Abstract
Background and purpose:
Angiotensin type 2 receptor (AT2 receptor) stimulation evokes vasodilator effects in vitro and in vivo that oppose the vasoconstrictor effects of angiotensin type 1 receptors (AT1 receptors). Recently, a novel non-peptide AT2 receptor agonist, Compound 21, was described, which exhibited high AT2 receptor selectivity.
Experimental approach:
Functional cardiovascular effects of the drug candidate Compound 21 were assessed, using mouse isolated aorta and rat mesenteric arteries in vitro and in conscious spontaneously hypertensive rats (SHR).
Key results:
Compound 21 evoked dose-dependent vasorelaxations in aortic and mesenteric vessels, abolished by the AT2 receptor antagonist, PD123319. In vivo, Compound 21 administered alone, at doses ranging from 50 to 1000 ng·kg−1·min−1 over 4 h did not decrease blood pressure in conscious normotensive Wistar-Kyoto rats or SHR. However, when given in combination with the AT1 receptor antagonist, candesartan, Compound 21 (300 ng·kg−1·min−1) lowered blood pressure in SHR only. Further analysis in separate groups of conscious SHR revealed that, at a sixfold lower dose, Compound 21 (50 ng·kg−1·min−1) still evoked a significant depressor response in adult SHR (∼30 mmHg) when combined with different doses of candesartan (0.01 or 0.1 mg·kg−1). Moreover, the Compound 21-evoked depressor effect was abolished when co-infused (50 µg·kg−1·min−1 for 2 h) with the AT2 receptor antagonist PD123319.
Conclusion and implications:
Collectively, our results indicate that acute administration of Compound 21 evoked blood pressure reductions via AT2 receptor stimulation. Thus Compound 21 can be considered an excellent drug candidate for further study of AT2 receptor function in cardiovascular disease.
Keywords: Compound 21, angiotensin, angiotensin AT2 receptor, spontaneously hypertensive rats, blood pressure, vascular function
Introduction
The octapeptide angiotensin II (Ang II) is the main biologically active mediator of the renin-angiotensin system and plays an important role in cardiovascular function by influencing vascular tone, structure, fluid and electrolyte balance via direct effects on endothelial and smooth muscle cells (Widdop et al., 2003; Jones et al., 2008). Two main receptor subtypes have been identified as binding sites for Ang II: angiotensin type 1 receptor (AT1 recepter) and type 2 receptor (AT2 receptor) (de Gasparo et al., 2000); nomenclature follows Alexander et al., 2008). Ang II has similar affinity for both AT1 receptors and AT2 receptors, whereas CGP42112 and PD123319 are the prototypical examples of an agonist and antagonist, respectively, at the AT2 receptor subtype. On the other hand, compounds such as candesartan and losartan are selective AT1 receptor antagonists that are used clinically for the treatment of hypertension. It is well established that most of the cardiovascular effects induced by Ang II, such as vasoconstriction, water and salt retention, are mediated via AT1 receptor (de Gasparo et al., 2000; Carey, 2005). In contrast, it has been suggested that the function of the AT2 receptor is to counter-regulate AT1 receptor-mediated actions, mainly based on experiments in which AT2 receptor function was deduced from the effects of AT2 receptor blockade, or altered responses in genetically modified animal models of AT2 receptor overexpression or deletion. However, demonstration of AT2 receptor-mediated effects, particularly in an in vivo setting, has been hampered by a lack of non-peptide AT2 receptor selective agonists and antagonists that exhibit oral bioavailability.
In this context, Wan et al. (2004b) have recently described the first non-peptide, selective AT2 receptor agonist, Compound 21 (N-butyloxycarbonyl-3-(4—imidazol-1-ylmethylphenyl)-5-isobutylthiophene-2-sulphonamide). Compound 21 was derived from a medicinal chemistry programme aimed at transforming the drug-like but non-selective AT1 and AT2 receptor agonist L-162313 (Wan et al., 2004a) into a selective AT2 receptor agonist. Compound 21 exhibits a Ki value of 0.4 nM for the AT2 receptor and a Ki > 10 µM for the AT1 receptor. In addition, due to the non-peptide nature of the drug, Compound 21 has an estimated oral bioavailability of 20–30% and 4 h half-life in rats (Wan et al., 2004b). Compound 21 has been shown to induce neurite outgrowth in cell culture and to increase duodenal mucosal alkalinization in the rat via stimulation of MAPK and NO/cGMP signalling pathways (Wan et al., 2004b). Furthermore, Compound 21 decreased mean arterial blood pressure (MAP) in anaesthetized spontaneously hypertensive rats (SHR), although detailed and systematic evaluation of haemodynamic responses to Compound 21 were not performed in this earlier study.
AT2 receptor-mediated relaxation is a well-established effect in isolated resistance vessels (Matrougui et al., 1999; Dimitropoulou et al., 2001; Widdop et al., 2002); conversely, there is less consensus regarding the influence of AT2 receptors on blood pressure regulation in vivo. Studies using AT2 receptor knockout mice support a role for AT2 receptors in haemodynamic control, as these animals exhibit elevated basal blood pressure and enhanced sensitivity to the vasopressor effects of Ang II (Hein et al., 1995; Ichiki et al., 1995). Conversely, overexpression of AT2 receptors in vasculature did not alter basal blood pressure, but markedly impaired Ang II-induced pressor activity (Tsutsumi et al., 1999). In conscious SHR, Ang II-mediated vasodilatation during AT1 receptor blockade was not observed (Gohlke et al., 1998), presumably because the hypotensive effect of AT2 receptor stimulation was masked by the concomitant, dominant AT1 receptor-mediated pressor action during Ang II infusion. In order to avoid such confounding influences of AT1 receptor stimulation on potential AT2 receptor vasodilator function, we and others have also assessed the effect of selective AT2 receptor agonists and antagonists during AT1 receptor blockade. Using this approach, selective stimulation of AT2 receptors by CGP42112 lowered blood pressure, provided that there was a background of AT1 receptor blockade in conscious SHR (Wistar-Kyoto rat, WKY) (Barber et al., 1999), and Sprague-Dawley rats (Carey et al., 2001) in a PD123319-reversible manner. Furthermore, this blood pressure-lowering response to AT2 receptor stimulation was shown to be associated with increased blood flow in renal, mesenteric and hindquarter circulations in conscious SHR suggesting widespread vasodilatation (Li and Widdop, 2004).
