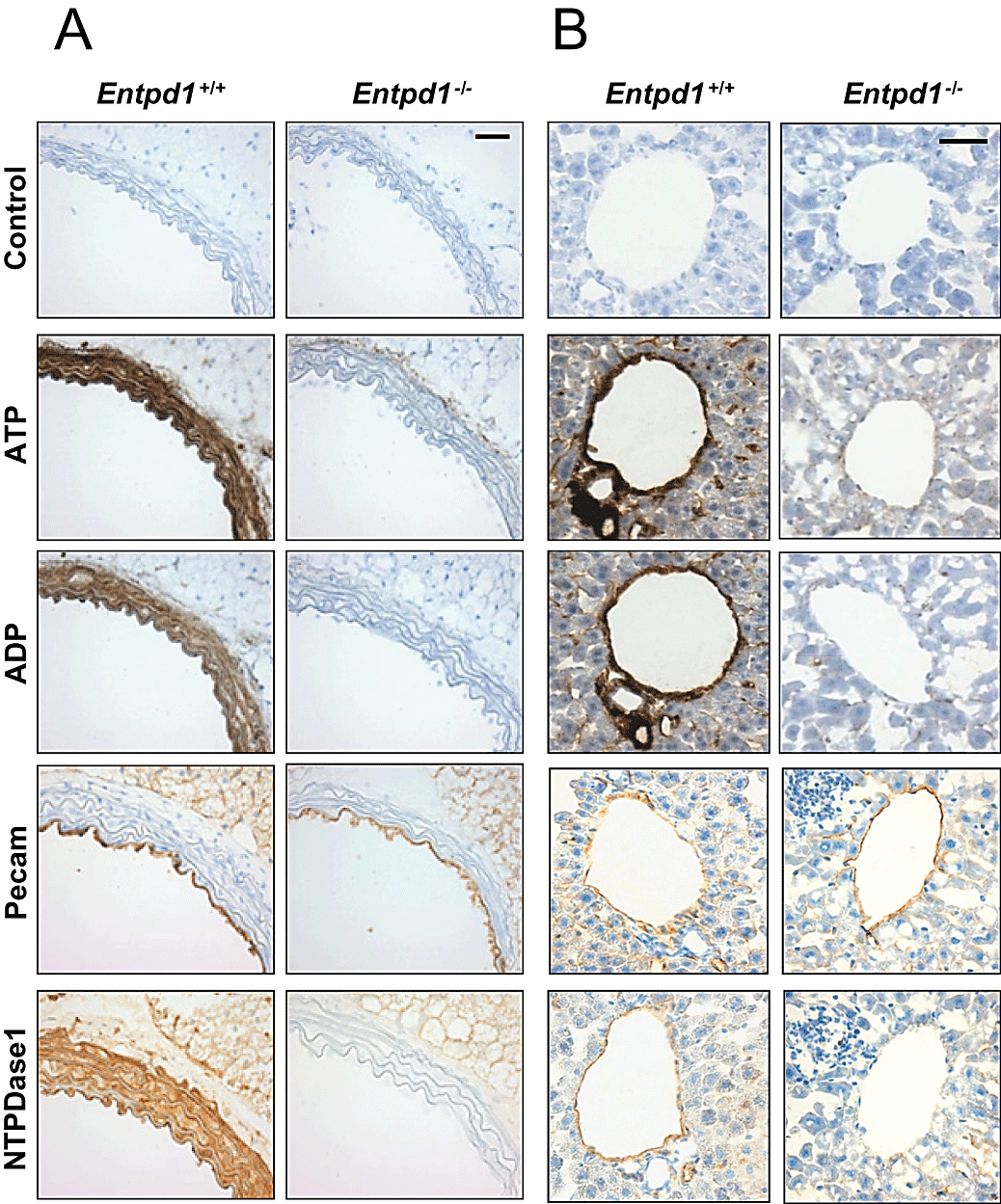
Deficit of nucleotidase activity in Entpd1−/− mouse vasculature in situ. (A) Aorta sections: Nucleotide hydrolysis is impaired in the wall of Entpd1−/− vessels compared with Entpd1+/+ aortas, which display significant ADPase and ATPase activity, as shown by the brown deposit on VSMCs and endothelial cells. The remaining ATPase activity in the adventitia is due to nucleoside triphosphate diphosphohydrolase-2 (NTPDase2). (B) Liver sections: The deficit in nucleotidase activity in the endothelium is more obvious in the hepatic central vein. The activity in canalicules is attributable to NTPDase8 (Fausther et al., 2007). (A and B) Control immunolabelling of the endothelium (PECAM) and NTPDase1 immunolabelling was performed as described. Note that NTPDase1 is expressed on both endothelial cells and VSMCs, in agreement with the ATPase and ADPase activities in these cells. Scale bars represent 50 µm.
Effect of intravenous injection of nucleotides
We questioned whether NTPDase1 could affect nucleotide-induced hypotensive responses in vivo. Thus, blood pressure was monitored on anaesthetized mice via a carotid catheter as described in the Methods section. Resting mean arterial pressure (MAP) was similar in Entpd1+/+ and Entpd1−/− mice (mean ± SEM: 62.1 ± 1.9 vs. 64.2 ± 1.3 mmHg, respectively).
