Abstract
Background and purpose:
Experimental and clinical data suggest that extracts of Ginkgo biloba improve cognitive function. However, the neurochemical correlates of these effects are not yet fully clarified. The purpose of this study was to examine the effects of acute and repeated oral administration of the standardized extract EGb 761® on extracellular levels of dopamine, noradrenaline and serotonin (5-HT), and the dopamine metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) in the prefrontal cortex (PFC) and striatum of conscious rats.
Experimental approach:
Monoamines and their metabolites were monitored by the use of microdialysis sampling and HPLC with electrochemical or fluorescence detection.
Key results:
A single oral dose of EGb 761 (100 mg·kg−1) had no effect on monoamine levels. However, following chronic (100 mg·kg−1/14 days/once daily) treatment, the same dose significantly increased extracellular dopamine and noradrenaline levels, while 5-HT levels were unaffected. Chronic treatment with EGb 761 showed dose-dependent increases in frontocortical dopamine levels and, to a lesser extent, in the striatum. The extracellular levels of HVA and DOPAC were not affected by either acute or repeated doses. Treatment with the main constituents of EGb 761 revealed that the increase in dopamine levels was mostly caused by the flavonol glycosides and ginkgolide fractions, whereas bilobalide treatment was without effect.
Conclusions and implications:
The present results demonstrate that chronic but not acute treatment with EGb 761 increased dopaminergic transmission in the PFC. This finding may be one of the mechanisms underlying the reported effects of G. biloba in improving cognitive function.
Keywords: Ginkgo biloba, microdialysis, monoamines, dopamine, noradrenaline, serotonin, prefrontal cortex, cognitive function
Introduction
Extracts of Ginkgo biloba (EGb 761®) have been shown to exert beneficial effects as cognitive enhancers in ageing, anti-stress agents and in therapy of age-related neurological disorders such as Alzheimer's disease (see DeFeudis and Drieu, 2000; DeFeudis, 2003; DeKosky and Furberg, 2008). A number of clinical studies have demonstrated that extracts of G. biloba, and particularly the standardized extract EGb 761, could ameliorate cognitive defects associated with mild to moderate Alzheimer's disease (Andrieu et al., 2003; Kanowski and Hoerr, 2003; Mazza et al., 2006; Napryeyenko et al., 2007; Scripnikov et al., 2007; Dodge et al., 2008). However, a recent Cochrane review on published randomized, double-blind, clinical trials has found that the use of G. biloba extracts was safe but the evidence for a significant benefit in people with dementia or cognitive impairment was inconsistent and unconvincing (Birks and Grimley Evans, 2007). In addition, a randomized, double-blind, placebo-controlled clinical study on 3069 volunteers aged 75 years or older receiving EGb 761 at 120 mg twice a day did not confirm the therapeutic efficacy of EGb 761 in reducing the overall incidence rate of dementia or Alzheimer's disease in elderly individuals with normal cognition or those with mild cognitive impairment (DeKosky et al., 2008). A similar long-lasting clinical study initiated in Europe is still in progress (Vellas et al., 2006).
In animal experiments, G. biloba extracts were shown to improve spatial memory deficits in aged rats (Wang et al., 2006; Blecharz-Klin et al., 2009) and a transgenic mouse model of Alzheimer's disease (Stackman et al., 2003) as well as to improve acquisition of working memory in rats (Satvat and Mallet, 2009). However, the precise neurochemical correlates of these behavioural effects of G. biloba are not known. Extracts of G. biloba exhibit potent antioxidant activity, scavenging various reactive oxygen species, including superoxide, peroxy and hydroxyl radicals (see DeFeudis and Drieu, 2000; Ahlemeyer and Krieglstein, 2003). G. biloba has been reported to enhance the activities of superoxide dismutase and catalase, and to decrease lipid peroxidation in the striatum, substantia nigra and hippocampus. The neuroprotective effects of G. biloba were demonstrated in animal models of Parkinson's disease, both in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned mice (Wu and Zhu, 1999; Rojas et al., 2008) and 6-hydroxydopamine-lesioned rats (Ahmad et al., 2005). In addition, the extracts of G. biloba were shown to reduce the activity of both forms of MAO-A and MAO-B in the rat (White et al., 1996), in the aged mice (Pardon et al., 2000) and mouse brain in vitro (Wu and Zhu, 1999). The activity of MAO is closely associated with mitochondrial function and production of hydrogen peroxide as a by-product of oxidation of monoamines. Accordingly, the proposed neuroprotective effects of G. biloba in Parkinson's and Alzheimer's diseases were attributed to the ability of Ginkgo extracts to stabilize mitochondrial function (Abdel-Kader et al., 2007).
Acute administration of G. biloba extracts was reported to reduce stress-induced increases in whole brain levels of catecholamines and serotonin (5-HT) in the rat (Shah et al., 2003). Similarly, chronic administration of EGb 761 enhanced copulatory behaviour and reduced serum prolactin levels in male rats, suggesting involvement of the dopaminergic system in the effects of G. biloba (Yeh et al., 2008). Based on these reports, we have hypothesized that there could be a causal link between the central protective effects of G. biloba and monoaminergic neurotransmission. Strong evidence exists for the role of prefrontal cortical dopamine and its receptors in modulating prefrontal cortical neurons, which are commonly viewed as a basis for cognitive operations (for review, see Goldman-Rakic et al., 2000; Mehta and Riedel, 2006; Phillips et al., 2008). Indeed, several studies have shown enhanced dopamine release in the rat prefrontal cortex (PFC) during various cognitive tasks (Rossetti and Carboni, 2005; Phillips et al., 2008). However, there are no data available demonstrating possible in vivo effects of G. biloba extracts on monoamine neurotransmitters in the brain areas implicated in regulating cognitive function, motivation and mood.
The purpose of the present study was to examine whether the acute and sub-chronic (14 days) daily administration of G. biloba extract and its main constituents, flavonol glycosides, ginkgolides and bilobalide, could affect basal extracellular levels of monoamines dopamine, noradrenaline and 5-HT monitored by microdialysis in the PFC and striatum of awake rats. Some preliminary data of this study have been reported at Gesellschaft für Arzneipflanzenforschung 2006 – International Congress and 54th Annual Meeting of the Society for Medicinal Plant Research, 29 August–2 September 2006, Helsinki, Finland.
Methods
Animals
All animal care and experimental procedures were approved by the local ethical committee following the directives of the ‘Principles of Laboratory Animal Care’ (National Institute of Health publication no. 8023) and the Council of the European Communities (86/809/EEC). Male Sprague-Dawley rats (weighing 250–350 g, total number of 75 rats) were used in the study. The rats (three animals/cage) were maintained on a 12 h light–dark cycle (light at 7 am), room temperature of 23 ± 2°C and humidity of 55–65%. All efforts were made to minimize animal suffering and the amount of animals used for the study.
