Abstract
Background and purpose:
Obesity is associated with deterioration in asthma outcomes. Although airways eosinophil accumulation is characteristic of lung allergic diseases, little is known about the influence of obesity on the allergic eosinophil trafficking from bone marrow to lung tissues, and recruitment to airways lumen. Here, we have assessed the effects of diet-induced obesity on allergic eosinophilic inflammation in mice, examining eosinophil trafficking from bone marrow to airways, and production of TH1/TH2 cytokines.
Experimental approach:
C57BL/6 mice fed for 10 weeks with standard chow or high-fat diet were sensitized and challenged with ovalbumin. At 24–96 h post-ovalbumin challenge, bronchoalveolar lavage (BAL) fluid, lung tissue and bone marrow were examined.
Key results:
The high-fat-fed mice exhibited increased body weight and epididymal fat, glucose intolerance and alterations in lipid profile compared with the lean mice. Obesity markedly elevated serum leptin and lowered adiponectin levels. Ovalbumin challenge in obese mice promoted a markedly higher eosinophil accumulation in bone marrow and connective tissue surrounding the bronchial and bronchiolar segments. Eosinophil number in BAL fluid of obese mice was lower at 24 and 48 h. Levels of interleukin (IL)-5, eotaxin, tumour necrosis factor-α and IL-10 in BAL fluid of obese mice were significantly higher than in lean mice.
Conclusions and implications:
Diet-induced obesity enhanced eosinophil trafficking from bone marrow to lung tissues, and delayed their transit through the airway epithelium into the airway lumen. Consequently, eosinophils remain longer in lung peribronchiolar segments due to overproduction of TH1/TH2 cytokines and chemokines.
Keywords: obesity, asthma, eosinophil, bone marrow, eotaxin, TH2 cytokines
Introduction
Epidemiological data show an increased incidence of asthma in overweight or obese children and adults. The relative risk of incident asthma increases with increasing body mass, which has been associated with enhancement of asthma symptoms, airway hyper-responsiveness and atopy (Shore, 2008). Obesity may be particularly important for severe asthma because more than 75% of asthmatic individuals visiting the emergency room are obese or overweight (Thomson et al., 2003). Obesity may also increase disease severity in subjects that already have asthma (Akerman et al., 2004), and appears to alter the efficacy of standard medications for asthma (Peters-Golden et al., 2006). Likewise, weight loss in obese, asthmatic subjects results in decreased symptoms and severity of asthma (Shore, 2008). Data from human and animal studies suggest that obesity is a pro-inflammatory state. Increased levels of leptin and decreased levels of adiponectin, as well as increased production of pro-inflammatory cytokines such as interleukin (IL)-6 and IL-1, have been detected in obese subjects (Fantuzzi, 2005). The marked presence of macrophages within adipose tissue is also believed to contribute to the amplification of this inflammatory condition via the release of tumour necrosis factor-α (TNF-α) and IL-6, among other mechanisms (Weisberg et al., 2003).
Selective accumulation of eosinophils into the airways has become a central concept of the asthma pathology (Rothenberg and Hogan, 2006; Palmqvist et al., 2007). Eosinophils synthesize and release pro-inflammatory substances, including the preformed, cytotoxic, granular basic proteins (major basic protein, eosinophil cationic protein, eosinophil peroxidase and eosinophil-derived neurotoxin) and de novo synthesized products such as arachidonic acid metabolites, platelet-activating factor, reactive oxygen species and neuropeptides (Kroegel et al., 1993). In addition, eotaxin plays important roles in attracting eosinophils into inflammatory sites via the chemokine receptor CCR-3 (Palmqvist et al., 2007). Accordingly, increased eotaxin levels have been detected in the serum, plasma and/or sputum of asthmatic patients and antigen-challenged animals (Lilly et al., 2001; Tateno et al., 2004; Li et al., 2005).
In the last few years, animal models of obesity have been used to increase our understanding of the pathophysiology of airway hyper-responsiveness and inflammation in allergic individuals (see Shore and Johnston, 2006). In non-allergic models of pulmonary inflammation, ozone exposure has been shown to enhance the airway hyper-responsiveness in both ob/ob mice (obese animals with a genetic defect in the gene encoding leptin) and db/db mice (obese animals lacking the leptin receptor; Shore et al., 2003; Rivera-Sanchez et al. 2004). Higher levels of eotaxin, IL-6, KC and MIP-2 in bronchoalveolar lavage (BAL) fluid, along with increased expressions of pulmonary IL-1β and TNF-α mRNA expressions, have also been recently found in ozone-exposed obese mice (Lu et al., 2006). Similar findings were observed in carboxypeptidase E mutant fat mice after ozone exposure (Johnston et al., 2006).
Although eosinophil accumulation into the airways is one of the main characteristics of allergic diseases, studies on the influence of obesity on eosinophil trafficking remain surprisingly few. Johnston et al. (2007) showed a decreased eosinophil number in BAL fluid of ob/ob mice sensitized and challenged with ovalbumin. However, given that obesity is more likely to aggravate airway inflammatory responses, our hypothesis was to examine whether consumption of a high-fat diet in a condition that more closely mimics the development of human obesity (Surwit et al., 1995; Ikemoto et al., 1996) aggravates the eosinophil recruitment to the airways in mice actively sensitized and challenged with ovalbumin.
Therefore, in this study, we have followed over time the eosinophil trafficking from bone marrow to peripheral blood, and their recruitment into the airways in obese and allergic mice, along with measurements of levels of TH1/TH2 cytokines, adipocytokines and eotaxin in ovalbumin-sensitized C57BL/6 obese mice, have been carried out in this study.
Methods
Animals
All animal care and experimental protocols were approved by the Ethical Principles in Animal Research adopted by the Brazilian College for Animal Experimentation (COBEA), and followed the Guide for the Care and Use of Laboratory Animals. Four-week-old male C57BL6/J mice were provided by the Central Animal House Services of State University of Campinas (UNICAMP). The animals were housed three per cage on a 12 h light–dark cycle, and fed for 10 weeks with either a standard chow diet (carbohydrate: 70%; protein: 20%; fat: 10%) or a high-fat diet that induces obesity (carbohydrate: 29%; protein: 16%; fat: 55%), according to our previous work (Tsukumo et al., 2007).
Measurement of lipid levels
The measurements of total cholesterol (TC), high-density lipoprotein (HDL) and triglycerides (TGs) in serum were carried out using commercially available kits (Katal Biotecnológica Indústria e Comércio Ltda, São Paulo, Brazil). Low-density lipoprotein (LDL) levels were calculated according to the instructions of the manufacturer.