Therefore, in the current study we determined the effects of Compound 21 on blood pressure in conscious SHR and WKY rats, as well as in isolated vasculature. In addition, AT2 receptor selectivity of Compound 21 was determined by simultaneous administration of the selective AT2 receptor antagonist, PD123319, to determine whether or not these effects were AT2 receptor-mediated.
Methods
Animals
All animal care and experimental procedures were approved by the Monash University Animal Ethics Committee and performed according to the guidelines of the National Health and Medical Research Council of Australia for animal experimentation.
Male 16- to 18-week-old SHR and WKY rats, weighing approximately 300 to 350 g and male 16-week-old FVB/N mice, weighing approximately 25–30 g were obtained from the Animal Resource Centre (Perth, WA, USA). Animals were maintained on a 12 h day/night cycle with standard laboratory rat or mice chow and water available ad libitum.
In vitro reactivity
Mice were killed by isoflurane inhalation followed by decapitation. The thoracic aorta was removed and cut transversely into ring segments for organ bath studies. Two stainless steel wires were threaded through the lumen of each aortic ring and the rings were then mounted and suspended in vertical 10 mL organ baths containing Krebs bicarbonate solution [composition (mM): NaCl 118, KCl 4.7, KH2PO4 1.2, MgSO4.7H2O 1.2, CaCl2 2.5, NaHCO3 25 and glucose 11.7; pH 7.4], which was maintained at 37°C and continuously bubbled with carbogen (95% oxygen and 5% CO2). Isometric tension was continuously measured via a force transducer (Grass FT03) interfaced to a MacLab data acquisition device (ADInstruments, Sydney, Australia) displayed on a Macintosh computer. Aortic rings were set to 0.5 g resting tension and allowed to equilibrate for 90 min, during which time Krebs bicarbonate solution was changed every 15 min. After equilibration, 0.3 µM of the thromboxane A2 receptor agonist, U46619, was used to obtain the maximum contractile response. Once a maximum response was determined, tissues were then washed with Krebs' solution and allowed to equilibrate for 30 min or until baseline had been reached.
Tissues were pre-contracted with U46619 to attain 30–40% of the maximum contractile response. In the first series of experiments, all vessels were pretreated with the AT1 receptor antagonist, losartan (0.1 µM). Cumulative dose–response curves at log intervals to Compound 21 (1 pM to 1 µM) were performed in absence or presence of the AT2 receptor antagonist PD123319 (0.1 µM). In a further series, AT1 receptor blockade was omitted and cumulative dose-response curves to Compound 21 were performed in the absence and presence of either the NOS inhibitor, L-NAME (10 µM) or PD123319. A parallel tissue served as a time control in which only U46619 was given. At the end of the experiment, 10 µM of the endothelium-independent vasodilator, sodium nitroprusside was added to the organ bath to test the integrity of the vascular smooth muscle cells.
In analogous studies, thoracic aortic rings from male SHR were set up at 2 g resting tension and a maximum response was obtained to 124 mM K+. After washing, tissues were pre-contracted with the α1-adrenoceptor agonist phenylephrine to 30–40% of maximum K+ response and cumulative dose–response curves to either Ang II (in the presence of 0.1 µM candesartan) or Compound 21 in the presence or absence of candesartan were obtained.
Mesenteric artery
Male WKY rats, approximately 16 weeks of age, were killed by isoflurane inhalation followed by decapitation and the gut was removed in order to dissect 3 to 5 mm long sections of the third order branch from mesenteric artery. The arterial sections were cannulated at both ends and mounted in a video-monitored perfusion system (Living Systems Instrumentation, Burlington, VT, USA), as previously described (Matrougui et al., 1999; Loufrani et al., 2001; Widdop et al., 2002). Mesenteric artery sections were bathed in 20 mL organ baths that contained Krebs solution to which the AT1 receptor antagonist, candesartan (1 µM) was added. The solution was bubbled with carbogen (95% O2 and 5% CO2), with temperature maintained at 37°C and the pH at 7.4. The arterial sections were superfused at a rate of 4 mL·min−1 and perfused at a rate of 100 µL·min−1. The intraluminal pressure was set at 75 mmHg. The diameter of the arterial sections was constantly measured and recorded with a video-monitoring system. Following an equilibration period of approximately 30 min, phenylephrine was added to achieve 20–30% of the maximum contractile response. Once the plateau was reached, a concentration response curve to Compound 21 (0.1 nM to 1 µM) was constructed. Analogous experiments were performed in which the AT2 receptor antagonist, PD123319 (1 µM) was added 30 min before Compound 21. An additional tissue was set up that served as a time control and was only pre-contracted with phenylephrine.
In vivo procedures
Rats were anaesthetized (ketamine and xylazine; 75 mg·kg−1 and 10 mg·kg−1, i.p., respectively; supplemented as required). Two catheters were inserted into the right jugular for i.v. drug administration. A catheter was inserted into the right carotid artery for direct blood pressure measurement as described previously (Barber et al., 1999; Li and Widdop, 2004; Walters et al., 2005). Rats were housed in individual cages and allowed free access to food and water while maintained on 12 h day/night cycle. The arterial catheter was infused overnight with heparinized saline using an infusion pump.
Twenty-four hours after the surgery, the arterial catheter was attached to a pressure transducer (Gould Inc., Eichstetten, Germany), connected to a MacLab-8 data acquisition system (ADInstruments) and interfaced to a Macintosh computer. Mean arterial pressure (MAP) and heart rate were computed from the phasic blood pressure signal.