Most of the nucleotides (ATP, ADP, UTP and UDP) are vasodilators in vitro through activation of endothelial P2 receptors (Erlinge and Burnstock, 2008), and accordingly, their i.v. injection induces a transient drop in MAP (Shah and Kadowitz, 2002). To assess the contribution of NTPDase1 in the nucleotide-dependent vasorelaxation and the resulting drop in MAP, we compared the effect of i.v. injections of ATP and UTP in Entpd1+/+ and Entpd1−/− mice (from 0.01 to 1000 nmole·kg−1). The drop in MAP following ATP injection was greater in Entpd1−/− mice (Figure 2A and B). UTP was as potent as ATP in decreasing blood pressure in Entpd1−/− mice, but it did not have any effects in wild-type animals (Figure 2A and B). Because of its ability to promote platelet aggregation, the effect of ADP was not tested in vivo. These results show that NTPDase1 activity limits the vasoactive effect of circulating ATP and UTP.
Figure 2.
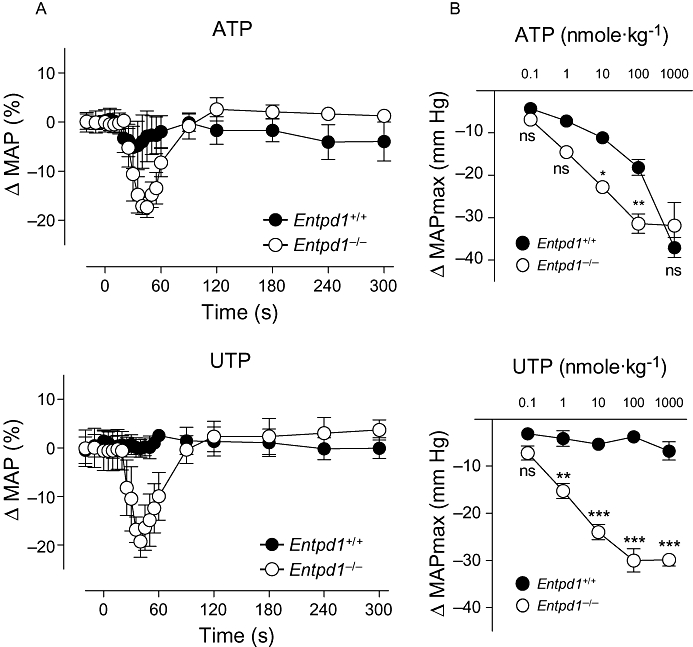
The absence of nucleoside triphosphate diphosphohydrolase-1 (NTPDase1) reveals a potent hypotensive effect of nucleotides. (A) Time course of the vascular response on the mean arterial pressure to injections of 10 nmole·kg−1 of ATP (upper panel) or UTP (lower panel) in wild-type (Entpd1+/+) and Entpd1−/− mice. (B) Concentration-response curves comparing the maximal decrease in mean arterial pressure (ΔMAP max) in response to i.v. injections of ATP (upper panel) or UTP (lower panel). Data are representative of the mean ± SEM of four to five experiments performed on different mice. *P < 0.05; **P < 0.01; ***P < 0.001.
P2Y receptor-induced relaxation in aortic rings
P2 receptors have already been characterized in mouse aortic rings: P2Y1 (ADP) and P2Y2 (ATP) receptors are responsible for adenine nucleotide-dependent vasorelaxation, and P2Y6 receptors mediates UDP and part of UTP-dependent relaxation (Guns et al., 2005; 2006; Bar et al., 2008). This relaxation depends on NO release, and is abolished in the presence of NO synthase inhibitors. To assess the role of NTPDase1 in P2Y1 and P2Y2-dependent relaxation, we evaluated ADP and ATP-induced relaxation of aortic rings from wild type and Entpd1−/− mice, pre-contracted by U46619 to 80% of the maximal response (Figure 3). U46619 induced similar contraction level in both strain of mice (mean ± SEM of 30 nM U46619-induced tension: 0.33 ± 0.07 vs. 0.35 ± 0.08 g of tension for Entpd1+/+ and Entpd1−/− aortic rings, respectively).
Figure 3.
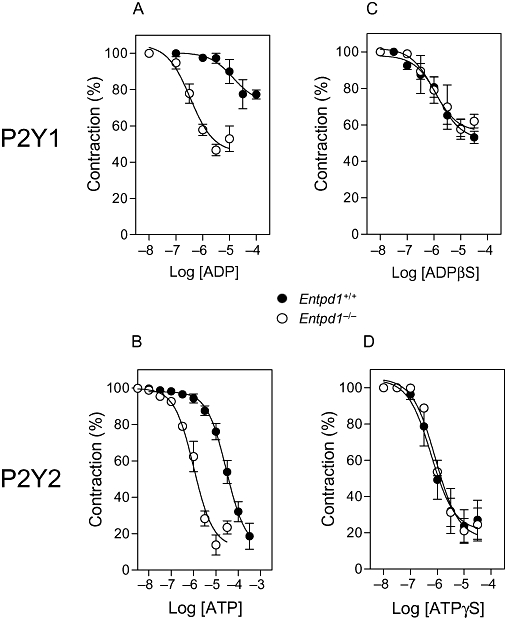
The relaxing effect of nucleotides is potentiated in Entpd1−/− aortas. The relaxing effect of nucleotides was evaluated on U46619-precontracted aortas (30 nM). ADP and ATP were used as P2Y1 and P2Y2 receptor agonists, respectively. Both ADP (A) and ATP-dependent (B) relaxations were enhanced in Entpd1−/− aortas. Relaxations induced by the non-hydrolysable analogues ADPβS (C) or ATPγS (D) were equivalent in both genotypes.