Oral glucose tolerance test (OGTT)
After 6 h of fasting, the mice (lean and obese) received a 20% glucose solution (2 g·kg−1) by gavage. The glucose concentration was measured in blood from the tail vein (Accu-Chek Performa, Roche Diagnostics, Indianapolis, IN, USA) immediately before and at 15, 30, 60 and 120 min after glucose loading.
Sensitization procedure and ovalbumin challenge
The mice were actively sensitized with a subcutaneous injection (0.4 mL) of 100 µg of ovalbumin (grade V; Sigma-Aldrich Co., St Louis, MO, USA) mixed with 1.6 mg Al(OH)3 in 0.9% NaCl (day 0). One week later (day 7), the mice received a second subcutaneous injection of 100 µg ovalbumin (0.4 mL; Lintomen et al., 2002). Non-sensitized mice received only a subcutaneous injection of Al(OH)3 (0.4 mL). At days 14 and 15, non-sensitized and sensitized mice were intranasally challenged with ovalbumin (10 µg/50 µL) twice a day, thus resulting in four challenges (at day 14, the first challenge occurred at time zero and the second challenge at 6 h later; at day 15, the third challenge occurred at time zero and the fourth challenge at 6 h later). As controls, both non-sensitized and sensitized mice were identically challenged with saline (50 µL). At 24, 48, 72 and/or 96 h after the first challenge, the mice were anaesthetized with isoflurane and exsanguinated, after which a sample of peripheral blood was collected from the abdominal vena cava. BAL was performed, and the femur was isolated to obtain the bone marrow (see below). Epididymal fat mass was also collected and weighed, and lungs were collected for morphological study, as detailed below.
BAL fluid
The mice were killed with isoflurane, and the trachea of animals was exposed and cannulated with a polyethylene tube connected to a syringe. The lungs were washed by flushing with phosphate-buffered saline (PBS) (5 × 300 µL) through the tracheal cannula. The fluid recovered after each wash was combined and centrifuged (500×g for 10 min at 4°C), and BAL fluid supernatant was stored at −80°C. The cell pellet was resuspended in 200 µL of PBS, and total (Neubauer) and differential (Diff-Quick stain) cell counts were done. A minimum of 300 cells were counted and classified as eosinophils, neutrophils and mononuclear cells based on normal morphological criteria.
Peripheral blood and bone marrow leukocytes
Blood samples were obtained from the abdominal vena cava, and were allowed to clot for 30 min at 37°C, and the serum was collected and stored at −20°C. The total cell counts were done (Neubauer), and cytospin smears (Diff-Quick stain) were used to obtain differential cell count. At least 300 cells were counted and classified as eosinophils, neutrophils and mononuclear cells based on normal morphological criteria.
The femurs of mice were also removed immediately after killing, and the epiphyses were cut transversely. Bone marrow cells were collected by flushing the two femurs with PBS (2.5 mL per femur), and the total (Neubauer) and differential (Diff-Quick stain) cell counts were done. A minimum of 300 cells were counted. The eosinophilic lineage was classified as immature eosinophils (myeloblast, promyelocyte and myelocyte) or mature eosinophils (metamyelocyte, band and mature) recognized by the intensely eosinophilic granules and morphological criteria.
Histological analysis
Lungs were removed and post-fixed by immersion for at least 24 h with 10% buffered formalin, after which they were macroscopically examined and cut transversally into slices of approximately 3 mm. Only the middle third of the caudal aspects of both lungs were sent to embedding in paraffin. Sections of these portions, 4–5 µm thick, were stained with haematoxylin–eosin, and evaluated for bronchiolitis under a Nikon Eclipse E200 microscope adapted to a Nikon Coolpix 995 camera (3 Mpixel Nikon, Melville NY, USA). For each animal, the extent of the lung infiltrate was determined by establishing the percentage of compromised bronchioli within 30 of such structures, randomly selected at low power fields (i.e. using a 4× objective). In addition, using the 40× objective, 18 random digital images per group (n= 6) were taken within areas of overt peri-bronchiolar inflammation. Total inflammatory and eosinophil cell counts were determined from these images, using the Imagelab Analysis software (version 2.4), and expressed as number of cells mm−2. Such quantification was focused on peri-bronchiolar areas, provided these regions were the main sites of inflammatory reaction. Parenchymal inflammation, represented by extension of the peri-bronchiolar infiltrates to alveoli, was mild/focal and only detected in few animals; thus, parenchymal infiltrates were not assessed quantitatively.
Measurement of cytokines and adipocytokine levels in BAL fluid and/or serum
The cytokines IL-5, IL-6, IL-10, TNF-α and eotaxin were measured in BAL fluid, whereas the adipocytokines leptin and adiponectin were measured in serum. All measurements were carried out using commercially available elisa kits (Mouse DuoSet elisa Development System), following the instructions of the manufacturer (R & D, Minneapolis, MN, USA).
Statistical analysis
Data are presented as the means ± SEM of n experiments. The program Instat (GraphPad software) and the SAS System for Windows (version 8.02) were used for statistical analysis. Two-way repeated measures anova was used to analyse the OGTT data. One-way anova was used to analyse the cell counts in BAL fluid, bone marrow, peripheral blood and pulmonary tissue, as well as cytokine levels in BAL fluid. In both cases, anova was followed by Tukey's test. Non-paired Student's t-test was used to analyse body weight, epididymal fat, TC, LDL, HDL and TGs. A value of P < 0.05 was accepted as significant.
Results
Weight gain, lipid profile and OGTT
The high-fat-fed mice exhibited a significant increase in body weight and epididymal fat compared with the mice receiving standard chow diet (Table 1). The obese mice presented increased serum levels of TC and LDL compared to the lean mice, but no significant differences in TGs and HDL levels between both groups were found (Table 1). Fasting glucose levels were not significantly different between the obese and lean mice (1979 ± 374 and 1522 ± 226 mg·L−1, respectively; n= 5–7). However, the OGTT showed that glucose levels remained increased up to 120 min after glucose consumption in the obese mice, while glucose levels in the lean mice returned to basal level at 60 min after the glucose load (two-way anova, F= 18.66, P < 0.0001; Figure 1).
Table 1.
Effect of a high-fat diet on mice anthropometric and lipid profiles
| Control | Obese | |
|---|---|---|
| Body weight (g) | 28.5 ± 0.32 | 39.2 ± 0.36* |
| Epididymal fat (g) | 0.3 ± 0.01 | 1.5 ± 0.08* |
| TC (mg·L−1) | 983 ± 40 | 1472 ± 55* |
| LDL (mg·L−1) | 321 ± 41 | 693 ± 83* |
| HDL (mg·L−1) | 548 ± 7 | 596 ± 35 |
| TGs (mg·L−1) | 769 ± 15 | 719 ± 52 |
Male C57BL6/J mice were fed with either a standard chow diet (control) or a high-fat diet (obese) during 10 weeks. Body weight, epididymal fat weight and serum levels of TC, LDL, HDL and TGs were evaluated. Values represent means ± SEM animal for n= 5.