Surgery and microdialysis experiments
The microdialysis experiments were carried out on conscious rats following the protocol described elsewhere (Osborne et al., 1990; Kehr, 1999; Kehr and Yoshitake, 2006). Fourteen days before the microdialysis experiment, the rats were anaesthetized with isoflurane (Forene®, Abbott Laboratories, Abbott Park, IL, USA) using a Univentor 400 anaesthesia unit (AgnThos, Lidingö, Sweden) and placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA, USA) in a flat skull position with the incisor bar set to −3.2 mm. The body temperature of the rat was controlled by a rectal thermometer and maintained at +37°C using a CMA/105 temperature controller (CMA/Microdialysis, Stockholm, Sweden). A mid-scalp incision of 2–3 cm was made, and the flaps were kept open with haemostat forceps. After exposing the skull, a hole for a probe and two holes for the fixing screws were made using a fine trephine drill. The guide cannula for a microdialysis probe (Eicom Corp., Kyoto, Japan) was implanted into the PFC (AP +3.2 mm, L −0.5 mm, V −1.1 mm; from the bregma and the dural surface, according to the stereotaxic atlas of Paxinos and Watson, 1997). In a separate group of rats, the guide cannula was implanted into the striatum at the following coordinates: AP +0.2 mm, L −3.0 mm, V −3.5 mm. The guide cannula was fixed firmly to the skull surface using dental cement. Following 14 days of recovery and repeated daily administration of the Ginkgo extract or carboxymethylcellulose (CMC) suspension alone, a microdialysis probe (Eicom A-I; 0.22 mm o.d., 2 mm membrane length with 50 000 Da cut-off) was inserted into the guide cannula of the conscious rat. A typical placement of the guide cannula and the microdialysis probe in the PFC is illustrated in Figure 1. The probe was perfused with Ringer's solution (NaCl, 147 mM; KCl, 4 mM; CaCl2, 2.3 mM) at a flow rate of 1 µL·min−1. After the initial stabilization period of 2–3 h, the microdialysis samples were collected in 20 min intervals. The first four samples were used for estimation of basal levels of dopamine, noradrenaline, 5-HT, 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA). Then, one dose of EGb 761 suspension in 0.3% w/v CMC or only CMC (vehicle-treated group) was given (p.o.), and the fractions were collected for a further 180 min.
Figure 1.
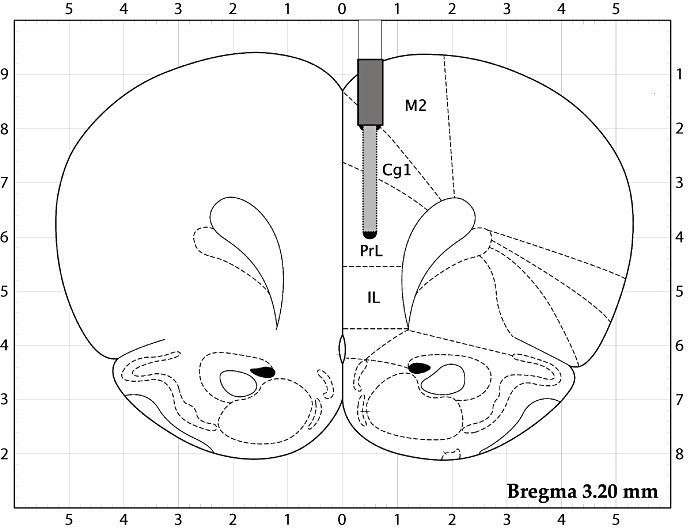
A schematic illustration of the placement of the guide cannula and the microdialysis probe in the rat prefrontal cortex. The membrane of the probe was placed in the cingulate cortex, area 1 (Cg1) and prelimbic cortex (PrL); the other marked areas are secondary motor cortex (M2) and infralimbic cortex (IL). Adapted from Paxinos and Watson (1997).
At the end of the experiment, the animals were killed by CO2 inhalation and dislocation of the neck. The brains were removed and examined for correct placement of the probe (the probe track) in the rat brain.
HPLC determination of monoamines and acidic metabolites
A highly sensitive liquid chromatographic method, based on precolumn derivatization and fluorescence detection, allowing simultaneous determination of 5-HT, noradrenaline and dopamine in brain microdialysis samples, was used following the method described elsewhere (Yoshitake et al., 2004a,b;) Briefly, 5-HT, noradrenaline and dopamine were derivatized with benzylamine and 1,2-diphenylethylenediamine in the presence of potassium hexacyanoferrate(III) and glycine, which yielded highly fluorescent and stable benzoxazoles. The derivatized monoamines were separated on a microbore column (150 × 1.0 mm i.d., packed with C18 silica, 5 µm) within 60 min. The mobile phase consisted of a mixture (32:68, v/v) of acetonitrile and Britton-Robinson buffer (composition: 25 mM boric acid, 25 mM acetic acid, 25 mM phosphoric acid and pH adjusted to 7.2 with 125 mM NaOH) containing 5 mM Na2EDTA and 5 mM octanesulfonic acid sodium salt. The detection limits (signal-to-noise ratio of 3) for 5-HT, noradrenaline and dopamine were 76, 42 and 95 amol·10 µL–1 injected on-column respectively.
Dopamine and its metabolites DOPAC and HVA were determined by ion-pair reversed-phase column liquid chromatography with electrochemical detection as described elsewhere (Kehr and Yoshitake, 2006). The HPLC system included a pump, a degasser and an amperometric detector (HTEC-500, Eicom Corp.). Dopamine and metabolites were separated on a 150 × 2.1 i.d. mm column (CA-5-ODS, Eicom Corp.). The mobile phase consisted of 0.1 M citric acid buffer at pH 3.5, 0.13 mM Na2EDTA, 2.3 mM sodium-1-octanesulfonate and 20% (v/v) methanol. The detection limit (signal-to-noise ratio = 3) for dopamine was 5·10−11 M, that is, 0.5 fmol in 10 µL injected onto the column respectively.
Data presentation and statistical analysis
The basal concentrations of monoamines and metabolites were expressed as mean ± standard error of the mean (SEM) of four fractions collected at −60 to 0 min from the rats in each treated group. These mean values were taken as 100%, and all the following values were related to these averaged levels. The overall effects of EGb 761 on dopamine levels were also expressed as the relative area under the curves (AUC) calculated as the mean percentage increase in extracellular dopamine levels over the entire 180 min post-treatment sampling period (nine samples) and compared with the corresponding value of the vehicle-treated group. Statistical analysis was performed using Prism 5 (GraphPad Software, San Diego, CA, USA) statistical software. Mean basal levels were compared by the use of one-way ANOVA followed by Newman–Keuls multiple comparison test. Differences between the groups and treatments were analysed by repeated-measures two-way ANOVA followed by Bonferroni's test.
Materials
The monoamines noradrenaline, 5-HT and dopamine, and the metabolites DOPAC and HVA, as well as all chemicals for mobile phase and reagent preparations, were purchased from Sigma-Aldrich (St. Louis, MO, USA), Wako Pure Chemical Co. (Osaka, Japan) or from Kisida Chemical Co. (Tokyo, Japan). Deionized and distilled water, purified with a Barnstead EASYpure RF (Hansen Co., Hyogo, Japan) system, was used for all aqueous solutions. Benzylamine hydrochloride was obtained from Tokyo Kasei Kogyo Co. (Tokyo, Japan) and was used after purification by recrystallization with absolute ethanol. 1,2-Diphenylethylenediamine was purchased from Tosoh (Tokyo, Japan). Standard solutions of monoamines were prepared in water and kept frozen (−20°C) in amber-coloured test tubes.
The standardized extract of G. biloba (EGb 761®) and its main constituents, flavonol glycosides, ginkgolides and bilobalide, were kindly provided by Willmar-Schwabe (Karlsruhe, Germany). The extract contains 24% flavonol glycosides, 6% terpene lactones and trace amounts of other substances including proanthocyanidins and organic acids (DeFeudis and Drieu, 2000; DeFeudis, 2003; Chan et al., 2007). The flavonol constituents are essentially flavonol-O-glycosides quercetin, kaempferol or isorhamnetin conjugated to D-glucose, L-rhamnose or glucorhamnose. The terpene trilactones are characteristic of G. biloba. Of those ginkgolides, A, B, C and J account for 3.1%, and bilobalide for 2.9% of the total extract. The dried extract or the constituents were resuspended in 0.3% w/v CMC (Sigma-Aldrich). Ginkgo suspension (EGb 761) was given orally (p.o.) via a gastro-oesophageal gavage, once daily for 14 days at doses of 30, 100 or 300 mg·kg−1. A separate group of rats received only the CMC solution (control group). The dose of each constituent fraction given p.o., once daily for 14 days, corresponded to its content in 300 mg·kg−1 of the EGb 761 extract: flavonol glycosides 72 mg·kg−1, ginkgolides 9.3 mg·kg−1 and bilobalide 8.7 mg·kg−1.