The relaxation induced by ATP was stable, and the shape of the dose response curves induced by cumulative or single concentrations of ATP was similar, suggesting that there was no or moderate desensitization of this response. In contrast, for ADP-induced relaxation, the concentration-response curve did not follow a sigmoid shape, suggesting that some desensitization occurred (data not shown). To avoid such desensitization, we constructed dose-response curves with a single concentration of each agonist per aortic ring. Both ADP and ATP-induced relaxation were potentiated in Entpd1−/− aortic rings (Figure 3 and Table 1), suggesting that NTPDase1 activity limited activation of endothelial cell P2Y1 and P2Y2 receptors, respectively. In contrast, the non-hydrolysable analogues ADPβS and ATPγS induced similar dose-dependent relaxation in either Entpd1+/+ or Entpd1−/− aortic rings (Figure 3C and D and Table 1), revealing that endothelial P2Y receptor functionality was comparable in vessels of both genotypes. The specificity of these non-hydrolysable agonists for P2Y1 and P2Y2 was verified by showing that ADPβS relaxation was blocked by the specific P2Y1 receptor antagonist MRS2500, while the response to ATPγS was absent in P2ry2−/− aortas (data not shown), in agreement with previous studies (Guns et al., 2006).
Table 1.
Relaxation of Entpd1+/+ and Entpd1−/− aortic rings in response to natural or non-hydrolysable agonists of P2Y1 and P2Y2 receptors
|
Emax (% of relaxation) |
EC50 (µM) |
|||
|---|---|---|---|---|
| Entpd1+/+ | Entpd1−/− | Entpd1+/+ | Entpd1−/− | |
| ADP | 54 | 27 | 14 | 0.35 |
| ADPβS | 44 | 48 | 1.4 | 0.99 |
| ATP | 96 | 96 | 29 | 1.0 |
| ATPγS | 83 | 79 | 0.6 | 0.86 |
Values shown in the table are derived from concentration-response curves obtained with a single concentration for each aortic ring. The relaxant effect of each agonist concentration was determined on aortic rings from three to five different mice.
We previously observed that uracil nucleotides induced a strong contractile response in Entpd1−/− denuded aortic rings (Kauffenstein et al., 2009). We have found here that this exacerbated contractile response was by far exceeding the relaxing effect of these nucleotides, making the study of endothelial P2Y6 receptor dependent relaxation impossible in Entpd1−/− tissues in this system (data not shown). In contrast, ATP and ADP did not display constrictor effect at concentrations below 30 µM, making the study of their relaxing effect possible with no interference resulting from the direct VSMC contractile responses.
Endothelial P2Y receptor desensitization
The ability of nucleotides to desensitize endothelial P2Y1 and P2Y2 receptors was tested in presence and absence of NTPDase1. For this, the relaxing effect of the non-hydrolysable analogues ADPβS and ATPγS were measured in wild type and Entpd1−/− aortas before and after exposure to different concentrations of the physiological agonists, ADP and ATP, at the P2Y1 and P2Y2 receptors, respectively (Figure 4). In Entpd1−/− aortic rings, P2Y1 receptors desensitized partially after being exposed to 10 µM ADP (44% of control relaxation), and completely after exposure to 100 µM ADP for 30 min, resulting in the absence of ADPβS-induced relaxation (Figure 4B and D). In contrast, in Entpd1+/+ aortic rings, ADPβS-induced relaxation was only moderately affected after exposure to a much higher concentration of ADP (10 mM; 56% of control relaxation remaining; Figure 4D), revealing that NTPDase1 provides an efficient protection of P2Y1 receptor against desensitization by ADP. The P2Y2 receptors presented a different behaviour: ATPγS-induced relaxation desensitized only weakly, even after exposing aortic rings to the high concentration of ATP (10 mM), and no significant difference between Entpd1+/+ and Entpd1−/− aortic rings could be detected (Figure 4C and E). Besides, carbachol-induced relaxation was not modified after ATP (10 mM) desensitization, showing that the functionality of relaxation was preserved (data not shown). Note that this concentration of ATP also desensitizes P2Y1 receptors (Enjyoji et al., 1999).
Figure 4.