P < 0.05 compared with the control group.
Figure 1.
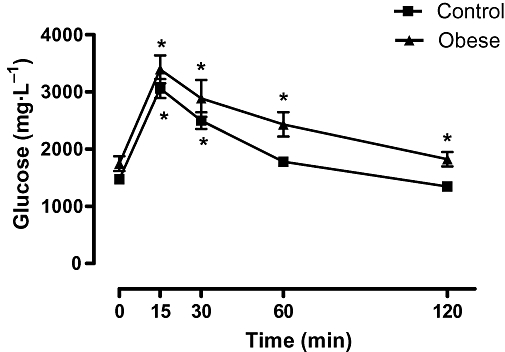
Effect of a 10 week high-fat diet or standard rodent chow diet on OGTT. The values represent means ± SEM for n= 5–7. *P < 0.0001 compared with respective baseline (time zero).
Cell counts in BAL fluid
The cells in BAL fluid from the non-sensitized mice challenged with PBS were >99% mononuclear cells in the lean and obese groups, as assessed from 24 to 96 h thereafter (data not shown, n= 5). In the non-sensitized mice challenged with ovalbumin, leukocytes in BAL fluid consisted mainly of mononuclear cells, with few neutrophils detected only at 24 h (16 and 11% for the lean and obese groups, respectively; n= 5). Similarly, in the ovalbumin-sensitized mice challenged with PBS, leukocytes in BAL fluid consisted of mononuclear cells, with few neutrophils (9.4 and 17% for the lean and obese groups, respectively; n= 4–5). There were virtually no eosinophils in both of these control groups (non-sensitized challenged with ovalbumin or ovalbumin-sensitized challenged with PBS), regardless of the time of measurement.
Ovalbumin challenge in the sensitized mice caused a significant increase in the number of total inflammatory cells (Figure 2A), and eosinophils (Figure 2B) in BAL fluid of both the lean and obese groups at 24, 48 and 72 h post-ovalbumin challenge, indicating the efficacy of the sensitization and challenge procedure. In the obese mice, the counts of total inflammatory cells in BAL fluid were significantly lower at 24 and 48 h post-ovalbumin challenge compared with the lean mice (Figure 2A). The counts of eosinophils in BAL fluid of the obese mice were also significantly lower at 24 and 48 h post-ovalbumin challenge compared with the lean mice (P < 0.05). On the contrary, at 72 h, the eosinophil count in BAL fluid of the obese mice was about 65% higher (P < 0.05) than those of the lean mice (Figure 2B).
Figure 2.
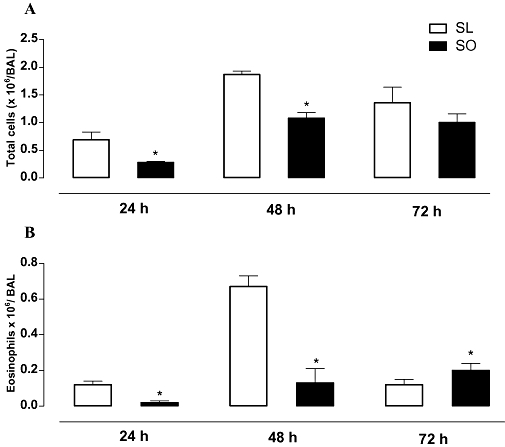
The effects of obesity on the number of total inflammatory cells (A) and eosinophils (B) in BAL fluid at 24, 48 and 72 h following intranasal challenge with ovalbumin in the sensitized mice. Each column represents the mean ± SEM (n= 7) for sensitized lean (SL) and sensitized obese (SO) mice. *P < 0.05 compared with the respective lean group (SL).
Lung histology
The lung histology of both non-sensitized and sensitized mice was performed at 48 and 72 h post-ovalbumin challenge. Histological examination of the lungs from the non-sensitized mice challenged with PBS showed normal tissue, with no inflammatory cells throughout pulmonary parenchyma and stroma, as observed in both the lean and obese mice. In the non-sensitized mice challenged with ovalbumin, this picture of pulmonary histology in the lean and obese animals was also observed (Figure 3A and B). The ovalbumin challenge in the sensitized lean mice produced a significant influx of total inflammatory cells (Figure 3A) and eosinophils (Figure 3B) into the connective tissue surrounding the bronchial and bronchiolar segments at both 48 and 72 h. However, in the sensitized obese mice, the total cell influx in response to ovalbumin challenge was about 2.3- and 2.1-fold higher than that of the lean mice at 48 and 72 h, respectively (Figure 3A). Similarly, the peribronchiolar eosinophil influx in response to ovalbumin challenge was markedly higher in the sensitized obese mice at both 48 h (about 5.3-fold) and 72 h (about 2.7-fold) compared with the sensitized lean mice (Figure 3B). Histology sections of connective tissue surrounding the bronchial and bronchiolar segments in all groups are shown in Figure 4.
Figure 3.
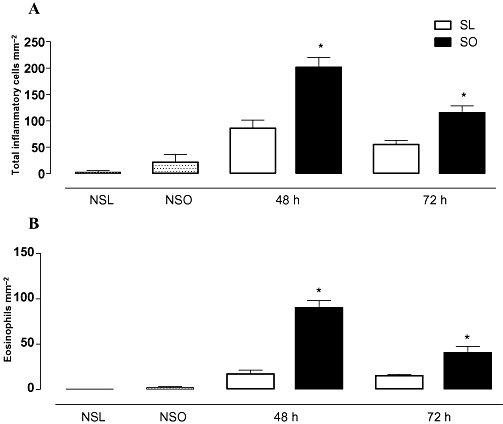
The effects of obesity on the number of total inflammatory cells (A) and eosinophils (B) in lung connective tissue surrounding the bronchial and bronchiolar segments at 48 and 72 h following intranasal challenge with ovalbumin in the sensitized mice. A control group of non-sensitized lean and obese mice challenged with ovalbumin (NSL and NSO, respectively) is also included (data from the non-sensitized mice at 48 and 72 h groups have been pooled). Each column represents mean ± SEM of the number of cells mm−2 for non-sensitized (NS), sensitized lean (SL) and sensitized obese (SO) mice. *P < 0.05 compared with the respective control group (SL).
Figure 4.