The nomenclature of drugs and molecular targets conforms to the ‘Guide to Receptors and Channels’ (Alexander et al., 2008).
Results
Basal extracellular levels of dopamine, noradrenaline, 5-HT, and metabolites DOPAC and HVA in the rat PFC
The basal concentrations (calculated in 10−10 M, i.e. fmol in 10 µL injected onto the column and expressed as mean ± SEM, n= 5) of monoamines, as well as the dopamine metabolites, DOPAC and HVA, in the dialysates from the PFC of conscious rats in all treated groups are summarized in Table 1. The basal levels of monoamines and metabolites did not significantly differ within the respective treated groups and analytical methods used.
Table 1.
Basal concentrations of dopamine (DA), noradrenaline (NA), 5-HT, and the metabolites DOPAC and HVA in the prefrontal cortex of vehicle- and EGb 761-treated rats
| 1.Analytical method: HPLC with electrochemical detection | ||||
|---|---|---|---|---|
| Treatment (EGb 761) | Basal concentrations (fmol in 10 µL, i.e. 10−10 M) | |||
| Dose (mg·kg−1) | Dosing schedule | DA | DOPAC | HVA |
| Vehicle | Once | 6.19 ± 0.39 | 987.7 ± 69.9 | 1489.2 ± 92.4 |
| 100 | Once | 5.92 ± 0.60 | 1091.3 ± 79.7 | 1527.3 ± 105.5 |
| Vehicle | Daily, 14 days | 5.84 ± 0.47 | 1022.2 ± 59.1 | 1662.3 ± 86.2 |
| 30 | Daily, 14 days | 5.63 ± 0.50 | – | – |
| 100 | Daily, 14 days | 6.55 ± 0.48 | – | – |
| 300 | Daily, 14 days | 7.61 ± 0.59 | 1280.0 ± 96.9 | 1784.4 ± 102.1 |
| Treatment (constituents) | ||||
| Vehicle | Daily, 14 days | 4.54 ± 0.53 | – | – |
| Ginkgolides | Daily, 14 days | 4.33 ± 0.41 | – | – |
| Bilobalide | Daily, 14 days | 4.71 ± 0.53 | – | – |
| Flavonoids | Daily, 14 days | 4.34 ± 0.59 | – | – |
| 2.Analytical method: HPLC with fluorescence detection | ||||
| Treatment (EGb 761) | DA | NA | 5-HT | |
| Vehicle | Daily, 14 days | 4.24 ± 0.42 | 3.41 ± 0.51 | 3.24 ± 0.63 |
| 100 | Daily, 14 days | 5.13 ± 0.46 | 4.01 ± 0.54 | 3.41 ± 0.62 |
The concentrations are expressed as mean ± SEM, n= 5 rats, where the basal value for each rat was calculated as the mean of four microdialysis samples collected at −60 to 0 min before the drug or vehicle administration. The basal levels of monoamines and metabolites did not significantly differ between the respective treated groups and analytical methods used.
DOPAC, 3,4-dihydroxyphenylacetic acid; HVA, homovanillic acid.
Effects of a single dose of EGb 761 on dopamine and metabolites in the rat PFC
A single dose of EGb 761 at a dose of 100 mg·kg−1 p.o. had no significant effect on basal extracellular levels of dopamine, DOPAC and HVA during the next 180 min, as shown in Figure 2. Similarly, the basal levels of noradrenaline and 5-HT were not affected by a single does of EGb 761 (data not shown).
Figure 2.
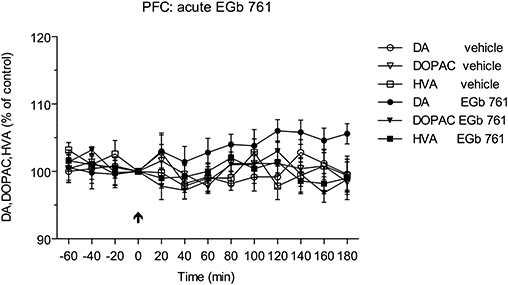
Effect of single administration of EGb 761 (100 mg·kg−1 p.o.) on the extracellular levels of dopamine (DA) and the metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) in the prefrontal cortex (PFC) of conscious rats. Administration of EGb 761 had no effect on the levels of dopamine and its metabolites.
Effects of sub-chronic administration of EGb 761 on monoamines and metabolites in the rat PFC
In the first part of this experiment, the monoamines dopamine, noradrenaline and 5-HT were determined by HPLC with fluorescence detection following precolumn derivatization. The basal concentrations of monoamines were not significantly different between the vehicle- and EGb 761-treated groups, as listed in Table 1. A single dose of EGb 761 (100 mg·kg−1 p.o.) following 2 weeks daily pretreatment with EGb 761 (100 mg·kg−1 p.o.) now significantly increased extracellular dopamine levels, within 40–180 min, reaching a maximal value at 140 min after the dose (Figure 3). The noradrenaline levels increased to a lesser extent within 80–180 min, with maximal value reached at 120 min, whereas EGb 761 showed no significant effect on concentrations of extracellular 5-HT. There was a significant difference between the groups both for the treatment [F(5,288)= 48.80; P < 0.0001] and the time points [F(12,288)= 21.42; P < 0.0001]. The time courses for each monoamine following chronic EGb 761 administration are shown in Figure 3.
Figure 3.
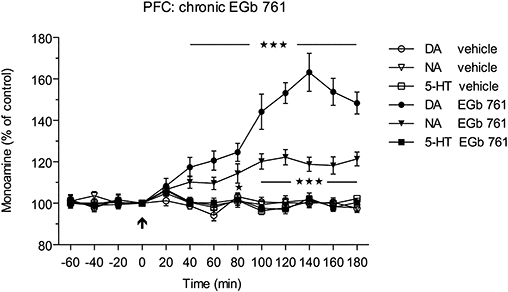
Effects of sub-chronic administration of EGb 761 (100 mg·kg−1 p.o. for 14 days, once daily) on extracellular levels of dopamine (DA), noradrenaline (NA) and 5-HT in the rat prefrontal cortex (PFC). Administration of a single dose of EGb 761 (100 mg·kg−1 p.o.) following 2 weeks daily pretreatment with EGb 761 caused a significant increase in extracellular dopamine levels within 40–180 min (***P < 0.001), whereas the NA levels increased to a lesser extent (*P < 0.05; ***P < 0.001) within 80–180 min after the single dose of EGb 761.
In the next experiment, separate groups of rats were treated for 14 days with EGb 761 at three doses (30, 100 and 300 mg·kg−1 p.o. once daily), and following the last EGb challenge on day 14, the dopamine, DOPAC and HVA levels in the microdialysates were determined by the use of HPLC with electrochemical detection. Basal dopamine levels in this group did not significantly differ from those receiving only single vehicle, or EGb 761 doses, whether measured by HPLC with electrochemical detection or HPLC with fluorescence detection (Table 1). Further, this experiment confirmed the previous results showing the significant [F(3,192)= 50.43; P < 0.0001] increase in extracellular dopamine levels that lasted for at least 3 h after the EGb 761 dose (Figure 4A). Interestingly, in spite of increased dopamine levels, the concentrations of metabolites DOPAC and HVA were unchanged even following sub-chronic treatment with EGb 761 at the highest dose (data not shown). The low dose (30 mg·kg−1) of EGb 761 extract had no effect on basal extracellular levels of dopamine, whereas both 100 mg·kg−1 and 300 mg·kg−1 EGb 761 doses caused marked and rapid increases in extracellular levels of dopamine from 40 and 60 min respectively. Following the 100 mg·kg−1 treatment, the dopamine levels gradually increased to a maximal value at 160 min, whereas the dose of 300 mg·kg−1 caused a faster and sharper increase to a maximal value at 100 min. The overall effect expressed as relative AUC(0–180 min) values for dopamine following vehicle, acute and sub-chronic treatments with three doses of EGb 761 is shown in Figure 4B. As seen, chronic EGb 761 caused dose-dependent increases in AUCs for the 100 mg·kg−1 and 300 mg·kg−1 EGb 761 doses.