Experimental protocol
Rats received drug combinations in a randomized fashion over a 4 or 5 day protocol, as described previously (Barber et al., 1999; Walters et al., 2005). Doses of candesartan and PD123319 were chosen on the basis of previous studies (Barber et al., 1999; Walters et al., 2005). Animals in group 1 (WKY; dose-ranging) and group 2 (SHR; dose-ranging) were randomized to receive following treatments: (i) a 4 h Compound 21 infusion (50, 100 or 300 ng·kg−1·min−1 in WKY rats or 100, 300 and 1000 ng·kg−1·min−1 in SHR); and (ii) a 4 h Compound 21 infusion (50 and 300 ng·kg−1·min−1 in WKY rats or 300 and 1000 ng·kg−1·min−1 in SHR) given simultaneously with candesartan (0.1 mg·kg−1 i.v.).
Based on the dose-ranging results, additional SHR (group 3) received the following treatments in randomized fashion: (i) candesartan (0.1 mg·kg−1 i.v.); (ii) Compound 21 infusion (50 ng·kg−1·min−1 for 4 h); (iii) a 4 h Compound 21 infusion together with candesartan; and (iv) a 4 h Compound 21 infusion in the presence of candesartan and PD123319 infusion (50 µg·kg−1·min−1 for 2 h). The dose of PD123319 was based on our previous experience using this compound in similar in vivo experiments (Barber et al., 1999; Li and Widdop, 2004). In analogous experiments in separate SHR (group 4), an identical protocol was repeated to that of group 3 but a 10-fold lower dose of candesartan (0.01 mg·kg−1 i.v.) was used. We have previously shown that basal BP recordings are stable over these time periods. Nevertheless, in this latter group, SHR also received a 4 h infusion (0.1 mL·kg−1·h−1 i.v.) of saline (0.9% NaCl) to confirm a lack of effect on MAP.
Statistical analysis
All data are presented as mean responses ± standard error of the mean (SEM). Differences in vasorelaxation or MAP between treatments were analysed using a two-way repeated measure, anova. Statistical analysis was performed using GraphPad Prism (Version 5.0). P-values <0.05 were considered statistically significant.
Materials
Compound 21 was provided by A Hallberg, Department of Medicinal Chemistry, Uppsala University; PD123319 and candesartan were kind gifts from Pfizer and AstraZeneca respectively. All other chemicals were purchased from commercial sources: L-NAME (Sigma), sodium nitroprusside (Sigma, Sydney, Australia), ketamine (Troy Laboratories, Sydney, Australia), xylazine (Troy Laboratories), phenylephrine (Sigma), isoflurane (Baxter, Deerfield, IL, USA) and U46619 (Saphire Bioscience, Sydney, Australia).
Results
In vitro relaxation evoked by Compound 21
Compound 21 caused a dose-dependent relaxation of mouse aorta in the presence of AT1 receptor blockade, which was markedly inhibited by PD123319 (Figure 1A). Furthermore, Compound 21-evoked relaxation was also evident in the absence of AT1 receptor blockade and was abolished by L-NAME (Figure 1B). In addition, Compound 21 caused vasodilatation in third order perfused rat mesenteric artery, which was also blocked by PD123319 (Figure 1C).
Figure 1.
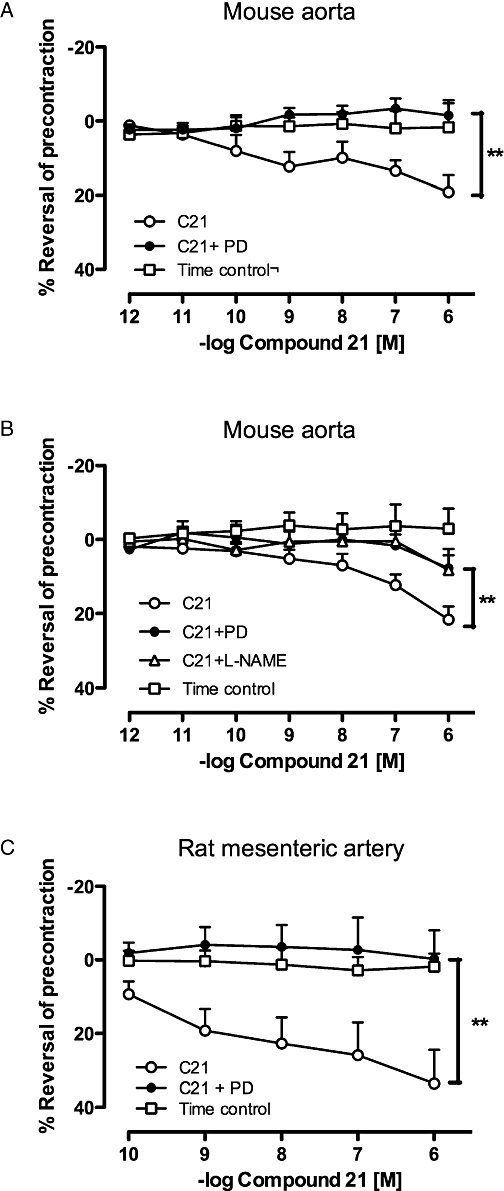
Dose–response curves to Compound 21 (C21) performed in (A) mouse aorta and (C) third order perfused rat mesenteric artery in the absence (n= 5 and 9 for aorta and mesenteric artery respectively) and presence (n= 5 and 3 for aorta and mesenteric artery respectively) of PD123319 (PD); all experiments in Figure 1A and C performed in the presence of angiotensin type 1 receptor (AT1 receptor) blockade, as described in the Methods. (B) Effect of Compound 21 in mouse aorta in the absence of AT1 receptor blockade and in the presence of PD123319 or L-NAME (n= 10 for each). Values represent mean ± SEM. **P < 0.01 for treatment effect between Compound 21 and Compound 21 + PD123319 or Compound 21 + L-NAME (two-way repeated measures anova).