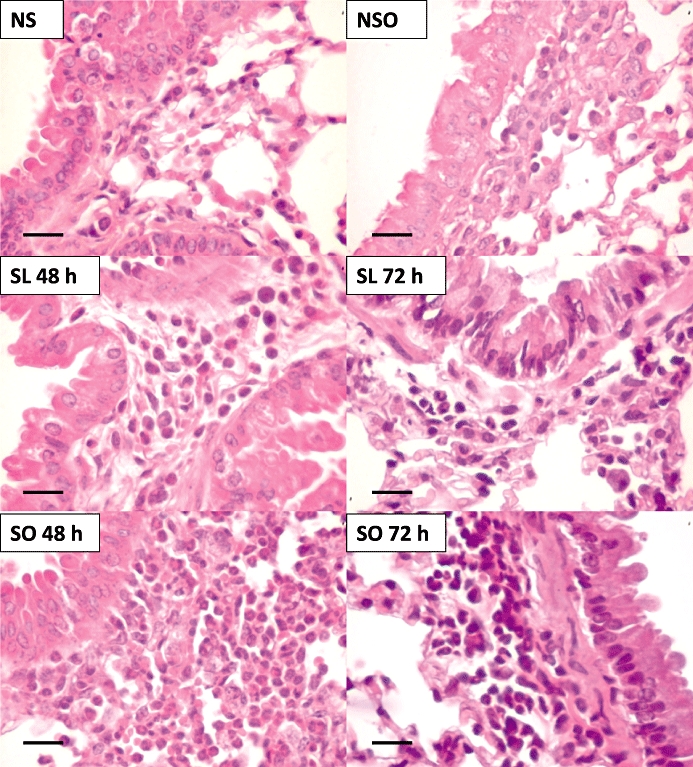
Representative high-power fields of bronchiolar structures from the following groups: non-sensitized (NS), NS obese (NSO), ovalbumin-sensitized lean (SL 48 and 72 h), ovalbumin-sensitized obese (SO 48 and 72 h), all after ovalbumin challenge. The mice lungs display variable degrees of peri-bronchiolar inflammation with greater magnitude in the ovalbumin-sensitized obese groups compared with the respective lean groups (n= 6). Haematoxylin–eosin, high magnification (bar represents 35 µm).
Bone marrow and peripheral blood eosinophil counts
In the non-sensitized mice, challenge with ovalbumin induced no alterations in the pattern of immature and mature eosinophil counts in the bone marrow of the lean and obese mice, as evaluated at 24–96 h after challenge (n= 5 each; Table 2).
Table 2.
Number of mature and immature eosinophils in bone marrow (106 cells mL−1) of the non-sensitized mice challenged with ovalbumin at 24, 48 and 72 h post-injection
| Group | 24 h | 48 h | 72 h | |
|---|---|---|---|---|
| Mature eosinophils | NSC | 0.05 ± 0.02 | 0.10 ± 0.04 | 0.12 ± 0.04 |
| NSO | 0.06 ± 0.03 | 0.15 ± 0.08 | 0.14 ± 0.06 | |
| Immature eosinophils | NSC | 0.03 ± 0.02 | 0.06 ± 0.04 | 0.09 ± 0.05 |
| NSO | 0.02 ± 0.02 | 0.12 ± 0.08 | 0.10 ± 0.05 |
Values represent the mean ± SEM (n= 5 each group).
NSC, non-sensitized, control (lean); NSO, non-sensitized, obese.
The intranasal challenge with ovalbumin in the sensitized mice significantly elevated the number of immature and mature forms of eosinophils in bone marrow, as expected (Figure 5). At 24 h post-ovalbumin challenge, the number of mature and immature forms of eosinophils was significantly lower in the obese mice than in the lean animals (Figure 5). However, at 48 h, the ovalbumin-challenged mice showed a markedly higher number of immature and mature eosinophils in the obese mice (about 3.5- and 11.0-fold, respectively) when compared with the ovalbumin-challenged lean mice. At 72 h post-ovalbumin challenge, the number of mature eosinophils (but not immature cells) was also higher (approximately twofold) in bone marrow of the obese group than in the lean mice (Figure 5).
Figure 5.
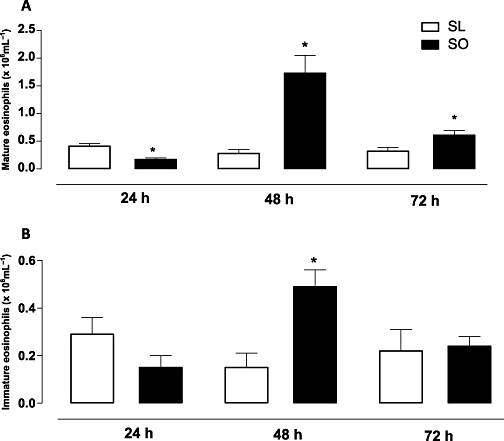
The effects of obesity on the counts of mature (A) and immature eosinophils (B) in bone marrow at 24, 48 and 72 h following intranasal challenge with ovalbumin in the sensitized mice. Each column represents the mean ± SEM (n= 7 each group) for ovalbumin-sensitized lean (SL) and ovalbumin-sensitized obese (SO) mice. *P < 0.05 compared with the respective SL group.
In peripheral blood, the counts of eosinophils at 48 and 72 h post-ovalbumin challenge did not significantly differ between the lean and obese sensitized groups (not shown; n= 5).
Cytokine levels in BAL fluid
The levels of IL-5, eotaxin, TNF-α, IL-10 and IL-6 in BAL fluid of the sensitized lean and obese mice were measured at 48 and 72 h post-ovalbumin challenge. The levels of IL-5, eotaxin, TNF-α and IL-10 were largely higher in the obese mice at 48 and 72 h post-ovalbumin challenge compared with those at the corresponding times in the lean mice (Figure 6). The IL-6 levels in the obese mice were lower at 48 h, but were increased at 72 h compared with the lean mice (Figure 6).
Figure 6.