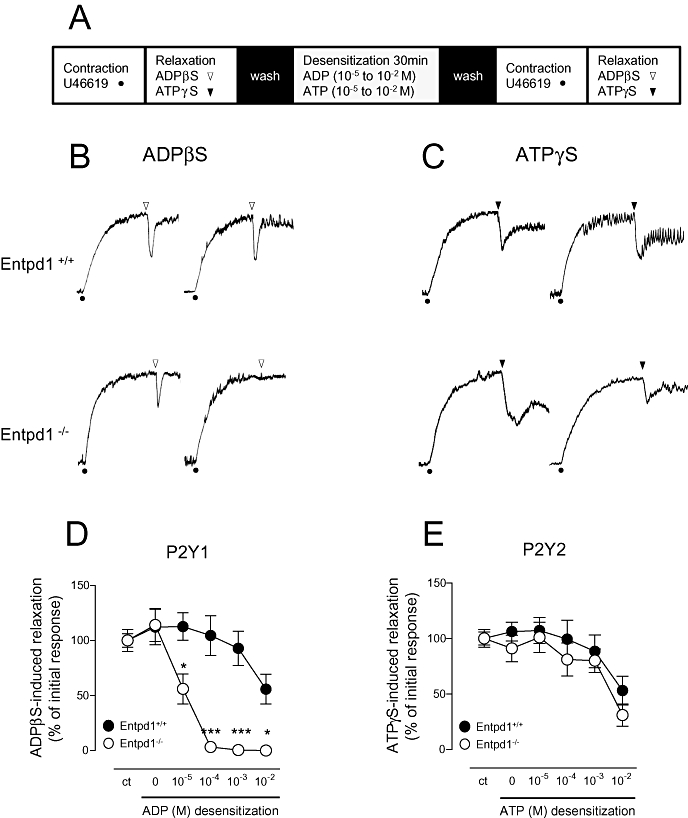
Nucleoside triphosphate diphosphohydrolase-1 (NTPDase1) differentially regulates the homologous desensitization of endothelial P2Y1 and P2Y2 receptors. Panel (A) represents the sequence of the protocol. Entpd1+/+ and Entpd1−/− aortic rings were contracted with 30 nM U46619 and challenged for a first relaxation with 10 µM of either ADPβS (white arrow) or ATPγS (black arrow). After extensive washing of the non-hydrolysable analogue, the rings were exposed respectively to ADP or ATP for 30 min (0.01 to 10 mM), washed and challenged a second time for their ability to relax in response to the same non-hydrolysable agonist (second set of arrows). Panels (B) and (C) show representative tracings of the relaxation in response to ADPβS (b) or ATPγS (C) before (left) and after (right) desensitization to 100 µM ADP (B) or ATP (C). Panels (D) and (E) summarize the effects of the various concentrations of ADP and ATP used to desensitize ADPβS and ATPγS responses, respectively. Results are expressed as percentage of the first relaxation produced before desensitization. Data are representative of the mean ± SEM of 4–10 experiments performed on different mice. *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion and conclusions
The study of Entpd1−/− mice brought to light a role of this enzyme in thromboregulation (Enjyoji et al., 1999; Pinsky et al., 2002), angiogenesis (Goepfert et al., 2001) and vascular permeability (Guckelberger et al., 2004). As circulating nucleotides are potent vasodilators through activation of endothelial P2 receptors (Erlinge and Burnstock, 2008), we assessed the role of NTPDase1 in the regulation of nucleotide-dependent relaxation. Both ATPase and ADPase activities were virtually absent in Entpd1−/− mouse vessels (Figure 1), suggesting that NTPDase1 is the major enzyme responsible for the hydrolysis of nucleotides at the endothelial surface, in agreement with previous observations showing that NTPDase1 was the major ectonucleotidase in cardiac endothelial cells (Enjyoji et al., 1999).
In vivo, the absence of NTPDase1 led to a more pronounced hypotension following i.v. injection of ATP and UTP. More striking was our finding that, when injected in vivo, UTP did not induce a hypotensive response (up to 1 µmole·kg−1) in wild-type mice, while it was as potent as ATP in Entpd1−/− mice (Figure 2). The fact that exogenous UTP did not modify arterial blood pressure in wild-type mice suggests that the receptor(s) involved (P2Y6 and/or P2Y2) is (are) tightly regulated by NTPDase1. These results also demonstrate that NTPDase1 differentially influences UTP and ATP responses in vivo.
An explanation for that discrepancy may be that the UTP receptor is located closer to NTPDase1 than the ATP receptor(s), as found for other P2 receptors. For example, P2Y1 and NTPDase1 have both been reported to be specifically located in the cholesterol-rich micro-domains of endothelial cells (Kaiser et al., 2002; Papanikolaou et al., 2005). Second, the vasodilator effect of adenine nucleotides may involve several P2Y (P2Y1,2,4) and P2X (P2X1,4) receptors, making the control of their activation by the action of NTPDase1 a more complex process. Finally, the major difference in the effect of UTP on blood pressure in wild-type and Entpd1−/− mice may also be due to the low redundancy of uracil nucleotide hydrolyzing enzymes compared with the wide range of adenine nucleotide metabolizing ecto-enzymes (Robson et al., 2006), making NTPDase1 particularly important in the regulation of uracil nucleotide action.
In aortic rings in vitro, we measured a leftward shift of the concentration-response curve of relaxation produced by the addition of either ADP or ATP. This reveals that NTPDase1 controls P2Y1 and P2Y2 receptor activation, as these are the receptors mediating the relaxant effects (Guns et al., 2005; 2006;) (Figure 3). The present observations in vitro and in vivo suggest that during conditions in which endothelial NTPDase1 activity is reduced, such as in vascular inflammation or oxidative stress (Robson et al., 1997), the vasodilator effects of nucleotides would be potentiated.