Figure 4.
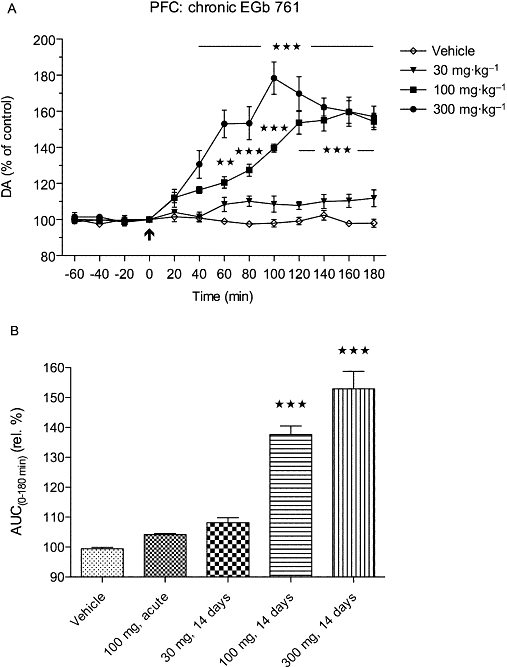
Sub-chronic (14 days) treatment with EGb 761 at three different doses caused dose-dependent and long-lasting increases in the extracellular dopamine (DA) levels in the rat prefrontal cortex as shown in (A) the upper panel for the time courses (**P < 0.01; ***P < 0.001) and (B) the lower panel summarizing the relative (rel.) area under the curve (AUC) values. The AUCs were significantly higher (***P < 0.001) for the 100 and 300 mg·kg−1 doses compared with the vehicle-treated group.
Effects of sub-chronic administration of EGb 761 on dopamine in the rat striatum
The basal concentration (expressed in 10−10 M) of dopamine in the dialysates from the striatum of conscious rats was 45.8 ± 5.5 (mean ± SEM, n= 5; vehicle-treated group). Following repeated (14 days) oral administration of 100 mg·kg−1 and 300 mg·kg−1 of EGb 761, the corresponding basal levels of dopamine were 47.9 ± 6.3 and 48.8 ± 7.1 respectively. A single dose of 100 mg·kg−1 given to rats treated with this dose for 14 days caused only a minor transient increase in dopamine levels at 80 min and at 100 min (P < 0.01), whereas a single dose of 300 mg·kg−1 following 14 days administration of EGb 761 at this dose caused a significant [F(2,156)= 15.27; P < 0.001] increase in extracellular dopamine levels, reaching a maximal value at 120 min after administration (Figure 5). The corresponding AUC(0–180 min) values for the lower and higher doses were 107.4 ± 2.4% and 119.6 ± 3.6% (P < 0.001 compared with the vehicle-treated group, and P < 0.01 compared with the 100 mg·kg−1-treated group), respectively, suggesting a dose-dependent effect.
Figure 5.
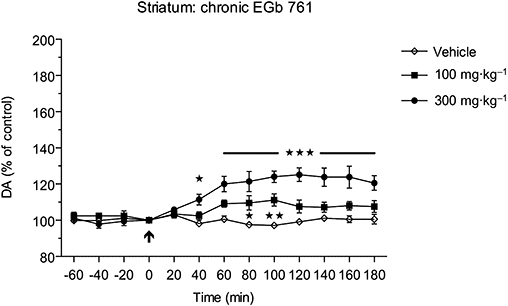
Sub-chronic (14 days) treatment with EGb 761 at doses of 100 and 300 mg·kg−1 p.o., respectively, caused only minor, but yet significant, (*P < 0.05; **P < 0.01; ***P < 0.001) increases in extracellular dopamine (DA) levels in the striatum of awake rats.
Effects of sub-chronic administration of EGb 761 constituents on dopamine levels in the rat PFC
The basal concentrations of dopamine in the dialysates from the PFC of conscious rats treated with EGb 761 constituents are shown in Table 1. Repeated (14 days) administration of flavonol glycosides (flavanoids) caused a significant [F(3,192)= 49.17; P < 0.001] increase in extracellular levels of dopamine starting at 40 min and increasing to a maximal value at 120 min compared with the vehicle-treated group (Figure 6). Correspondingly, repeated administration of ginkgolides caused only a moderate but significant increase in dopamine levels within 100–180 min, reaching a maximal value at 140 min, and treatment with bilobalide extract had no effect on extracellular levels of dopamine. The calculated AUC(0–180 min) values for bilobalide, ginkgolide and flavonoid groups compared with the vehicle-treaded group were 102.8 ± 0.7%, 110.1 ± 1.0% and 127.3 ± 3.0% respectively. Thus, the relative AUC(0–180 min) calculated as the sum of the increments of all three constituents was 40.2% above the AUC of the vehicle-treated group, which can be compared with the corresponding value of 52.9% observed for the treatment with 300 mg·kg−1 of the EGb 761 extract (shown in Figure 4B).
Figure 6.
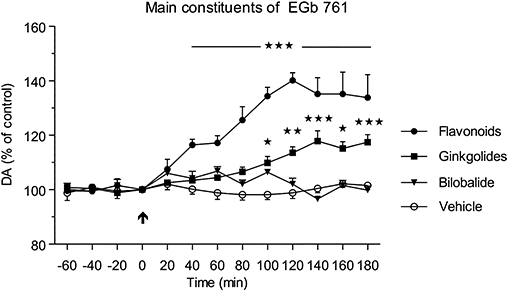
Effects of sub-chronic administration of flavonol glycosides, ginkgolides or bilobalide, the major constituents of the EGb 761 extract, on extracellular levels of dopamine (DA) in the rat prefrontal cortex. Administration of flavonol glycosides (flavanoids; 72 mg·kg−1 p.o.) following 2 weeks daily treatment caused a significant increase in extracellular DA levels within 40–180 min (***P < 0.001), whereas ginkgolides increased the DA levels to a lesser extent (*P < 0.05; **P < 0.01; ***P < 0.001) within 100–180 min after the administration.