Experiments were also performed using aortic rings obtained from naive SHR, a preparation that is reported to be unresponsive to the AT2 receptor-mediated vasorelaxant effects of Ang II (Cosentino et al., 2005). Indeed, Ang II did not cause relaxation; on the other hand, Compound 21 evoked dose-dependent relaxation that was similar in the presence or absence of AT1 receptor blockade (Figure 2).
Figure 2.
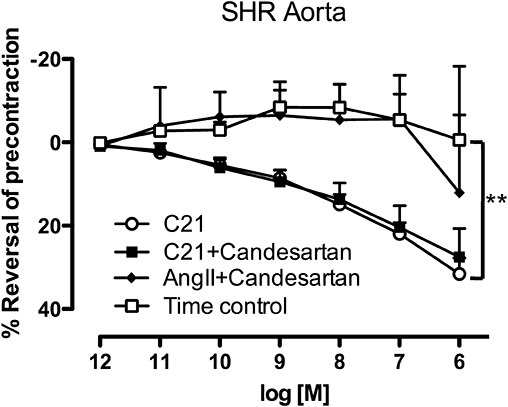
Dose–response curves to either angiotensin II (Ang II) or Compound 21 (C21) performed in aorta obtained from naive spontaneously hypertensive rat (SHR) in the absence (Compound 21) and presence (Ang II & Compound 21) of candesartan (0.1 µM) (n= 5 for each). Values represent mean ± SEM. **P < 0.01 for treatment effect between Compound 21 and time control (two-way repeated measures anova).
In vivo effect of Compound 21 in conscious rats
Basal MAP of SHR over the four or five experimental days for each group are listed in the Table 1. There was no significant difference between resting MAP over the experimental period for any of the treatment groups, suggesting that none of the acute treatments had effects that lasted more than 24 h, and therefore did not influence baseline MAP on subsequent days.
Table 1.
Resting mean arterial pressure (MAP) of spontaneously hypertensive rat recorded on separate days before drug treatments, as indicated
| Treatment | MAP (mmHg) |
|---|---|
| Group 2 (n= 5) | |
| Compound 21 (100 ng·kg−1·min−1) | 174 ± 8 |
| Compound 21 (300 ng·kg−1·min−1) | 177 ± 8 |
| Compound 21 (1000 ng·kg−1·min−1) | 165 ± 5 |
| Compound 21 (300 ng·kg−1·min−1) & candesartan (0.1 mg·kg−1) | 164 ± 8 |
| Compound 21 (1000 ng·kg−1·min−1) & candesartan (0.1 mg·kg−1) | 163 ± 5 |
| Group 3 (n= 7) | |
| Compound 21 (50 ng·kg−1·min−1) | 178 ± 6 |
| Candesartan (0.1 mg·kg−1) | 191 ± 6 |
| Compound 21 & candesartan | 190 ± 7 |
| Compound 21, candesartan & PD123319 (50 µg·kg−1·min−1) | 177 ± 7 |
| Group 4 (n= 7) | |
| Saline | 175 ± 6 |
| Compound 21 (50 ng·kg−1·min−1) | 179 ± 7 |
| Candesartan (0.01 mg·kg−1) | 184 ± 8 |
| Compound 21 & candesartan | 190 ± 7 |
| Compound 21, candesartan & PD123319 (50 µg·kg−1·min−1) | 170 ± 10 |
The values shown in the Table are means ± SEM. n= 5–7 per group.
In both WKY (Figure 3) and SHR (Figure 4), infusion of Compound 21 alone, at doses ranging from 50 to 300 ng·kg−1·min−1, had no significant effect on MAP. Combined administration of Compound 21 (100 and 300 ng·kg−1·min−1) and candesartan (0.1 mg·kg−1) also had no effect on MAP in WKY rats (Figure 3); however, the combination of Compound 21 (300 ng·kg−1·min−1) and candesartan, significantly decreased MAP in SHR compared with Compound 21 alone (P < 0.001) (Figure 4). Interestingly, at the highest dose tested (1000 ng·kg−1·min−1), Compound 21 alone caused an increase in MAP in SHR (P < 0.05), suggesting a lack of selectivity of Compound 21 at this dose. This Compound 21-mediated pressor effect was attenuated by simultaneous AT1 receptor blockade (Figure 4).
Figure 3.
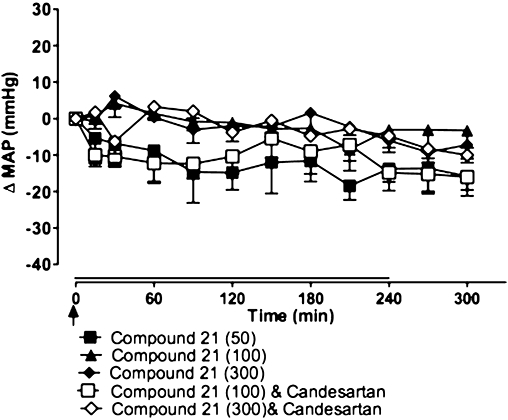
Effects of Compound 21 (50, 100 and 300 ng·kg−1·min−1), administered as a 4 h infusion (shown by horizontal line), on mean arterial pressure (MAP) in Wistar-Kyoto rats (n= 5). Compound 21 was given in the presence or absence of the angiotensin type 1 receptor antagonist, candesartan (0.1 mg·kg−1 i.v. bolus; shown by an arrow). Values represent mean ± SEM.
Figure 4.