As a counterpart, the absence of NTPDase1 revealed the propensity of endothelial P2Y1 receptors to undergo desensitization when exposed to ADP in vitro (Figure 4). The latter is in agreement with the rapid and complete P2Y1 receptor desensitization in the presence of its natural ligand ADP (Baurand et al., 2000). The desensitization of P2Y1 receptors has been particularly examined on blood platelets, since it is responsible for the well-known refractory state of stored platelets (Holme and Holmsen, 1975). Indeed, apyrase, a potato enzyme that hydrolyses nucleotides similarly to NTPDase1, has been used since a long time to circumvent desensitization during platelet isolation (Ardlie et al., 1971). This function is fulfilled by NTPDase1 in vivo. As a consequence, the absence of NTPDase1 leads to a bleeding disorder specifically linked to platelet P2Y1 receptor desensitization and a failure of aggregation (Enjyoji et al., 1999). Here we found that NTPDase1 protects endothelial P2Y1 receptors and its associated relaxation against desensitization. The fact that ADPβS-dependent relaxation was unchanged between Entpd1+/+ and Entpd1−/− aortas in these in vitro experiments suggests that these receptors are not desensitized in the long term. However, we cannot exclude that endothelial P2Y1 receptors may desensitize when exposed to significant ADP concentrations released by blood cells, as from aggregating platelets (Enjyoji et al., 1999). The in vivo hypotensive effect of ADP was not tested due to its pro-aggregatory properties.
On the other hand, P2Y2 receptor was only poorly desensitized in our experimental conditions. High concentrations of ATP were required to diminish P2Y2-dependent relaxation (Figure 4). These results clearly indicate that, in the same arterial preparation, P2Y2 receptors are less prone to desensitization than P2Y1 receptors and correlate with the earlier observation that in bovine aortic endothelial cells, IP3 accumulation induced by 2MeS-ADP- (a P2Y1 agonist) was more easily desensitized than responses to ATP/UTP (P2Y2 agonists) (Motte et al., 1993; Wilkinson et al., 1994). Similarly, repeated stimulation with 2MeS-ADP resulted in desensitization of the relaxing response in rat aorta, while UTP-induced relaxation was not affected (Dol-Gleizes et al., 1999). Altogether, these data reveal that P2Y1 receptors are more likely to undergo homologous desensitization when compared with P2Y2 receptors, and that NTPDase1 plays a role in the protection of the former receptor.
Thus, even if P2Y1 and P2Y2 receptors are both coupled to Gq, they may display distinct desensitizing mechanisms. Indeed, it was recently shown that P2Y1 receptors recruit the G-protein-coupled receptor kinase-2 (GRK2), while P2Y2 receptors recruit GRK1 for their desensitization (Hoffmann et al., 2008). Moreover, in endothelial cells, the effect of 2MeS-ATP (a P2Y1 agonist) on IP3 was significantly inhibited after a short exposure to phorbol 12-myristate 13-acetate as compared with the effect of UTP (a P2Y2 agonist), suggesting that the P2Y1 receptor response was more sensitive to protein kinase C (PKC)-dependent desensitization than that of P2Y2 receptors (Communi et al., 1995). Thus, sensitivity to PKC probably also contributes to the difference in desensitization of P2Y1 and P2Y2 receptors. Nevertheless, P2Y2 receptors did show a weaker desensitization in comparison with that of P2Y1 receptors, as its associated dependent relaxing effect was only diminished by half after 10 mM ATP exposure (Figure 4). Interestingly, NTPDase1 did not exert a protective effect on P2Y2, as it did for P2Y1 receptors. It is noteworthy that this high ATP concentration necessary for desensitization suggests that such desensitization of P2Y2 receptors does not occur in vivo.
In Entpd1−/− arteries, the accumulation of UTP and UDP at the surface of VSMCs mediated a significant contraction (Kauffenstein et al., 2009). The net effect of uracil nucleotides resulted in a constriction in Entpd1−/− aortas instead of the relaxation obtained in the same vessels in Entpd1+/+. For this reason, it was not possible to use Entpd1−/− mice in this model to study the role of NTPDase1 on the function of endothelial P2Y6 receptors, which have been proposed to mediate UDP- and a part of UTP-dependent relaxation (Bar et al., 2008). This apparent greater contribution of NTPDase1 to vasoconstriction in vitro is likely to be linked to the smaller intercellular space and the slower diffusion in the multi-layer-organized VSMCs, favouring the exposure of nucleotides to ectonucleotidases, limiting their effects. In our experimental model, the exogenous nucleotides had free access to both endothelial cells and VSMCs. Therefore, the bioavailability of nucleotides within smooth muscle would be strongly influenced by NTPDase1 action, and the absence of this enzyme would have more impact on VSMC activation (contraction) than on the endothelial function (relaxation), as observed here. This makes the full picture more complex to analyse, as P2Y1, P2Y2 and P2Y6 receptors are all expressed by both endothelial cells and VSMCs, making the bioavailability of nucleotides a crucial factor in the regulation of the vascular tone.
Our results suggest that physio(patho)logical modulation of NTPDase1 expression or activity in endothelial cells may differently affect P2Y1, P2Y2, and probably other endothelial P2 receptor activation and the consequent relaxation. Hypoxia was shown to dramatically increase NTPDase1 expression (Eltzschig et al., 2003). The increased endothelial nucleotidase activity may diminish the potency of vasodilator effect of nucleotides, and, as a consequence, increase vascular tone. On the other hand, a reduced NTPDase1 activity has been reported in inflammatory and oxidative environments (Robson et al., 1997). We can speculate that moderate down-regulation of NTPDase1 would facilitate P2 receptor activation, while a more profound drop in activity would lead to P2Y1 receptor desensitization and a loss in nucleotide-dependent relaxation. In considering NTPDase1 as a regulator of vascular tone, it is important to integrate the contribution of NTPDase1 at the surface of smooth muscles and endothelial cells, because variations in the expression or the activity of the enzyme will have an opposite effect on vascular tone modulation, a drop in the activity enhancing the constrictor effect of nucleotides and vice versa. The final outcome of NTPDase1 modulation on vascular tone will depend on its respective endothelial versus muscular expression, as well as on the site of nucleotide release.