Discussion
The purpose of the present study was to examine the effects of acute and chronic treatment with the extract of G. biloba (EGb 761) on the extracellular levels of the monoamines dopamine, noradrenaline and 5-HT in the PFC of conscious rats. In addition, the three main constituents of the extract, flavonol glycosides, ginkgolides and bilobalide, were tested in order to evaluate their role in mediating the effects of repeated administration of the EGb 761 extract on dopamine signalling in the rat PFC. The rationale for monitoring the levels of monoamines was based on the earlier studies suggesting that G. biloba reduced the activity of both MAO-A and MAO-B forms in the rat (White et al., 1996) and mouse (Wu and Zhu, 1999; Pardon et al., 2000) brains. We have applied the microdialysis technique in combination with highly sensitive liquid chromatographic methods for determination of the extracellular levels of dopamine, noradrenaline and 5-HT, as well as the acidic metabolites DOPAC and HVA, in the PFC of conscious rats. A single oral administration of EGb 761 had no effect on the levels of basal extracellular monoamines and metabolites. Likewise, an acute dose of G. biloba extract had no effect on brain tissue levels of catecholamines or 5-HT (Shah et al., 2003). However, the present data show that a single dose of EGb 761, following a sub-chronic daily treatment with the same dose for 14 days, caused a marked elevation of extracellular levels of dopamine and a moderate increase in noradrenaline levels, but had no effect on extracellular 5-HT values in the rat PFC. Interestingly, the levels of DOPAC and HVA were not affected by any dose and treatment. In the subsequent studies, the sub-chronic administration of EGb 761 at three different doses revealed a dose-dependent and long-lasting (>3 h) effect on increasing the dopamine levels in the rat PFC and, to a lesser extent, in the striatum. The finding that striatal dopamine levels increased only marginally is in good agreement with our behavioural observations (Yoshitake et al., 2004a), where no significant increases in locomotor activity were recorded. The EGb 761 doses of 100 and 300 mg·kg−1 used in this study are considered to be relevant to the currently recommended clinical doses of 240 mg·kg−1 daily (Schwabe, Tebonin konzent, Fachinformation, 2008).
A major finding of the study is that the sub-chronic treatment with EGb 761 preferentially increased extracellular dopamine in the PFC of conscious rats. In addition, the same treatment with the three main constituents of EGb 761, at the doses corresponding to their respective contents in the EGb 761 extract, revealed that flavonol glycosides and, to a lesser extent, the ginkgolide fractions were the main components contributing to the observed effects of the EGb 761 extract on the dopamine levels. The sum of the overall effects of the three major components on the increase of dopamine levels was comparable with that of chronic treatment with the corresponding dose of the whole EGb 761 extract. This finding indicates that the effects of the EGb 761 extract, at least those observed for the dopamine levels in the PFC, are simply additive, that is, they reflect a sum of contributions of active constituents, rather than a synergistic potentiation effect, triggered by the individual components, or a ‘polyvalent’ action as often proposed for the mechanisms of action of herbal extracts, including EGb 761 (DeFeudis and Drieu, 2000).
Several studies have indicated that G. biloba extracts may affect the brain dopamine system and dopamine-mediated functions (Shah et al., 2003; Szasz et al., 2008; Yeh et al., 2008; Fehske et al., 2009). However, this is the first report linking the effects of EGb 761 to the PFC, that is, the brain area that is neuroanatomically relevant to learning and memory processing. Previous reports have suggested that G. biloba extracts could increase monoaminergic function via inhibition of MAO activity (White et al., 1996; Wu and Zhu, 1999; Pardon et al., 2000). However, chronic (14 days) daily treatment with EGb 761 (100 mg·kg−1, p.o.) had no effect on MAO-A or MAO-B activities in the homogenates of mice brains (Fehske et al., 2009). Our findings on extracellular levels of monoamines and metabolites following repeated treatment with EGb 761 support the conclusions of the latter study.
Thus, chronic, but not acute, administration of EGb 761 increased dopamine levels. However, the concentrations of noradrenaline were increased only marginally, while the levels of 5-HT remained unaltered, and there was no effect on the acidic metabolites DOPAC and HVA. This differs from the effects of MAO inhibitors, which increase extracellular levels of all three monoamines and decrease the levels of their respective metabolites, even after a single injection. For example, in our previous study (Yoshitake et al., 2004a), we have shown that systemic administration of MAO-A/B inhibitor phenelzine (5 mg·kg−1 i.p.) caused a gradual increase in extracellular dopamine, noradrenaline and 5-HT levels, which at 120 min reached peak values of 171, 121 and 140% of the pre-drug levels respectively. The corresponding extracellular levels of DOPAC and 5-hydroxyindoleacetic acid (5-HIAA) decreased to 41 and 83% respectively (Yoshitake et al., 2004a). These findings are in agreement with an earlier study showing that chronic treatment with the selective MAO-A inhibitor, clorgyline, (1 mg·kg−1) or MAO-B inhibitor [(-)-selegiline, 10 mg·kg−1] produced sustained elevations of concentrations of dopamine and 5-HT, and decreased their deaminated metabolites in the rat forebrain tissue (Yeghiayan et al., 1997). Thus, we postulate that the EGb 761 extract affects brain dopaminergic systems through other more specific and treatment time-dependent mechanisms than inhibiting the MAO or COMT activities.
A possible explanation for the effects exerted by chronic EGb 761 administration could involve desensitization or down-regulation of receptors modulating dopamine and noradrenaline release in the mesocortical and mesolimbic structures. For example, it was shown that chronic but not acute treatment with sertraline caused an increase in the noradrenaline levels in the frontal cortex but not in the hippocampus of rats, most likely as a consequence of desensitization of cortical α2-adrenoceptors (Thomas et al., 1998). In addition, blockade of α2-adrenoceptors with the α2-antagonist idazoxan was shown to preferentially increase extracellular dopamine levels in the PFC (Hertel et al., 1999) and potentiate the effects of venlafaxine on dopamine and noradrenaline levels (Weikop et al., 2004). In addition, modulation of other receptors such as 5-HT or muscarinic acetylcholine receptors by chronic EGb 761 treatment may also account for the increased extracellular dopamine levels in the rat PFC. There is evidence that exists for the role of 5-HT2A/2C antagonists or inverse agonists, 5-HT6 or 5-HT7 receptor antagonists (see Meltzer and Huang, 2008), 5-HT1A and muscarinic M1 receptor agonists (Li et al., 2009) in enhancing cortical dopamine transmission, particularly when given in combination with typical antipsychotic drugs such as haloperidol, as well as atypical antipsychotic drugs, which possess intrinsic affinities for these and other receptor subtypes. Some data are available on the effects of EGb 761 and its main constituents on binding affinity to central neurotransmitter receptors. Thus, the G. biloba extract reduced [3H]ketanserin binding to 5-HT2A receptors in the frontal cortex of MAO-A knockout mice (Shin et al., 2000). Chronic treatment with EGb 761 increased binding (Bmax values) to muscarinic acetylcholine receptors (Taylor, 1986) and α2-adrenoceptors (Huguet and Tarrade, 1992) in the hippocampal membranes, and 5-HT1A receptors (Huguet et al., 1994) in cortical membranes of aged, but not young, rats. Administration of EGb 761 to adult rats increased noradrenaline turnover in the cerebral cortex only after sub-chronic treatment (Brunello et al., 1985). More recent findings demonstrate that ginkgolides, active constituents of G. biloba, are effective blockers of the glycine receptor pore (Chatterjee et al., 2003; Heads et al., 2008) and recombinant human GABAA receptors (Huang et al., 2004). However, it is difficult to predict the functional outcome of these effects on behaviour as blockers of glycine and GABAA receptors are typically proconvulsant. Taken together, although several reports indicate that EGb 761 and its constituents interact with seven-transmembrane receptors and transmitter-gated ion channel receptors, these data are inconclusive and support only a tentative explanation for the findings of this study.