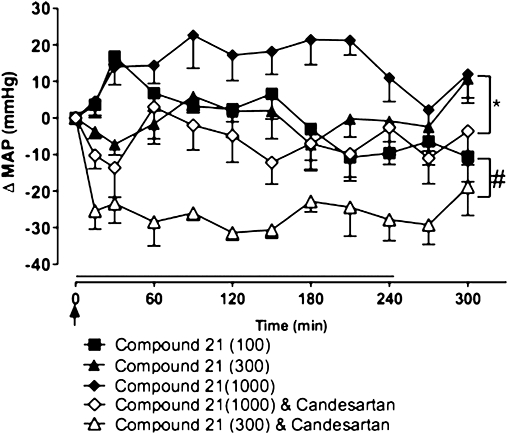
Effects of Compound 21 (100, 300 and 1000 ng·kg−1·min−1), administered as a 4 h infusion (shown by horizontal line), on mean arterial pressure (MAP) in spontaneously hypertensive rats (n= 5). Compound 21 was given in the presence or absence of the angiotensin type 1 receptor agonist, candesartan (0.1 mg·kg−1 i.v. bolus, shown by an arrow). Values represent mean ± SEM. #P < 0.001 for treatment effect between Compound 21 (300 ng·kg−1·min−1) + candesartan and Compound 21(300 ng·kg−1·min−1) (two-way repeated measures anova); *P < 0.05 for treatment effect between Compound 21(1000 ng·kg−1·min−1) + candesartan and Compound 21(1000 ng·kg−1·min−1) (two-way repeated measures anova).
Given the lack of response to Compound 21 in WKY rats, further examination of the effect of Compound 21 on MAP was performed in separate groups of SHR, to determine the effects of lower dose of Compound 21 in combination with AT1 receptor block. As had been previously determined, Compound 21 infusion alone (50 ng·kg−1·min−1) had no effect on MAP in SHR (Figure 5). However, when combined with either high-dose (0.1 mg·kg−1; Figure 5) or low-dose (0.01 mg·kg−1; Figure 6) candesartan, Compound 21 caused a significant reduction in MAP in SHR (P < 0.001). Importantly, when the AT2 receptor antagonist, PD123319 (50 µg·kg−1·min−1), was co-infused for 2 h with Compound 21 and candesartan, this blood pressure-lowering effect was abolished, indicating AT2 receptor selectivity of Compound 21 (P < 0.001). Furthermore, infusion of saline had no effect on blood pressure (Figure 6).
Figure 5.
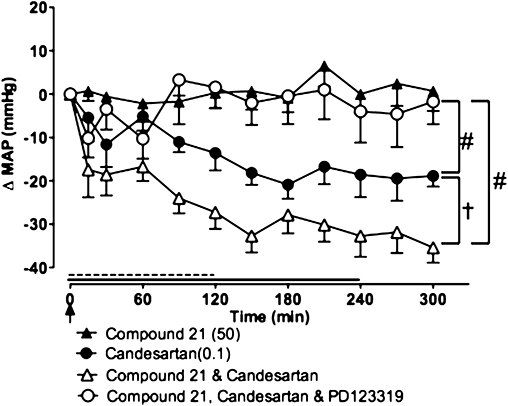
Effect of Compound 21 (50 ng·kg−1·min−1; 4 h infusion shown by full line), high-dose candesartan (0.1 mg·kg−1 bolus i.v.; shown by an arrow), Compound 21 + candesartan and Compound 21 + candesartan + PD123319 (50 µg·kg−1·min−1 for 2 h; (dashed line), on mean arterial pressure (MAP) in spontaneously hypertensive rat (n= 7). Values represent mean ± SEM. #P < 0.001 for overall effect of treatment versus Compound 21 (two-way repeated measures anova); †P < 0.01 for treatment effect between Compound 21 + candesartan and candesartan (two-way repeated measures anova).
Figure 6.
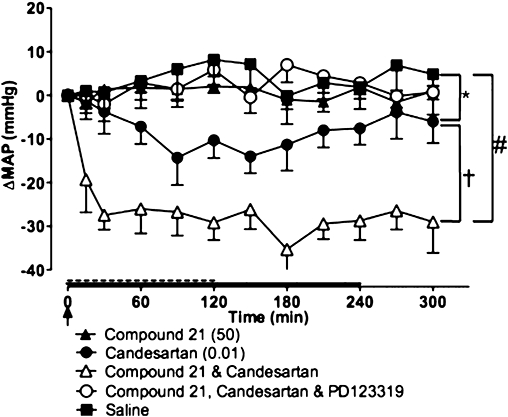
Effect of Compound 21 (50 ng·kg−1·min−1; 4 h infusion shown by full line), low-dose candesartan (0.01 mg·kg−1 bolus i.v.; shown by an arrow), Compound 21 + candesartan and Compound 21 + candesartan + PD123319 (50 µg·kg−1·min−1 for 2 h; shown by dashed line), on mean arterial pressure (MAP) in spontaneously hypertensive rat (n= 7). Values represent mean ± SEM. *P < 0.05 for treatment effect between Compound 21 and candesartan; #P < 0.001 for overall effect of individual treatment versus Compound 21 (two-way repeated measures anova); †P < 0.01 for treatment effect between Compound 21 + candesartan and candesartan alone (two-way repeated measures anova).
Discussion
The main finding of the current study was that the novel non-peptide AT2 receptor agonist, Compound 21, evoked vasorelaxation in vitro, which translated into vasodepressor responses in conscious SHR against a background of AT1 receptor blockade. To our knowledge, this study represents the first systematic study of the vascular effects of Compound 21, particularly in hypertension.
Compound 21 exhibits a similar binding profile at AT2 receptors to that of Ang II and CGP42112 but with little affinity for AT1 receptors. In mouse aortic rings, Compound 21, in the presence of AT1 receptor antagonists, caused concentration-dependent vasorelaxation that was inhibited by the AT2 receptor antagonist, PD123319. This in vitro effect was not dependent on background AT1 receptor blockade, as it was in vivo, and was also inhibited by L-NAME. Thus, Compound 21 elicited classical AT2 receptor-mediated NO signalling, a hallmark of AT2 receptors in vascular tissue (Widdop et al., 2003; Jones et al., 2008). This compound was also tested using rat isolated mesenteric arteries, a preparation well recognized as exhibiting AT2 receptor-mediated vasorelaxation (Matrougui et al., 1999; Henrion et al., 2001; Widdop et al., 2002). Indeed in these resistance-like vessels, Compound 21 caused vasorelaxation that was inhibited by PD123319, confirming selective AT2 receptor-mediated actions of the compound.