In conclusion, NTPDase1 constitutes the major ectoenzyme hydrolysing extracellular nucleotides at the surface of the vascular endothelium. Its absence allows a facilitated relaxation in vitro and a hypotensive effect in vivo, in response to nucleotides. The enzyme prevents endothelial P2 receptor overactivation and provides an efficient protection of P2Y1 receptors against desensitization. Endothelial P2Y2 receptors exhibited a limited desensitization that was not affected by NTPDase1 activity. In addition, Entpd1−/− mice provide a useful model to highlight the predominant P2 receptors implicated in particular vascular fields. Further work is required to establish the in vivo relevance of such enzymatic control of vascular tone, taking into consideration physio(patho)logical conditions where the expression or activity of NTPDase1 is modified.
Acknowledgments
The authors are grateful to Drs SC Robson and K Enjyoji (HMS, Boston, MA, USA) for providing Entpd1−/− mice, Dr B Robaye (ULB, Belgium) for P2ry2−/− mice, and I Brochu (Sherbrooke University, QC, Canada) for technical assistance. This work was supported by the Canadian Institutes of Health Research (CIHR) and by a small pilot grant from the Fonds de la Recherche en Santé du Québec (FRSQ). GK received a fellowship from the Institut National de la Santé et de la Recherche Médicale de France (INSERM) in partnership with the FRSQ, which was followed by an award from the Heart and Stroke Foundation of Canada in partnership with the CIHR and the Canadian Stroke Network. CRF was a recipient of a fellowship for visiting scholar at Université Laval sponsored by CAPES (Brazilian Ministry of Education), and JS was a recipient of a new investigator award from the CIHR and of a Junior 2 scholarship from the FRSQ.
Glossary
Abbreviations:
- ADPβS
adenosine 5′-O-(2-thiodiphosphate)
- ARL67156
6-N, N-diethyl-D-β,γ-dibromomethyleneATP trisodium salt
- ATPγS
adenosine 5′-O-(3-thio) triphosphate
- Entpd1−/−
E-NTPDase1 knock-out mice
- MRS2500
2-iodo-N6-methyl-(N)-methanocarba-2′-deoxyadenosine 3′,5′-bisphosphate
- NTPDase1
nucleoside triphosphate diphosphohydrolase-1
- P2ry2−/−
P2Y2 receptor knock-out mice
- PGF2α
prostaglandin F2α
- U46619
9, 11-dideoxy-9α, 11α-methanoepoxy PGF2α
- VSMC
vascular smooth muscle cell
Statement of conflicts of interest
The authors state no conflict of interest.
References
- Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, et al. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58(3):281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad S, Ahmad A, Ghosh M, Leslie CC, White CW. Extracellular ATP-mediated signaling for survival in hyperoxia-induced oxidative stress. J Biol Chem. 2004;279(16):16317–16325. doi: 10.1074/jbc.M313890200. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 3rd edition. Br J Pharmacol. 2008;153(Suppl 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardlie NG, Perry DW, Packham MA, Mustard JF. Influence of apyrase on stability of suspensions of washed rabbit platelets. Proc Soc Exp Biol Med. 1971;136(4):1021–1023. doi: 10.3181/00379727-136-35419. [DOI] [PubMed] [Google Scholar]
- Bar I, Guns PJ, Metallo J, Cammarata D, Wilkin F, Boeynams JM, et al. Knockout mice reveal a role for P2Y6 receptor in macrophages, endothelial cells, and vascular smooth muscle cells. Mol Pharmacol. 2008;74(3):777–784. doi: 10.1124/mol.108.046904. [DOI] [PubMed] [Google Scholar]
- Baurand A, Eckly A, Bari N, Leon C, Hechler B, Cazenave JP, et al. Desensitization of the platelet aggregation response to ADP: differential down-regulation of the P2Y1 and P2cyc receptors. Thromb Haemost. 2000;84(3):484–491. [PubMed] [Google Scholar]
- Boarder MR, Hourani SM. The regulation of vascular function by P2 receptors: multiple sites and multiple receptors. Trends Pharmacol Sci. 1998;19(3):99–107. doi: 10.1016/s0165-6147(98)01170-5. [DOI] [PubMed] [Google Scholar]
- Bodin P, Bailey D, Burnstock G. Increased flow-induced ATP release from isolated vascular endothelial cells but not smooth muscle cells. Br J Pharmacol. 1991;103(1):1203–1205. doi: 10.1111/j.1476-5381.1991.tb12324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun N, Sévigny J, Robson SC, Enjyoji K, Guckelberger O, Hammer K, et al. Assignment of ecto-nucleoside triphosphate diphosphohydrolase-1/cd39 expression to microglia and vasculature of the brain. Eur J Neurosci. 2000;12(12):4357–4366. [PubMed] [Google Scholar]
- Burnstock G. Purinergic signaling and vascular cell proliferation and death. Arterioscler Thromb Vasc Biol. 2002;22(3):364–373. doi: 10.1161/hq0302.105360. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol Rev. 2006;58(1):58–86. doi: 10.1124/pr.58.1.5. [DOI] [PubMed] [Google Scholar]