The monoamines noradrenaline, 5-HT and, possibly, dopamine are traditionally implicated in the aetiology of depression and related mood disorders, whereas the progressive impairment of learning and memory functions, observed in Alzheimer's disease, is strongly associated with the atrophy and loss of cholinergic neurons in the basal forebrain (Bartus et al., 1982; Coyle et al., 1983; Terry and Buccafusco, 2003). However, an increasing body of evidence exists for the role of the non-cholinergic neurotransmitter systems in Alzheimer's disease pathology (Terry, 1994; Francis, 1996; Dringenberg, 2000). Thus, the dysfunction of cortical and hippocampal glutamatergic and GABAergic networks, and the major afferent systems such as 5-hydroxytryptaminergic, noradrenergic and histaminergic systems has been implicated in the neuropathology of Alzheimer's disease and appears to affect the majority of patients with this condition (Cross et al., 1981; Bowen and Davison, 1986; Rossor and Lversen, 1986; Baker and Reynolds, 1989; Cross, 1990). A body of evidence exists for the role of prefrontal cortical dopamine and dopamine receptors in modulating the neurons essential for working memory (Goldman-Rakic, 1995; Goldman-Rakic et al., 2000; Castner and Goldman-Rakic, 2004; Paspalas and Goldman-Rakic, 2005). Thus, cognitive deficits have been observed experimentally in rhesus monkeys following dopamine depletion in the PFC (Brozoski et al., 1979; Williams and Goldman-Rakic, 1995), as well as following modulation of dopamine D1 and D2 receptors in humans (Müller et al., 1998). In addition, a microdialysis study in freely moving rats during a spatial working memory task provided direct evidence that both noradrenaline and dopamine were increased in the PFC (Rossetti and Carboni, 2005). The dopamine increase was primarily attributed to reward expectancy in tasks involving the memory-guided search for food, whereas the noradrenaline increase was more likely associated with the attention necessary for behavioural activation during the task.
These reports together with the recent findings on the stimulatory effects of EGb 761 on dopamine and noradrenaline transmission in the rat PFC may provide a working hypothesis for the mechanism of action of EGb 761 in relation to improved cognitive performance in aged and healthy people. Recently, a placebo-controlled, double-blind study conducted on 177 test persons between the ages of 45 years and 60 years, and receiving a daily dose of 240 mg Ginkgo extract for 6 weeks revealed a significant twofold improvement in so-called implied learning (the ability needed in everyday situations to quickly reach a correct solution from the new information and to learn from this). The retention capacity (e.g. remembering dates after a 45 min diversion) increased in the EGb-treated group by 26%, but not in the placebo group (Kaschel, 2007). However, the therapeutic efficacy of G. biloba extracts in reducing the incidence of cognitive decline was not confirmed in a recent clinical study (DeKosky et al., 2008).
In summary, the present data demonstrate for the first time that repeated administration of G. biloba extract EGb 761 results in increased dopaminergic and noradrenergic transmission in the frontocortical brain areas, which may be one of the underlying mechanisms behind the clinically observed effects of G. biloba on improved cognitive function. In addition, G. biloba extracts, besides their reported neuroprotective effects and improved memory function, may possess mood/motivation-enhancing properties in disorders associated with abnormal monoaminergic and, in particular, dopaminergic function.
Acknowledgments
The study was supported by Willmar-Schwabe GmbH & Co, Karlsruhe, Germany. The authors thank Dr M Nöldner for the kind supply of G. biloba EGb 761 extracts; the purified fractions containing flavonol glycosides, ginkgolides and bilobalide; and for the valuable comments to the manuscript.
Glossary
Abbreviations:
- CMC
carboxymethylcellulose
- DOPAC
3,4-dihydroxyphenylacetic acid
- HPLC
high-performance liquid chromatography
- HVA
homovanillic acid
- MAO
monoamine oxidase
- PFC
prefrontal cortex
Conflict of interest
The authors declare no conflict of interest.
References
- Abdel-Kader R, Hauptmann S, Keil U, Scherping I, Leuner K, Eckert A, et al. Stabilization of mitochondrial function by Ginkgo biloba extract (EGb 761) Pharmacol Res. 2007;56:493–502. doi: 10.1016/j.phrs.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Ahlemeyer B, Krieglstein J. Neuroprotective effects of Ginkgo biloba extract. Cell Mol Life Sci. 2003;60:1779–1792. doi: 10.1007/s00018-003-3080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad M, Saleem S, Ahmad AS, Yousuf S, Ansari MA, Khan MB, et al. Ginkgo biloba affords dose-dependent protection against 6-hydroxydopamine-induced parkinsonism in rats: neurobehavioural, neurochemical and immunohistochemical evidences. J Neurochem. 2005;93:94–104. doi: 10.1111/j.1471-4159.2005.03000.x. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 3rd edition. Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrieu S, Gillette S, Amouyal K, Nourhashemi F, Reynish E, Ousset PJ, et al. EPIDOS study. Association of Alzheimer's disease onset with Ginkgo biloba and other symptomatic cognitive treatments in a population of women aged 75 years and older from the EPIDOS study. J Gerontol A Biol Sci Med Sci. 2003;58:372–377. doi: 10.1093/gerona/58.4.m372. [DOI] [PubMed] [Google Scholar]
- Baker GB, Reynolds GP. Biogenic amines and their metabolites in Alzheimer's disease: noradrenaline, 5-hydroxytryptamine and 5-hydroxyindole-3-acetic acid depleted in hippocampus but not in substantia innominata. Neurosci Lett. 1989;100:335–339. doi: 10.1016/0304-3940(89)90709-x. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Dean RL, 3rd, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- Birks J, Grimley Evans J. Ginkgo biloba for cognitive impairment and dementia. Cochrane Database Syst Rev. 2007;2:CD003120. doi: 10.1002/14651858.CD003120.pub2. [DOI] [PubMed] [Google Scholar]
- Blecharz-Klin K, Piechal A, Joniec I, Pyrzanowska J, Widy-Tyszkiewicz E. Pharmacological and biochemical effects of Ginkgo biloba extract on learning, memory consolidation and motor activity in old rats. Acta Neurobiol Exp (Wars) 2009;69:217–231. doi: 10.55782/ane-2009-1747. [DOI] [PubMed] [Google Scholar]
- Bowen DM, Davison AN. Biochemical studies of nerve cells and energy metabolism in Alzheimer's disease. Br Med Bull. 1986;42:75–80. doi: 10.1093/oxfordjournals.bmb.a072102. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–932. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- Brunello N, Racagni G, Clostre F, Drieu K, Braquet P. Effects of an extract of Ginkgo biloba on noradrenergic systems of rat cerebral cortex. Pharmacol Res Commun. 1985;17:1063–1072. doi: 10.1016/0031-6989(85)90112-2. [DOI] [PubMed] [Google Scholar]