In the present study, dose–response analysis was performed in conscious SHR and WKY rats in which multiple doses were tested in the same animals on different days, allowing within-animal analysis. When tested over a wide dose range, Compound 21 alone did not reduce MAP in SHR or WKY rats except during AT1 receptor blockade in SHR, as seen previously with CGP42112 (Barber et al., 1999; Li and Widdop, 2004). It is likely that these results can be explained by the fact that circulating endogenous Ang II itself exerts tonic AT1 receptor-mediated vasoconstriction that, once removed, allows AT2 receptor-mediated vasodilatation to be manifest. These data are consistent with previous studies in conscious SHR in which Ang II did not cause vasodilatation during AT1 receptor blockade (Gohlke et al., 1998), presumably because the hypotensive effect of AT2 receptor stimulation was masked by the concomitant, dominant AT1 receptor-mediated pressor action during Ang II infusion. A differential effect on vascular tone to AT2 receptor stimulation has also been noted by others using SHR and WKY rats (Savoia et al., 2005), and is consistent with a lack of effect of Compound 21 on blood pressure in anaesthetized normotensive rats (Wan et al., 2004b). This lack of effect of Compound 21 on BP in normotensive animals may relate to the fact that subtle AT2 receptor-mediated depressor responses are more easily observed from a higher basal blood pressure. Alternatively, it may represent strain-dependent differences in sensitivity to AT2 receptor stimulation or drug-induced changes in vascular AT2 receptor expression. In this context, aortic AT2 receptor expression is higher in adult SHR compared with age-matched WKY, whereas mesenteric AT2 receptor expression is increased in young SHR but decreased in adult SHR (see Widdop et al., 2008).
Limited in vivo studies in anaesthetized SHR implied a role of this compound on vascular tone as, when given as bolus i.v. injections to anaesthetized SHR, Compound 21 lowered BP (Wan et al., 2004b). The discrepancy between the two studies most likely reflects the different experimental designs between the current and previous studies (Wan et al., 2004b). The highest effective dose (0.05 mg·kg−1) of Compound 21 previously tested (Wan et al., 2004b) was probably higher than our maximally effective depressor dose achieved during a 4 h infusion (i.e. 300 ng·kg−1·min−1∼ 0.072 mg·kg−1 total; during AT1 receptor blockade), once half-life and pharmacokinetic considerations are taken into account, although it should be noted that a higher dose of Compound 21 (1000 ng·kg−1·min−1) alone also did not decrease blood pressure. Another important difference is that the previous study (Wan et al., 2004b) tested barbiturate-anaesthetized SHR, which are less physiological than consciously instrumented SHR that would be more able to buffer potential blood pressure reductions by homeostatic reflex mechanisms. Moreover, we were keen to be able to make direct comparisons with previous studies that also reported AT2 receptor-mediated depressor effects of the selective agonist CGP42112 during AT1 receptor blockade (Barber et al., 1999; Li and Widdop, 2004).
Curiously, AT2 receptor stimulation does not generally cause vasorelaxation in vessels isolated from SHR strains (Matrougui et al., 2000; Cosentino et al., 2005; Savoia et al., 2005; You et al., 2005). Chronic treatment with AT1 receptor antagonists is associated with increased AT2 receptor expression in aortae from SHR (Cosentino et al., 2005; Savoia et al., 2005), and human subcutaneous gluteal arteries (Savoia et al., 2007), as well as in mesenteric arteries from SHR, which normally exhibited decreased AT2 receptor expression under basal conditions (You et al., 2005). Indeed, sartan-induced up-regulation of AT2 receptors unmasked ex vivo AT2 receptor-mediated vasorelaxation in otherwise unresponsive vessels (Yayama et al., 2004; Cosentino et al., 2005; Savoia et al., 2005; Savoia et al., 2007). Therefore, it is conceivable that enhanced in vivo sensitivity of untreated SHR, compared with WKY rats, to Compound 21 was due to higher AT2 receptor expression, although elevated MAP per se could still contribute to AT2 receptor-mediated depressor activity in vivo. In any case, whether or not ex vivo AT2 receptor-mediated relaxation evoked by Compound 21 is more manifest following chronic treatment with an AT1 receptor antagonist awaits further investigation. However, we did test the in vitro effects of Compound 21 acutely in naive SHR. As expected, Ang II did not evoke vasorelaxation whereas, strikingly, Compound 21 relaxed aortae. Thus, in aortic tissue known to be refractory to acute AT2 receptor-mediated effects of Ang II (Cosentino et al., 2005; Savoia et al., 2005), Compound 21 caused vasorelaxation, in SHR, both in vitro and in vivo, at least in the presence of AT1 receptor blockade. Thus, the current study highlights the importance of using subtype selective compounds.
At the highest dose tested (1000 ng·kg−1·min−1), Compound 21 alone actually increased MAP in SHR, most likely representing a lack of AT2 receptor selectivity at this concentration. Although the sensitivity of this pressor effect of Compound 21 to blockade by PD123319 was not tested in the current study, simultaneous AT1 receptor inhibition restored MAP responses to baseline. This finding could indicate that Compound 21 caused AT1 receptor stimulation at higher doses, which may relate to the higher AT1/AT2 receptor ratio in vasculature, or that the hypotensive effect of candesartan offset Compound 21-mediated vasoconstriction via other mechanisms. The fact that a lower dose of Compound 21 (300 ng·kg−1·min−1), in combination with candesartan, significantly lowered MAP in these same SHR makes it likely that a more selective AT2 receptor vasodilator effect was manifest at doses <1000 ng·kg−1·min−1. Similarly, pressor doses of Ang II infused in the presence of AT1 receptor blockade do not always reduce blood pressure (Gohlke et al., 1998), most likely for the same reasons of opposing vascular effects of AT1 and AT2 receptor stimulation (Barber et al., 1999; Li and Widdop, 2004).