- Communi D, Raspe E, Pirotton S, Boeynaems JM. Coexpression of P2Y and P2U receptors on aortic endothelial cells. Comparison of cell localization and signaling pathways. Circ Res. 1995;76(2):191–198. doi: 10.1161/01.res.76.2.191. [DOI] [PubMed] [Google Scholar]
- Cote YP, Filep JG, Battistini B, Gauvreau J, Sirois P, Beaudoin AR. Characterization of ATP-diphosphohydrolase activities in the intima and media of the bovine aorta: evidence for a regulatory role in platelet activation in vitro. Biochim Biophys Acta. 1992;1139(1–2):133–142. doi: 10.1016/0925-4439(92)90092-2. [DOI] [PubMed] [Google Scholar]
- Dol-Gleizes F, Mares AM, Savi P, Herbert JM. Relaxant effect of 2-methyl-thio-adenosine diphosphate on rat thoracic aorta: effect of clopidogrel. Eur J Pharmacol. 1999;367(2–3):247–253. doi: 10.1016/s0014-2999(98)00985-6. [DOI] [PubMed] [Google Scholar]
- Eltzschig HK, Ibla JC, Furuta GT, Leonard MO, Jacobson KA, Enjyoji K, et al. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J Exp Med. 2003;198(5):783–796. doi: 10.1084/jem.20030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enjyoji K, Sévigny J, Lin Y, Frenette PS, Christie PD, Esch JS, 2nd, et al. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med. 1999;5(9):1010–1017. doi: 10.1038/12447. [DOI] [PubMed] [Google Scholar]
- Erlinge D, Burnstock G. P2 receptors in cardiovascular regulation and disease. Purinergic Signal. 2008;4(1):1–20. doi: 10.1007/s11302-007-9078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausther M, Lecka J, Kukulski F, Levesque SA, Pelletier J, Zimmermann H, et al. Cloning, purification, and identification of the liver canalicular ecto-ATPase as NTPDase8. Am J Physiol Gastrointest Liver Physiol. 2007;292(3):G785–G795. doi: 10.1152/ajpgi.00293.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitterman DP, Evans RJ. Nerve evoked P2X receptor contractions of rat mesenteric arteries; dependence on vessel size and lack of role of L-type calcium channels and calcium induced calcium release. Br J Pharmacol. 2001;132(6):1201–1208. doi: 10.1038/sj.bjp.0703925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goepfert C, Sundberg C, Sévigny J, Enjyoji K, Hoshi T, Csizmadia E, et al. Disordered cellular migration and angiogenesis in cd39-null mice. Circulation. 2001;104(25):3109–3115. doi: 10.1161/hc5001.100663. [DOI] [PubMed] [Google Scholar]
- Gordon JL. Extracellular ATP: effects, sources and fate. Biochem J. 1986;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guckelberger O, Sun XF, Sévigny J, Imai M, Kaczmarek E, Enjyoji K, et al. Beneficial effects of CD39/ecto-nucleoside triphosphate diphosphohydrolase-1 in murine intestinal ischemia-reperfusion injury. Thromb Haemost. 2004;91(3):576–586. doi: 10.1160/TH03-06-0373. [DOI] [PubMed] [Google Scholar]
- Guns PJ, Korda A, Crauwels HM, Van Assche T, Robaye B, Boeynaems JM, et al. Pharmacological characterization of nucleotide P2Y receptors on endothelial cells of the mouse aorta. Br J Pharmacol. 2005;146(2):288–295. doi: 10.1038/sj.bjp.0706326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guns PJ, Van Assche T, Fransen P, Robaye B, Boeynaems JM, Bult H. Endothelium-dependent relaxation evoked by ATP and UTP in the aorta of P2Y2-deficient mice. Br J Pharmacol. 2006;147(5):569–574. doi: 10.1038/sj.bjp.0706642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LS, Evans RJ, Wray J, Norling L, Swales KE, Vial C, et al. Purinergic 2X1 receptors mediate endothelial dependent vasodilation to ATP. Mol Pharmacol. 2007;72(5):1132–1136. doi: 10.1124/mol.107.037325. [DOI] [PubMed] [Google Scholar]
- Heine P, Braun N, Heilbronn A, Zimmermann H. Functional characterization of rat ecto-ATPase and ecto-ATP diphosphohydrolase after heterologous expression in CHO cells. Eur J Biochem. 1999;262(1):102–107. doi: 10.1046/j.1432-1327.1999.00347.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann C, Ziegler N, Reiner S, Krasel C, Lohse MJ. Agonist-selective, Receptor-specific Interaction of Human P2Y Receptors with {beta}-Arrestin-1 and -2. J Biol Chem. 2008;283(45):30933–30941. doi: 10.1074/jbc.M801472200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holme S, Holmsen H. ADP-induced refractory state of platelets in vitro. I. Methodological studies on aggregation in platelet rich plasma. Scand J Haematol. 1975;15(2):96–103. doi: 10.1111/j.1600-0609.1975.tb01060.x. [DOI] [PubMed] [Google Scholar]
- Joseph SM, Buchakjian MR, Dubyak GR. Colocalization of ATP release sites and ecto-ATPase activity at the extracellular surface of human astrocytes. J Biol Chem. 2003;278(26):23331–23342. doi: 10.1074/jbc.M302680200. [DOI] [PubMed] [Google Scholar]