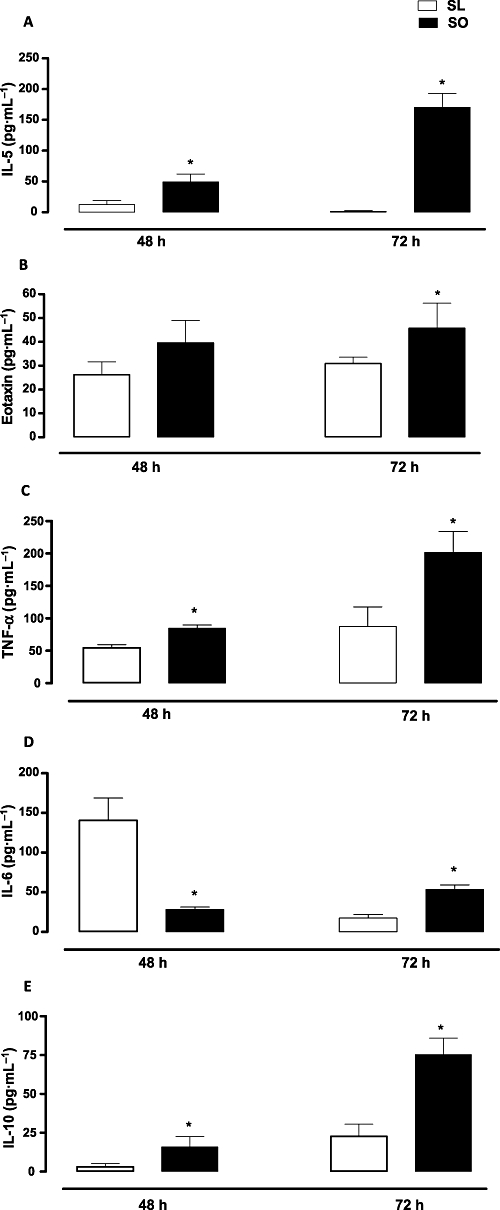
Effects of obesity on the levels of IL-5 (A), eotaxin (B), TNF-α (C), IL-6 (D) and IL-10 (E) in BAL fluid at 48 and 72 h following intranasal instillation of ovalbumin in previously sensitized mice. Each column represents the mean ± SEM (n= 5 each group) for ovalbumin-sensitized lean (SL) and ovalbumin-sensitized obese (SO) mice. *P < 0.05 compared with the respective SL group.
Serum levels of leptin and adiponectin
The serum levels of leptin in the sensitized obese mice were markedly higher than in the sensitized lean mice at both 48 and 72 h post-ovalbumin challenge. In contrast, the serum adiponectin levels in the obese mice were significantly lower than the lean group (Figure 7).
Figure 7.
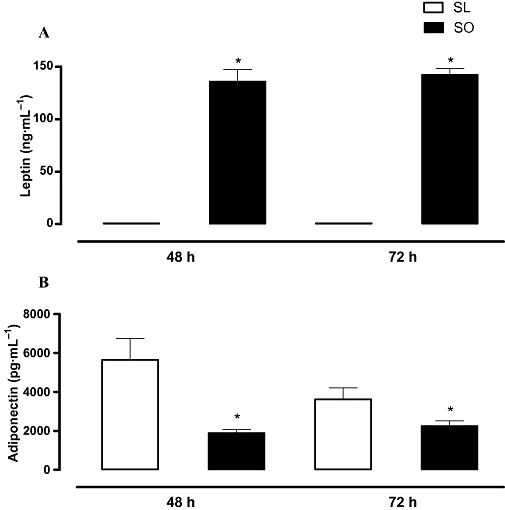
Effects of obesity on the levels of leptin (A) and adiponectin (B) in serum at 48 and 72 h following intranasal challenge with ovalbumin in the sensitized mice. Each column represents the mean ± SEM (n= 5 each group) for the ovalbumin-sensitized lean (SL) and ovalbumin-sensitized obese (SO) mice. *P < 0.05 compared with the respective SL group.
Discussion and conclusions
The current study carried out in high-fat-diet-fed mice showed that diet-induced obesity enhanced eosinophil trafficking from bone marrow to pulmonary tissue, and delayed the eosinophil transit into the airway lumen, allowing these cells to remain longer in the connective tissue surrounding the bronchial and bronchiolar segments of the lung.
Human obesity is mostly derived from consumption of high-fat diets combined with low expenditure of energy (Galgani and Ravussin, 2008). Different mouse strains fed with diets rich in fat have been used as a model of obesity, but C57BL/6J mice are reported to be particularly sensitive to the effects of a high-fat diet (Surwit et al., 1995; Ikemoto et al., 1996). Accordingly, in our present study, C57BL/6J mice fed with a high-fat diet for 10 weeks developed obesity, as shown by the increase in body weight and epididymal fat mass, accompanied by increased serum levels of TC and LDL cholesterol. Additionally, in the obese mice, the OGTT showed that the glucose levels remained increased at 60 and 120 min after glucose consumption, indicating impaired glucose tolerance and a potential pre-diabetic state in these obese animals (Gallou-Kabani et al., 2007).
Adipose tissue has been considered as an endocrine organ releasing several substances, including the adipokines adiponectin and leptin (Tilg and Moschen, 2006). Low plasma levels of the anti-inflammatory factor adiponectin characterize obesity and insulin resistance (Ouchi et al., 2003; Fantuzzi, 2005). Exogenous adiponectin administration reduced airway hyper-responsiveness and inflammation in mice (Shore and Johnston, 2006). A recent study also showed that adiponectin-deficient mice (APN−/−) exhibit greater accumulation of eosinophils and monocytes in the airways associated with elevated lung chemokine levels (Medoff et al., 2009). In contrast to adiponectin, elevated levels of the pro-inflammatory hormone leptin are reported to be crucial in connecting obesity to allergic airway inflammation (Shore et al., 2005). Airway hyper-responsiveness and inflammation were both enhanced in ob/ob and db/db mice (Shore et al., 2003; Rivera-Sanchez et al., 2004; Lu et al., 2006), as well as in carboxypeptidase E mutant fat mice exposed to ozone (Johnston et al., 2006). Higher leptin (and lower adiponectin) levels in serum of obese mice have been found in our study, reinforcing the suitability of this murine model to further our understanding of the pathophysiology of asthma in human obesity.
It is well established that allergen exposure in rodent models and humans leads to the recruitment of eosinophils into tissues, which is followed by the appearance of intraluminal eosinophils in the BAL fluid. Although eosinophils within the lumina of airways are reported to exert important functions (Shi et al., 2000), most studies have focused on the mechanisms contributing to eosinophil trafficking that culminates in eosinophil chemotaxis through the airway epithelium into the airway lumen (Rosenberg et al., 2007). In the pulmonary tissue, eosinophils possess effector functions in promoting the pathogenesis of airway diseases via the release of cationic granule proteins and a number of inflammatory mediators, which has been correlated with CD4+ T cells and production of Th2-cytokines and chemokines (Gleich et al., 1987; Hogan et al., 2008).
In our study, ovalbumin challenge in the sensitized lean mice markedly increased the eosinophil counts in peribronchiolar regions and BAL fluid that peaked at 48 h post-challenge, indicating the efficacy of the ovalbumin sensitization and challenge procedure. In the obese mice, the eosinophil number was significantly lower in BAL fluid at 48 h post-ovalbumin challenge compared with the lean animals; in contrast, a marked peribronchiolar eosinophil accumulation was seen at both 48 and 72 h post-ovalbumin challenge. These findings strongly suggest that eosinophil transit through the airway epithelium into the lumen is largely impaired in the allergic obese mice. Interestingly, ovalbumin challenge in female C57BL/6J mice fed with isocaloric high-saturated-fat diet that do not develop obesity actually reduced airway eosinophilia, indicating that a high-fat content in the diet per se does not predispose towards allergic inflammation (de Vries et al., 2009). Besides using non-obese female mice, de Vries et al. performed different strategies of ovalbumin challenge, that is, they carried out functional analysis on the seventh day after the first ovalbumin challenge, whereas in the present study we have measured eosinophil recruitment within 3 days after the first ovalbumin challenge.