When infused at a sixfold lower dose, the depressor effect of Compound 21 (50 ng·kg−1·min−1) was similar in groups concomitantly administered either high- or low-dose candesartan, suggesting that the maximum achievable fall in MAP had been reached. By contrast, using CGP42112, we did not find additional depressor effects when combined with high-dose candesartan (Barber et al., 1999), which may reflect a difference in metabolic fate of these two compounds as Compound 21 is a non-peptide compound. The additive effect of Compound 21 and candesartan may also reflect the fact that sartan-induced elevation in Ang II levels was probably not maximal. In any case, we have found that AT2 receptors do not functionally desensitize even in the face of raised Ang II levels (Widdop et al., 2002), therefore the non-peptide AT2 receptor agonist may exert a prolonged effect. Importantly, in these same animals, we also tested PD123319, which completely abolished the depressor effect of combined Compound 21 and candesartan; consistent with the AT2 receptor selectivity demonstrated by both the current in vitro data and radioligand binding assays performed with this compound (Wan et al., 2004b). Interestingly, although tested in separate animal groups, it also appeared that there was no difference in the maximal Compound 21-mediated depressor effect using either 50 or 300 ng·kg−1·min−1. In this context, a bell-shaped dose–response relationship for the effect of Compound 21 on BP was also reported by Wan et al. (2004b), who found that the depressor effect of bolus Compound 21 administration in anaesthetized SHR was present at lower doses, but lost at higher doses (>0.05 mg·kg−1).
Collectively, these data implicate the AT2 receptor as a potential target for the treatment of hypertension, although until now, there have been no drug-like candidates available to directly test this premise. In this context, the effects of Compound 21 have recently been reported in the setting of myocardial infarction. In that study, Compound 21, given for 7 days after myocardial infarction, improved systolic and diastolic function and reduced infarct size (Kaschina et al., 2008), thus illustrating the potential use of this non-peptide compound in a number of cardiovascular settings. Indeed, our current findings suggest additive effects of AT1 receptor blockade and AT2 receptor stimulation would be beneficial for BP reduction, and fit with clinical findings of increased vascular AT2 receptor expression after long-term sartan treatment (Savoia et al., 2007), highlighting the need for future determination of the chronic effects of Compound 21 in hypertensive settings.
In conclusion, we have established that Compound 21 evoked vasorelaxation in mouse and SHR aortae or rat mesenteric arteries, and vasodepressor responses in conscious SHR, via AT2 receptor stimulation. The BP-lowering effect of Compound 21 was additive to candesartan when the latter compound was given at a dose that itself lowered BP. Further studies are warranted on the chronic effects of Compound 21, alone and in combination with AT1 receptor antagonists, in hypertensive-related diseases. These studies implicate the AT2 receptor as a potential therapeutic target in the setting of hypertension and related cardiovascular diseases.
Acknowledgments
These studies were funded in part by grants from the National Health and Medical Research Council of Australia and the Swedish Research Council.
Glossary
Abbreviations:
- Ang II
angiotensin II
- AT1 receptor
angiotensin type 1 receptor
- AT2 receptor
angiotensin type 2 receptor
- MAP
mean arterial pressure
- SHR
spontaneously hypertensive rat
- WKY rat
Wistar-Kyoto rat
Conflicts of interest
None.
References
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 3rd edition. Br J Pharmacol. 2008;153:S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber MN, Sampey DB, Widdop RE. AT2 receptor stimulation enhances antihypertensive effect of AT1 receptor antagonist in hypertensive rats. Hypertension. 1999;34:1112–1116. doi: 10.1161/01.hyp.34.5.1112. [DOI] [PubMed] [Google Scholar]
- Carey RM. Update on the role of the AT(2) receptor. Curr Opin Nephrol Hypertens. 2005;14:67–71. doi: 10.1097/00041552-200501000-00011. [DOI] [PubMed] [Google Scholar]
- Carey RM, Howell NL, Jin X-H, Siragy HM. Angiotensin type 2 receptor-mediated hypotension in angiotensin type-1 receptor-blocked rats. Hypertension. 2001;38:1272–1277. doi: 10.1161/hy1201.096576. [DOI] [PubMed] [Google Scholar]
- Cosentino F, Savoia C, De Paolis P, Francia P, Russo A, Maffei A, et al. Angiotensin II type 2 receptors contribute to vascular responses in spontaneously hypertensive rats treated with angiotensin II type 1 receptor antagonists. Am J Hypertens. 2005;18:493–499. doi: 10.1016/j.amjhyper.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Dimitropoulou C, White RE, Fuchs L, Zhang HF, Catravas JD, Carrier GO. Angiotensin II relaxes microvessels via the AT(2) receptor and Ca2+-activated K+ (BKCa) channels. Hypertension. 2001;37:301–307. doi: 10.1161/01.hyp.37.2.301. [DOI] [PubMed] [Google Scholar]
- de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52:415–472. [PubMed] [Google Scholar]
- Gohlke P, Pees C, Unger T. AT(2) receptor stimulation increases aortic cyclic GMP in SHRSP by a kinin-dependent mechanism. Hypertension. 1998;31:349–355. doi: 10.1161/01.hyp.31.1.349. [DOI] [PubMed] [Google Scholar]