- Castner SA, Goldman-Rakic PS. Enhancement of working memory in aged monkeys by a sensitizing regimen of dopamine D1 receptor stimulation. J Neurosci. 2004;24:1446–1450. doi: 10.1523/JNEUROSCI.3987-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan PC, Xia Q, Fu PP. Ginkgo biloba leave extract: biological, medicinal, and toxicological effects. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2007;25:211–244. doi: 10.1080/10590500701569414. [DOI] [PubMed] [Google Scholar]
- Chatterjee SS, Kondratskaya EL, Krishtal OA. Structure-activity studies with Ginkgo biloba extract constituents as receptor-gated chloride channel blockers and modulators. Pharmacopsychiatry. 2003;36(Suppl. 1):S68–S77. doi: 10.1055/s-2003-40455. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Price DL, DeLong MR. Alzheimer's disease: a disorder of cortical cholinergic innervation. Science. 1983;219:1184–1190. doi: 10.1126/science.6338589. [DOI] [PubMed] [Google Scholar]
- Cross AJ. Serotonin in Alzheimer-type dementia and other dementing illnesses. Ann N Y Acad Sci. 1990;600:405–415. doi: 10.1111/j.1749-6632.1990.tb16897.x. [DOI] [PubMed] [Google Scholar]
- Cross AJ, Crow TJ, Perry EK, Perry RH, Blessed G, Tomlinson BE. Reduced dopamine-beta-hydroxylase activity in Alzheimer's disease. Br Med J. 1981;282:93–94. doi: 10.1136/bmj.282.6258.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFeudis FV. A brief history of EGb 761 and its therapeutic uses. Pharmacopsychiatry. 2003;36(Suppl. 1):S2–S7. doi: 10.1055/s-2003-40450. [DOI] [PubMed] [Google Scholar]
- DeFeudis FV, Drieu K. Ginkgo biloba extract (EGb 761) and CNS functions: basic studies and clinical applications. Curr Drug Targets. 2000;1:25–58. doi: 10.2174/1389450003349380. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Furberg CD. Turning over a new leaf: Ginkgo biloba in prevention of dementia? Neurology. 2008;70:1730–1731. doi: 10.1212/01.wnl.0000311449.76944.6b. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Williamson JD, Fitzpatrick AL, Kronmal RA, Ives DG, Saxton JA, et al. Ginkgo biloba for prevention of dementia. A randomized controlled trial. JAMA. 2008;300:2253–2262. doi: 10.1001/jama.2008.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge HH, Zitzelberger T, Oken BS, Howieson D, Kaye J. A randomized placebo-controlled trial of Ginkgo biloba for the prevention of cognitive decline. Neurology. 2008;70:1809–1817. doi: 10.1212/01.wnl.0000303814.13509.db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringenberg HC. Alzheimer's disease: more than a ‘cholinergic disorder’– evidence that cholinergic-monoaminergic interactions contribute to EEG slowing and dementia. Behav Brain Res. 2000;115:235–249. doi: 10.1016/s0166-4328(00)00261-8. [DOI] [PubMed] [Google Scholar]
- Fehske CJ, Leuner K, Müller WE. Ginkgo biloba extract (EGb761) influences monoaminergic neurotransmission via inhibition of NE uptake, but not MAO activity after chronic treatment. Pharmacol Res. 2009;60:68–73. doi: 10.1016/j.phrs.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Francis PT. Pyramidal neurone modulation: a therapeutic target for Alzheimer's disease. Neurodegeneration. 1996;5:461–465. doi: 10.1006/neur.1996.0063. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Muly EC, 3rd, Williams GV. D(1) receptors in prefrontal cells and circuits. Brain Res Brain Res Rev. 2000;31:295–301. doi: 10.1016/s0165-0173(99)00045-4. [DOI] [PubMed] [Google Scholar]
- Heads JA, Hawthorne RL, Lynagh T, Lynch JW. Structure-activity analysis of ginkgolide binding in the glycine receptor pore. J Neurochem. 2008;105:1418–1427. doi: 10.1111/j.1471-4159.2008.05244.x. [DOI] [PubMed] [Google Scholar]
- Hertel P, Nomikos GG, Svensson TH. Idazoxan preferentially increases dopamine output in the rat medial prefrontal cortex at the nerve terminal level. Eur J Pharmacol. 1999;371:153–158. doi: 10.1016/s0014-2999(99)00175-2. [DOI] [PubMed] [Google Scholar]
- Huang SH, Duke RK, Chebib M, Sasaki K, Wada K, Johnston GA. Ginkgolides, diterpene trilactones of Ginkgo biloba, as antagonists at recombinant alpha1beta2gamma2L GABAA receptors. Eur J Pharmacol. 2004;494:131–138. doi: 10.1016/j.ejphar.2004.04.051. [DOI] [PubMed] [Google Scholar]
- Huguet F, Drieu K, Piriou A. Decreased cerebral 5-HT1A receptors during ageing: reversal by Ginkgo biloba extract (EGb 761) J Pharm Pharmacol. 1994;46:316–318. doi: 10.1111/j.2042-7158.1994.tb03802.x. [DOI] [PubMed] [Google Scholar]
- Huguet F, Tarrade T. Alpha 2-adrenoceptor changes during cerebral ageing. The effect of Ginkgo biloba extract. J Pharm Pharmacol. 1992;44:24–27. doi: 10.1111/j.2042-7158.1992.tb14357.x. [DOI] [PubMed] [Google Scholar]
- Kanowski S, Hoerr R. Ginkgo biloba extract EGb 761 in dementia: intent-to-treat analyses of a 24-week, multi-center, double-blind, placebo-controlled, randomized trial. Pharmacopsychiatry. 2003;36:297–303. doi: 10.1055/s-2003-45117. [DOI] [PubMed] [Google Scholar]
- Kaschel R. Specific memory effects of Ginkgo biloba extract EGb 761® in middle aged healthy volunteers. 2007. Data presented at the 14th Annual Symposium on Complementary Health Care, Exeter, 11–13 December 2007. [DOI] [PubMed]
- Kehr J. Monitoring chemistry of brain microenvironment: biosensors, microdialysis and related techniques. In: Windhorst U, Johansson H, editors. Modern Techniques in Neuroscience Research. Heidelberg, Germany: Springer-Verlag GmbH; 1999. pp. 1149–1198. Chapter 41. In. [Google Scholar]
- Kehr J, Yoshitake T. Monitoring brain chemical signals by microdialysis. In: Grimes CA, Dickey EC, Pishko MV, editors. Encyclopedia of Sensors. Valencia, CA: American Scientific Publishers; 2006. pp. 287–312. Vol. 6. [Google Scholar]
- Li Z, Prus AJ, Dai J, Meltzer HY. Differential effects of M1 and 5-hydroxytryptamine1A receptors on atypical antipsychotic drug-induced dopamine efflux in the medial prefrontal cortex. J Pharmacol Exp Ther. 2009;330:948–955. doi: 10.1124/jpet.109.155663. [DOI] [PubMed] [Google Scholar]
- Mazza M, Capuano A, Bria P, Mazza S. Ginkgo biloba and donepezil: a comparison in the treatment of Alzheimer's dementia in a randomized placebo-controlled double-blind study. Eur J Neurol. 2006;13:981–985. doi: 10.1111/j.1468-1331.2006.01409.x. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Riedel WJ. Dopaminergic enhancement of cognitive function. Curr Pharm Des. 2006;12:2487–2500. doi: 10.2174/138161206777698891. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Huang M. In vivo actions of atypical antipsychotic drug on serotonergic and dopaminergic systems. Prog Brain Res. 2008;172:177–197. doi: 10.1016/S0079-6123(08)00909-6. [DOI] [PubMed] [Google Scholar]
- Müller U, von Cramon DY, Pollmann S. D1- versus D2-receptor modulation of visuospatial working memory in humans. J Neurosci. 1998;18:2720–2728. doi: 10.1523/JNEUROSCI.18-07-02720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napryeyenko O, Borzenko I, GINDEM-NP Study Group Ginkgo biloba special extract in dementia with neuropsychiatric features. A randomised, placebo-controlled, double-blind clinical trial. Arzneimittelforschung. 2007;57:4–11. doi: 10.1055/s-0031-1296579. [DOI] [PubMed] [Google Scholar]
- Osborne PG, O'Conner WT, Kehr J, Ungerstedt U. In vivo characterisation of extracellular dopamine, GABA, and acetylcholine from the dorsolateral striatum of conscious freely-moving rats by chronic microdialysis. J Neurosci Methods. 1990;37:93–102. doi: 10.1016/0165-0270(91)90119-k. [DOI] [PubMed] [Google Scholar]