It is well established that bone marrow plays a pivotal role in allergic inflammatory responses (Wood et al., 2002). Eosinophils are derived in the bone marrow from myeloid precursors in response to cytokine activation, and, following antigen challenge, they are released into the circulation and recruited to tissues. Several pathways have been implicated in this process, including stimulation of resident bone marrow cells, release of allergen-induced haematopoietic growth factors and cell trafficking (Mohle et al., 1997). In our study, the ovalbumin sensitization markedly increased the mature and immature forms of eosinophils in the bone marrow of the control mice, which is in agreement with previous studies carried out in ovalbumin-challenged mice (Chin et al., 1998) and asthmatic subjects exposed to antigen inhalation (Wood et al., 1998). In the obese mice, the bone marrow eosinophil counts were markedly higher than that of the lean mice, about 3- and 10-fold for immature and mature eosinophils respectively. Interestingly, such increases in bone marrow eosinophil production in the obese mice were detected at 48 h post-ovalbumin challenge, a time by which a marked eosinophil accumulation was seen in the bronchiolar structures, achieving maximal accumulation at 72 h. The higher eosinophil production by bone marrow in the obese mice is likely to reflect an accelerated dynamic of the eosinophil lineage (bone marrow → blood → lung), thus providing a more sustained eosinophil trafficking to enter the lung tissues, before crossing the airway epithelium to reach the lumen. This reinforces the concept that communications between lung and bone marrow play an important role in the pathogenesis of allergen-induced asthmatic responses (Kung et al., 1994; Li et al., 2005), and that this communication in obese state may be more effective. Unexpectedly, we did not detect statistical differences in blood eosinophilia between the lean and obese mice at any time analysed; however, it is possible that the blood eosinophilia may be different at some time between 24 and 48 h post-ovalbumin challenge.
IL-5 is a central factor mediating eosinophil expansion, priming, recruitment and prolonged tissue survival in response to allergic stimuli at the level of bone marrow, blood and tissue (Rosenberg et al., 2007). Moreover, eotaxin plays important roles in attracting eosinophils into inflammatory sites via the chemokine receptor CCR-3 (Pease and Williams, 2006). Increased circulating levels of eotaxin and mRNA eotaxin expression in visceral adipose tissue have been shown in obese mice and humans (Vasudevan et al., 2006). In our study, the IL-5 levels in BAL fluid of the obese mice were markedly higher at 48 h, peaking at 72 h post-ovalbumin challenge, in comparison with the lean mice. The eotaxin levels in BAL fluid were also higher in the obese mice, suggesting that eotaxin, along with IL-5, plays important roles in enhancing the attraction of eosinophils into the airways and in prolonging their survival into the lung parenchyma of obese individuals. In agreement with this, earlier studies have shown that eosinophil priming with IL-5 synergized with eotaxin to allow transendothelial migration, possibly via a change in the affinity of VLA-4 in responding leukocytes (Lamkhioued et al., 1997; Mould et al., 1997; Palframan et al., 1998).
The TNF-α levels in BAL fluid of the obese mice were also higher than in the lean mice at 48 h, peaking at 72 h post-ovalbumin challenge. This is consistent with studies showing that TNF-α exerts immunoregulatory activities and can switch the immune system towards a Th2 cytokine profile (Nakae et al., 2007). The systemic and lung TNF-α levels are increased in allergic animals and patients with bronchial asthma (Kips et al., 1993; Brightling et al., 2008). Moreover, the TNF-α signalling pathway seems to be a common feature to both obesity and asthma, and may undergo up-regulation by the presence of both conditions (MacEwan, 2002; Weiss, 2005). Of interest, TNF-α induces the synthesis of eotaxin in several cell types, including human eosinophils (Wong et al., 2002).
IL-6 is secreted by cells of the innate immune system and induces the expansion of the Th2 effector cells. This cytokine influences different aspects of the immune response, especially under pathological conditions. IL-6 can bind to soluble or membrane-bound IL-6R, inducing cell proliferation and controlling Th2 cells in the lung (Doganci et al., 2005). In the present study, IL-6 levels in BAL fluid of the lean mice peaked at 48 h post-ovalbumin challenge decaying to basal levels at 72 h; on the contrary, in the obese mice, the IL-6 levels steadily increased from 48 to 72 h. This is consistent with an earlier study showing that ozone exposure in IL-6-deficient mice produced lower levels of inflammatory markers such as levels of soluble TNFR1 and TNFR2 in BAL fluid (Johnston et al., 2005). Additionally, in a diet-induced obesity model, ozone exposure increased the IL-6 levels in BAL fluid at 4 h post-exposure (Johnston et al., 2007).
IL-10 is a major anti-inflammatory cytokine that plays a crucial role in the regulation of the immune system (Moore et al., 2001). Our data from IL-10 measurements in BAL fluid showed a time-dependent increase in the obese mice (48 and 72 h post-ovalbumin challenge) compared with respective lean groups. It is likely therefore that IL-10 acts as a compensatory mechanism, down-regulating the inflammatory state seen in the obese mice. However, it should be recognized that any correlation between IL-10 production and obesity is complex and not yet fully elucidated (Moore et al., 2001; Esposito et al., 2003).
In summary, diet-induced obesity enhanced eosinophil trafficking from bone marrow to lung tissues, and delayed eosinophil transit into the airway lumen, allowing these cells to remain longer in the peribronchiolar structures of the lung. Taking into consideration that obesity has increasingly been recognized as a low-grade chronic inflammation that may precede asthma, it is plausible that the resulting pulmonary inflammation observed in the obese mice is a consequence of overproduction of TH1/TH2 cytokines, chemokines and adipokines.
Acknowledgments
Marina C. Calixto, Leticia Lintomen and Edson Antunes thank CAPES and FAPESP for financial support.