- Hein L, Barsh GS, Pratt RE, Dzau VJ, Kobilka BK. Behavioral and cardiovascular effects of disrupting the angiotensin-ii type-2 receptor gene in mice. Nature. 1995;377:744–747. doi: 10.1038/377744a0. [DOI] [PubMed] [Google Scholar]
- Henrion D, Kubis N, Levy BI. Physiological and pathophysiological functions of the AT2 subtype receptor of angiotensin II: from large arteries to the microcirculation. Hypertension. 2001;38:1150–1157. doi: 10.1161/hy1101.096109. [DOI] [PubMed] [Google Scholar]
- Ichiki T, Hogan BLM, Ichikawa I, Inagami T. Targeted disruption of angiotensin-II type-2 receptor (AT2) gene. Hypertension. 1995;26:546–546. [Google Scholar]
- Jones ES, Vinh A, McCarthy CA, Gaspari TA, Widdop RE. AT2 receptors: functional relevance in cardiovascular disease. Pharmacol Ther. 2008;120:292–316. doi: 10.1016/j.pharmthera.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaschina E, Grzesiak A, Li J, Foryst-Ludwig A, Timm M, Rompe F, et al. Angiotensin II type 2 receptor stimulation: a novel option of therapeutic interference with the renin-angiotensin system in myocardial infarction? Circulation. 2008;118:2523–2532. doi: 10.1161/CIRCULATIONAHA.108.784868. [DOI] [PubMed] [Google Scholar]
- Li XC, Widdop RE. AT2 receptor-mediated vasodilatation is unmasked by AT1 receptor blockade in conscious SHR. Br J Pharmacol. 2004;142:821–830. doi: 10.1038/sj.bjp.0705838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loufrani L, Matrougui K, Gorny D, Duriez M, Blanc I, Levy BI, et al. Flow (shear stress)-induced endothelium-dependent dilation is altered in mice lacking the gene encoding for dystrophin. Circulation. 2001;103:864–870. doi: 10.1161/01.cir.103.6.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrougui K, Loufrani L, Heymes C, Levy BI, Henrion D. Activation of AT(2) receptors by endogenous angiotensin II is involved in flow-induced dilation in rat resistance arteries. Hypertension. 1999;34:659–665. doi: 10.1161/01.hyp.34.4.659. [DOI] [PubMed] [Google Scholar]
- Matrougui K, Levy BI, Henrion D. Tissue angiotensin II and endothelin-1 modulate differently the response to flow in mesenteric resistance arteries of normotensive and spontaneously hypertensive rats. Br J Pharmacol. 2000;130:521–526. doi: 10.1038/sj.bjp.0703371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoia C, Tabet F, Yao GY, Schiffrin EL, Touyz RM. Negative regulation of RhoA/Rho kinase by angiotensin II type 2 receptor in vascular smooth muscle cells: role in angiotensin II-induced vasodilation in stroke-prone spontaneously hypertensive rats. J Hypertens. 2005;23:1037–1045. doi: 10.1097/01.hjh.0000166845.49850.39. [DOI] [PubMed] [Google Scholar]
- Savoia C, Touyz RM, Volpe M, Schiffrin EL. Angiotensin type 2 receptor in resistance arteries of type 2 diabetic hypertensive patients. Hypertension. 2007;49:341–346. doi: 10.1161/01.HYP.0000253968.95136.b8. [DOI] [PubMed] [Google Scholar]
- Tsutsumi Y, Matsubara H, Masaki H, Kurihara H, Murasawa S, Takai S, et al. Angiotensin II type 2 receptor overexpression activates the vascular kinin system and causes vasodilation. J Clin Invest. 1999;104:925–935. doi: 10.1172/JCI7886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters PE, Gaspari TA, Widdop RE. Angiotensin-(1-7) acts as a vasodepressor agent via angiotensin II type 2 receptors in conscious rats. Hypertension. 2005;45:960–966. doi: 10.1161/01.HYP.0000160325.59323.b8. [DOI] [PubMed] [Google Scholar]
- Wan Y, Wallinder C, Johansson B, Holm M, Mahalingam AK, Wu X, et al. First reported nonpeptide AT1 receptor agonist (L-162,313) acts as an AT2 receptor agonist in vivo. J Med Chem. 2004a;47:1536–1546. doi: 10.1021/jm031031i. [DOI] [PubMed] [Google Scholar]
- Wan YQ, Wallinder C, Plouffe B, Beaudry H, Mahalingam AK, Wu XY, et al. Design, synthesis, and biological evaluation of the first selective nonpeptide AT(2) receptor agonist. J Med Chem. 2004b;47:5995–6008. doi: 10.1021/jm049715t. [DOI] [PubMed] [Google Scholar]
- Widdop RE, Matrougui K, Levy BI, Henrion D. AT2 receptor-mediated relaxation is preserved after long-term AT1 receptor blockade. Hypertension. 2002;40:516–520. doi: 10.1161/01.hyp.0000033224.99806.8a. [DOI] [PubMed] [Google Scholar]
- Widdop RE, Jones ES, Hannan RE, Gaspari TA. Angiotensin AT2 receptors: cardiovascular hope or hype? Br J Pharmacol. 2003;140:809–824. doi: 10.1038/sj.bjp.0705448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdop RE, Vinh A, Henrion D, Jones ES. Vascular angiotensin AT2 receptors in hypertension and ageing. Clin Exp Pharmacol Physiol. 2008;35:386–390. doi: 10.1111/j.1440-1681.2008.04883.x. [DOI] [PubMed] [Google Scholar]
- Yayama K, Horii M, Hiyoshi H, Takano M, Okamoto H, Kagota S, et al. Up-regulation of angiotensin II type 2 receptor in rat thoracic aorta by pressure-overload. J Pharmacol Exp Ther. 2004;308:736–743. doi: 10.1124/jpet.103.058420. [DOI] [PubMed] [Google Scholar]
- You D, Loufrani L, Baron C, Levy BI, Widdop RE, Henrion D. High blood pressure reduction reverses angiotensin II type 2 receptor-mediated vasoconstriction into vasodilation in spontaneously hypertensive rats. Circulation. 2005;111:1006–1011. doi: 10.1161/01.CIR.0000156503.62815.48. [DOI] [PMC free article] [PubMed] [Google Scholar]