- Pardon MC, Joubert C, Perez-Diaz F, Christen Y, Launay JM, Cohen-Salmon C. In vivo regulation of cerebral monoamine oxidase activity in senescent controls and chronically stressed mice by long-term treatment with Ginkgo biloba extract (EGb 761) Mech Ageing Dev. 2000;113:157–168. doi: 10.1016/s0047-6374(99)00107-4. [DOI] [PubMed] [Google Scholar]
- Paspalas CD, Goldman-Rakic PS. Presynaptic D1 dopamine receptors in primate prefrontal cortex: target-specific expression in the glutamatergic synapse. J Neurosci. 2005;25:1260–1267. doi: 10.1523/JNEUROSCI.3436-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Sterotaxic Coordinates. Sydney, Australia: Academic Press; 1997. [Google Scholar]
- Phillips AG, Vacca G, Ahn S. A top-down perspective on dopamine, motivation and memory. Pharmacol Biochem Behav. 2008;90:236–249. doi: 10.1016/j.pbb.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Rojas P, Serrano-García N, Mares-Sámano JJ, Medina-Campos ON, Pedraza-Chaverri J, Ogren SO. EGb761 protects against nigrostriatal dopaminergic neurotoxicity in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinsonism in mice: role of oxidative stress. Eur J Neurosci. 2008;28:41–50. doi: 10.1111/j.1460-9568.2008.06314.x. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Carboni S. Noradrenaline and dopamine elevations in the rat prefrontal cortex in spatial working memory. J Neurosci. 2005;25:2322–2329. doi: 10.1523/JNEUROSCI.3038-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossor M, Lversen LL. Non-cholinergic neurotransmitter abnormalities in Alzheimer's disease. Br Med Bull. 1986;42:70–74. doi: 10.1093/oxfordjournals.bmb.a072101. [DOI] [PubMed] [Google Scholar]
- Satvat E, Mallet PE. Chronic administration of a Ginkgo biloba leaf extract facilitates acquisition but not performance of a working memory task. Psychopharmacology (Berl) 2009;202:173–185. doi: 10.1007/s00213-008-1223-7. [DOI] [PubMed] [Google Scholar]
- Scripnikov A, Khomenko A, Napryeyenko O, GINDEM-NP Study Group Effects of Ginkgo biloba extract EGb 761 on neuropsychiatric symptoms of dementia: findings from a randomised controlled trial. Wien Med Wochenschr. 2007;157:295–300. doi: 10.1007/s10354-007-0427-5. [DOI] [PubMed] [Google Scholar]
- Shah ZA, Sharma P, Vohora SB. Ginkgo biloba normalises stress-elevated alterations in brain catecholamines, serotonin and plasma corticosterone levels. Eur Neuropsychopharmacol. 2003;13:321–325. doi: 10.1016/s0924-977x(03)00005-1. [DOI] [PubMed] [Google Scholar]
- Shin JC, Chen K, Ridd MJ, Seif I. Ginkgo biloba abolishes aggression in mice lacking MAO A. Antioxid Redox Signal. 2000;2:467–471. doi: 10.1089/15230860050192242. [DOI] [PubMed] [Google Scholar]
- Stackman RW, Eckenstein F, Frei B, Kulhanek D, Nowlin J, Quinn JF. Prevention of age-related spatial memory deficits in a transgenic mouse model of Alzheimer's disease by chronic Ginkgo biloba treatment. Exp Neurol. 2003;184:510–520. doi: 10.1016/s0014-4886(03)00399-6. [DOI] [PubMed] [Google Scholar]
- Szasz BK, Lenkey N, Barth AM, Mike A, Somogyvari Z, Farkas O, et al. Converging effects of Ginkgo biloba extract at the level of transmitter release, NMDA and sodium currents and dendritic spikes. Planta Med. 2008;74:1235–1239. doi: 10.1055/s-2008-1081292. [DOI] [PubMed] [Google Scholar]
- Taylor JE. Neuromediator binding to receptors in the rat brain. The effect of chronic administration of Ginkgo biloba extract. (French) Presse Med. 1986;15:1491–1493. [PubMed] [Google Scholar]
- Terry AV, Buccafusco JJ. The cholinergic hypothesis of age and Alzheimer's disease-related cognitive deficits: recent challenges and their implications for novel drug development. J Pharmacol Exp Ther. 2003;306:821–827. doi: 10.1124/jpet.102.041616. [DOI] [PubMed] [Google Scholar]
- Terry RD. Neuropathological changes in Alzheimer disease. Prog Brain Res. 1994;101:383–390. doi: 10.1016/s0079-6123(08)61964-0. [DOI] [PubMed] [Google Scholar]
- Thomas DN, Nutt DJ, Holman RB. Sertraline, a selective serotonin reuptake inhibitor modulates extracellular noradrenaline in the rat frontal cortex. J Psychopharmacol. 1998;12:366–370. doi: 10.1177/026988119801200406. [DOI] [PubMed] [Google Scholar]
- Vellas B, Andrieu S, Ousset PJ, Ouzid M, Mathiex-Fortunet H, GuidAge Study Group The GuidAge study: methodological issues. A 5-year double-blind randomized trial of the efficacy of EGb 761 for prevention of Alzheimer disease in patients over 70 with a memory complaint. Neurology. 2006;67(Suppl. 3):S6–S11. doi: 10.1212/wnl.67.9_suppl_3.s6. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang L, Wu J, Cai J. The in vivo synaptic plasticity mechanism of EGb 761-induced enhancement of spatial learning and memory in aged rats. Br J Pharmacol. 2006;148:147–153. doi: 10.1038/sj.bjp.0706720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weikop P, Kehr J, Scheel-Krüger J. The role of alpha1- and alpha2-adrenoreceptors on venlafaxine-induced elevation of extracellular serotonin, noradrenaline and dopamine levels in the rat prefrontal cortex and hippocampus. J Psychopharmacol. 2004;18:395–403. doi: 10.1177/026988110401800311. [DOI] [PubMed] [Google Scholar]
- White HL, Scates PW, Cooper BR. Extracts of Ginkgo biloba leaves inhibit monoamine oxidase. Life Sci. 1996;58:1315–1321. doi: 10.1016/0024-3205(96)00097-5. [DOI] [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- Wu WR, Zhu XZ. Involvement of monoamine oxidase inhibition in neuroprotective and neurorestorative effects of Ginkgo biloba extract against MPTP-induced nigrostriatal dopaminergic toxicity in C57 mice. Life Sci. 1999;65:157–164. doi: 10.1016/s0024-3205(99)00232-5. [DOI] [PubMed] [Google Scholar]
- Yeghiayan SK, Andersen SL, Baldessarini RJ. Lack of effect of chronic clorgyline or selegiline on dopamine and serotonin transporters in rat caudate-putamen or nucleus accumbens septi. Neurosci Lett. 1997;236:147–150. doi: 10.1016/s0304-3940(97)00777-5. [DOI] [PubMed] [Google Scholar]
- Yeh KY, Pu HF, Kaphle K, Lin SF, Wu LS, Lin JH, et al. Ginkgo biloba extract enhances male copulatory behavior and reduces serum prolactin levels in rats. Horm Behav. 2008;53:225–231. doi: 10.1016/j.yhbeh.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Yoshitake T, Kehr J, Yoshitake S, Fujino K, Nohta H, Yamaguchi M. Determination of serotonin, noradrenaline, dopamine and their metabolites in rat brain extracts and microdialysis samples by column liquid chromatography with fluorescence detection following derivatization with benzylamine and 1,2-diphenylethylenediamine. J Chromatogr B. 2004a;807:177–183. doi: 10.1016/j.jchromb.2004.03.069. [DOI] [PubMed] [Google Scholar]
- Yoshitake T, Yoshitake S, Fujino K, Nohta H, Yamaguchi M, Kehr J. High-sensitive liquid chromatographic method for determination of neuronal release of serotonin, noradrenaline and dopamine monitored by microdialysis in the rat prefrontal cortex. J Neurosci Methods. 2004b;140:163–168. doi: 10.1016/j.jneumeth.2004.04.041. [DOI] [PubMed] [Google Scholar]