Glossary
Abbreviations:
- BAL
bronchoalveolar lavage
- CCR
CC chemokine receptor
- IL
interleukin
- OGTT
oral glucose tolerance test
- TNF-α
tumour necrosis factor-α
- VLA-4
very late antigen-4
References
- Akerman MJ, Calacanis CM, Madsen MK. Relationship between asthma severity and obesity. J Asthma. 2004;41:521–526. doi: 10.1081/jas-120037651. [DOI] [PubMed] [Google Scholar]
- Brightling C, Berry M, Amrani Y. Targeting TNF-α: a novel therapeutic approach for asthma. J Allergy Clin Immunol. 2008;121:5–10. doi: 10.1016/j.jaci.2007.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin JE, Winterrowd GE, Hatfield CA, Brashler JR, Griffin RL, Vonderfecht SL, et al. Involvement of intercellular adhesion molecule-1 in the antigen-induced infiltration of eosinophils and lymphocytes into the airways in a murine model of pulmonary inflammation. Am J Respir Cell Mol Biol. 1998;18:158–167. doi: 10.1165/ajrcmb.18.2.2565m. [DOI] [PubMed] [Google Scholar]
- Doganci A, Sauer K, Karwot R, Finotto S. Pathological role of IL-6 in the experimental allergic bronchial asthma in mice. Clin Rev Allergy Immunol. 2005;28:257–270. doi: 10.1385/CRIAI:28:3:257. [DOI] [PubMed] [Google Scholar]
- Esposito K, Pontillo A, Giugliano F, Giugliano G, Marfella R, Nicoletti G, et al. Association of low interleukin-10 levels with the metabolic syndrome in obese women. J Clin Endocrinol Metab. 2003;88:1055–1058. doi: 10.1210/jc.2002-021437. [DOI] [PubMed] [Google Scholar]
- Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Galgani J, Ravussin E. Energy metabolism, fuel selection and body weight regulation. Int J Obes (Lond) 2008;32:S109–S119. doi: 10.1038/ijo.2008.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallou-Kabani C, Vigé A, Gross MS, Rabès JP, Boileau C, Larue-Achagiotis C, et al. C57BL/6J and A/J mice fed a high-fat diet delineate components of metabolic syndrome. Obesity (Silver Spring) 2007;15:1996–2005. doi: 10.1038/oby.2007.238. [DOI] [PubMed] [Google Scholar]
- Gleich GJ, Motojima S, Frigas E, Kephart GM, Fujisawa T, Kravis LP. The eosinophilic leukocyte and the pathology of fatal bronchial asthma: evidence for pathologic heterogeneity. J Allergy Clin Immunol. 1987;80:412–415. doi: 10.1016/0091-6749(87)90063-7. [DOI] [PubMed] [Google Scholar]
- Hogan SP, Rosenberg HF, Moqbel R, Phipps S, Foster PS, Lacy P, et al. Eosinophils: biological properties and role in health and disease. Clin Exp Allergy. 2008;38:709–750. doi: 10.1111/j.1365-2222.2008.02958.x. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Takahashi M, Tsunoda N, Maruyama K, Itakura H, Ezaki O. High-fat diet-induced hyperglycemia and obesity in mice: differential effects of dietary oils. Metabolism. 1996;45:1539–1546. doi: 10.1016/s0026-0495(96)90185-7. [DOI] [PubMed] [Google Scholar]
- Johnston RA, Schwartzman IN, Flynt L, Shore SA. Role of interleukin-6 in murine airway responses to ozone. Am J Physiol Lung Cell Mol Physiol. 2005;288:L390–L397. doi: 10.1152/ajplung.00007.2004. [DOI] [PubMed] [Google Scholar]
- Johnston RA, Theman TA, Shore SA. Augmented responses to ozone in obese carboxypeptidase E-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2006;290:126–133. doi: 10.1152/ajpregu.00306.2005. [DOI] [PubMed] [Google Scholar]
- Johnston RA, Zhu M, Rivera-Sanchez YM, Lu FL, Theman TA, Flynt L, et al. Allergic airway responses in obese mice. Am J Respir Crit Care Med. 2007;176:650–658. doi: 10.1164/rccm.200702-323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kips JC, Tavernier JH, Joos GF, Peleman RA, Pauwels RA. The potential role of tumour necrosis factor alpha in asthma. Clin Exp Allergy. 1993;23:247–250. doi: 10.1111/j.1365-2222.1993.tb00317.x. [DOI] [PubMed] [Google Scholar]
- Kroegel C, Virchow JC, Walker C. Asthma. N Engl J Med. 1993;328:1639–1640. [PubMed] [Google Scholar]
- Kung TT, Stelts D, Zurcher JA, Watnick AS, Jones H, Mauser PJ, et al. Mechanisms of allergic pulmonary eosinophilia in the mouse. J Allergy Clin Immunol. 1994;94:1217–1224. doi: 10.1016/0091-6749(94)90335-2. [DOI] [PubMed] [Google Scholar]
- Lamkhioued B, Renzi PM, Abi-Younes S, Garcia-Zepada EA, Allakhverdi Z, Ghaffar O, et al. Increased expression of eotaxin in bronchoalveolar lavage and airways of asthmatics contributes to the chemotaxis of eosinophils to the site of inflammation. J Immunol. 1997;159:4593–4601. [PubMed] [Google Scholar]
- Li J, Saito H, Crawford L, Inman MD, Cyr MM, Denburg JA. Hemopoietic mechanisms in murine allergic upper and lower airways inflammation. Immunology. 2005;114:386–396. doi: 10.1111/j.1365-2567.2005.02109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly CM, Nakamura H, Belostotsky OI, Haley KJ, Garcia-Zepeda EA, Luster AD, et al. Eotaxin expression after segmental allergen challenge in subjects with atopic asthma. Am J Respir Crit Care Med. 2001;163:1669–1675. doi: 10.1164/ajrccm.163.7.9812044. [DOI] [PubMed] [Google Scholar]
- Lintomen L, Elsas MI, Maximiano ES, de Paula Neto HA, Joseph D, Vargaftig BB, et al. Allergenic sensitization prevents upregulation of haemopoiesis by cyclo-oxygenase inhibitors in mice. Br J Pharmacol. 2002;135:1315–1323. doi: 10.1038/sj.bjp.0704580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu FL, Johnston RA, Flynt L, Theman TA, Terry RD, Schwartzman IN, et al. Increased pulmonary responses to acute ozone exposure in obese db/db mice. Am J Physiol Lung Cell Mol Physiol. 2006;290:L856–L865. doi: 10.1152/ajplung.00386.2005. [DOI] [PubMed] [Google Scholar]