- Kaiser RA, Oxhorn BC, Andrews G, Buxton IL. Functional compartmentation of endothelial P2Y receptor signaling. Circ Res. 2002;91(4):292–299. doi: 10.1161/01.res.0000030711.21521.ac. [DOI] [PubMed] [Google Scholar]
- Kauffenstein G, Drouin A, Thorin-Trescases N, Bachelard H, Robaye B, D'Orleans-Juste P, et al. NTPDase1 (CD39) controls nucleotide-dependent vasoconstriction in mouse. Cardiovasc Res. 2009 doi: 10.1093/cvr/cvp265. Epub ahead of print] doi: 10.1093/cvr/cvp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kugelgen I, Haussinger D, Starke K. Evidence for a vasoconstriction-mediating receptor for UTP, distinct from the P2 purinoceptor, in rabbit ear artery. Naunyn Schmiedebergs Arch Pharmacol. 1987;336(5):556–560. doi: 10.1007/BF00169313. [DOI] [PubMed] [Google Scholar]
- Marcus AJ, Safier LB, Hajjar KA, Ullman HL, Islam N, Broekman MJ, et al. Inhibition of platelet function by an aspirin-insensitive endothelial cell ADPase. Thromboregulation by endothelial cells. J Clin Invest. 1991;88(5):1690–1696. doi: 10.1172/JCI115485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Satué M, Lavoie EG, Pelletier J, Fausther M, Csizmadia E, Guckelberger O, et al. Localization of plasma membrane bound NTPDases in the murine reproductive tract. Histochem Cell Biol. 2009;131(5):615–628. doi: 10.1007/s00418-008-0551-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motte S, Pirotton S, Boeynaems JM. Heterogeneity of ATP receptors in aortic endothelial cells. Involvement of P2y and P2u receptors in inositol phosphate response. Circ Res. 1993;72(3):504–510. doi: 10.1161/01.res.72.3.504. [DOI] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82(4):1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Ostrom RS, Gregorian C, Insel PA. Cellular release of and response to ATP as key determinants of the set-point of signal transduction pathways. J Biol Chem. 2000;275(16):11735–11739. doi: 10.1074/jbc.275.16.11735. [DOI] [PubMed] [Google Scholar]
- Papanikolaou A, Papafotika A, Murphy C, Papamarcaki T, Tsolas O, Drab M, et al. Cholesterol-dependent lipid assemblies regulate the activity of the ecto-nucleotidase CD39. J Biol Chem. 2005;280(28):26406–26414. doi: 10.1074/jbc.M413927200. [DOI] [PubMed] [Google Scholar]
- Pinsky DJ, Broekman MJ, Peschon JJ, Stocking KL, Fujita T, Ramasamy R, et al. Elucidation of the thromboregulatory role of CD39/ectoapyrase in the ischemic brain. J Clin Invest. 2002;109(8):1031–1040. doi: 10.1172/JCI10649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson SC, Kaczmarek E, Siegel JB, Candinas D, Koziak K, Millan M, et al. Loss of ATP diphosphohydrolase activity with endothelial cell activation. J Exp Med. 1997;185(1):153–163. doi: 10.1084/jem.185.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson SC, Sévigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signalling. 2006;2:409–430. doi: 10.1007/s11302-006-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sévigny J, Sundberg C, Braun N, Guckelberger O, Csizmadia E, Qawi I, et al. Differential catalytic properties and vascular topography of murine nucleoside triphosphate diphosphohydrolase 1 (NTPDase1) and NTPDase2 have implications for thromboregulation. Blood. 2002;99(8):2801–2809. doi: 10.1182/blood.v99.8.2801. [DOI] [PubMed] [Google Scholar]
- Shah MK, Kadowitz PJ. Cyclic adenosine monophosphate-dependent vascular responses to purinergic agonists adenosine triphosphate and uridine triphosphate in the anesthetized mouse. J Cardiovasc Pharmacol. 2002;39(1):142–149. doi: 10.1097/00005344-200201000-00015. [DOI] [PubMed] [Google Scholar]
- Wilkinson GF, Purkiss JR, Boarder MR. Differential heterologous and homologous desensitization of two receptors for ATP (P2y purinoceptors and nucleotide receptors) coexisting on endothelial cells. Mol Pharmacol. 1994;45(4):731–736. [PubMed] [Google Scholar]
- Yamamoto K, Sokabe T, Matsumoto T, Yoshimura K, Shibata M, Ohura N, et al. Impaired flow-dependent control of vascular tone and remodeling in P2X4-deficient mice. Nat Med. 2006;12(1):133–137. doi: 10.1038/nm1338. [DOI] [PubMed] [Google Scholar]
- Yang S, Cheek DJ, Westfall DP, Buxton IL. Purinergic axis in cardiac blood vessels. Agonist-mediated release of ATP from cardiac endothelial cells. Circ Res. 1994;74(3):401–407. doi: 10.1161/01.res.74.3.401. [DOI] [PubMed] [Google Scholar]
- Yeung G, Mulero JJ, McGowan DW, Bajwa SS, Ford JE. CD39L2, a gene encoding a human nucleoside diphosphatase, predominantly expressed in the heart. Biochemistry. 2000;39(42):12916–12923. doi: 10.1021/bi000959z. [DOI] [PubMed] [Google Scholar]
- Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch Pharmacol. 2000;362(4–5):299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]