- MacEwan DJ. TNF ligands and receptors – a matter of life and death. Br J Pharmacol. 2002;135:855–875. doi: 10.1038/sj.bjp.0704549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medoff BD, Okamoto Y, Leyton P, Weng M, Sandall BP, Raher MJ, et al. Adiponectin-deficiency increases allergic airway inflammation and pulmonary vascular remodeling. Am J Respir Cell Mol Biol. 2009;41:397–406. doi: 10.1165/rcmb.2008-0415OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohle R, Salemi P, Moore MAS, Rafii S. Expression of interleukin-5 by human bone marrow microvascular endothelial cells: implications for the regulation of eosinophilopoiesis in vivo. Br J Haematol. 1997;99:732–738. doi: 10.1046/j.1365-2141.1997.4713279.x. [DOI] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Mould AW, Matthaei KI, Young IG, Foster PS. Relationship between interleukin-5 and eotaxin in regulating blood and tissue eosinophilia in mice. J Clin Invest. 1997;99:1064–1071. doi: 10.1172/JCI119234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae S, Ho LH, Yu M, Monteforte R, Iikura M, Suto H, et al. Mast cell-derived TNF contributes to airway hyperreactivity, inflammation, and TH2 cytokine production in an asthma model in mice. J Allergy Clin Immunol. 2007;120:48–55. doi: 10.1016/j.jaci.2007.02.046. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Kihara S, Funahashi T, Matsuzawa Y, Walsh K. Obesity, adiponectin and vascular inflammatory disease. Curr Opin Lipidol. 2003;14:561–566. doi: 10.1097/00041433-200312000-00003. [DOI] [PubMed] [Google Scholar]
- Palframan RT, Collins PD, Severs NJ, Rothery S, Williams TJ, Rankin SM. Mechanisms of acute eosinophil mobilization from the bone marrow stimulated by interleukin 5: the role of specific adhesion molecules and phosphatidylinositol 3-kinase. J Exp Med. 1998;188:1621–1632. doi: 10.1084/jem.188.9.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmqvist C, Wardlaw AJ, Bradding P. Chemokines and their receptors as potential targets for the treatment of asthma. Br J Pharmacol. 2007;151:725–736. doi: 10.1038/sj.bjp.0707263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pease JE, Williams TJ. The attraction of chemokines as a target for specific anti-inflammatory therapy. Br J Pharmacol. 2006;147:S212–S221. doi: 10.1038/sj.bjp.0706475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters-Golden M, Swern A, Bird SS, Hustad CM, Grant E, Edelman JM. Influence of body mass index on the response to asthma controller agents. Eur Respir J. 2006;27:495–503. doi: 10.1183/09031936.06.00077205. [DOI] [PubMed] [Google Scholar]
- Rivera-Sanchez YM, Johnston RA, Schwartzman IN, Valone J, Silverman ES, Fredberg JJ, et al. Differential effects of ozone on airway and tissue mechanics in obese mice. J Appl Physiol. 2004;96:2200–2206. doi: 10.1152/japplphysiol.00960.2003. [DOI] [PubMed] [Google Scholar]
- Rosenberg HF, Phipps S, Foster PS. Eosinophil trafficking in allergy and asthma. J Allergy Clin Immunol. 2007;119:1311–1312. doi: 10.1016/j.jaci.2007.03.048. [DOI] [PubMed] [Google Scholar]
- Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- Shi H-Z, Humbles A, Gerard C, Jin Z, Weller PF. Lymph node trafficking and antigen presentation by endobronchial eosinophils. J Clin Invest. 2000;105:945–953. doi: 10.1172/JCI8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore SA. Obesity and asthma: possible mechanisms. J Allergy Clin Immunol. 2008;121:1087–1093. doi: 10.1016/j.jaci.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Shore SA, Johnston RA. Obesity and asthma. Pharmacol Ther. 2006;110:83–102. doi: 10.1016/j.pharmthera.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Shore SA, Rivera-Sanchez YM, Schwartzman IN, Johnston RA. Responses to ozone are increased in obese mice. J Appl Physiol. 2003;95:938–945. doi: 10.1152/japplphysiol.00336.2003. [DOI] [PubMed] [Google Scholar]
- Shore SA, Schwartzman IN, Mellema MS, Flynt L, Imrich A, Johnston RA. Effect of leptin on allergic airway responses in mice. J Allergy Clin Immunol. 2005;115:103–109. doi: 10.1016/j.jaci.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Surwit RS, Feinglos MN, Rodin J, Sutherland A, Petro AE, Opara EC, et al. Differential effects of fat and sucrose on the development of obesity and diabetes in C57BL/6J and A/J mice. Metabolism. 1995;44:645–651. doi: 10.1016/0026-0495(95)90123-x. [DOI] [PubMed] [Google Scholar]
- Tateno H, Nakamura H, Minematsu N, Nakajima T, Takahashi S, Nakamura M, et al. Plasma eotaxin level and severity of asthma treated with corticosteroid. Respir Med. 2004;98:782–790. doi: 10.1016/j.rmed.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Thomson CC, Clark S, Camargo CA., Jr Body mass index and asthma severity among adults presenting to the emergency department. Chest. 2003;124:795–802. doi: 10.1378/chest.124.3.795. [DOI] [PubMed] [Google Scholar]
- Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, Prada PO, Hirabara SM, Schenka AA, et al. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes. 2007;56:1986–1998. doi: 10.2337/db06-1595. [DOI] [PubMed] [Google Scholar]
- Vasudevan AR, Wu H, Xydakis AM, Jones PH, Smith EO, Sweeney JF, et al. Eotaxin and obesity. J Clin Endocrinol Metab. 2006;91:256–261. doi: 10.1210/jc.2005-1280. [DOI] [PubMed] [Google Scholar]
- de Vries A, Hazlewood L, Fitch PM, Seckl JR, Foster P, Howie SE. High-fat feeding redirects cytokine responses and decreases allergic airway eosinophilia. Clin Exp Allergy. 2009;39:731–739. doi: 10.1111/j.1365-2222.2008.03179.x. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss ST. Obesity: insight into the origins of asthma. Nat Immunol. 2005;6:537–539. doi: 10.1038/ni0605-537. [DOI] [PubMed] [Google Scholar]
- Wong CK, Zhang JP, Ip WK, Lam CW. Activation of p38 mitogen-activated protein kinase and nuclear factor-kappaB in tumour necrosis factor-induced eotaxin release of human eosinophils. Clin Exp Immunol. 2002;128:483–489. doi: 10.1046/j.1365-2249.2002.01880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood LJ, Inman MD, Denburg JA, O'Byrne PM. Allergen challenge increases cell traffic between bone marrow and lung. Am J Respir Cell Mol Biol. 1998;18:759–767. doi: 10.1165/ajrcmb.18.6.3006. [DOI] [PubMed] [Google Scholar]
- Wood LJ, Sehmi R, Dorman S, Hamid Q, Tulic MK, Watson RM, et al. Allergen-induced increases in bone marrow T lymphocytes and interleukin-5 expression in subjects with asthma. Am J Respir Crit Care Med. 2002;166:883–889. doi: 10.1164/rccm.2108015. [DOI] [PubMed] [Google Scholar]


