Abstract
Background and purpose:
Although resveratrol is currently being evaluated in pre-clinical studies as a potential cancer chemopreventive agent and cardiovascular stress-releasing compound, treatment with resveratrol severely delays healing of pre-existing gastric ulcers. Resveratrol treatment can also induce endothelial NOS (eNOS) expression. Here, we have attempted to modulate NO production via eNOS in order to alleviate the pro-ulcer effects of resveratrol.
Experimental approach:
Gastric ulcers were induced in mice with a single dose of indomethacin. The effects of pretreatment with l-arginine on the pro-ulcer effects of resveratrol in these mice were then assessed. We measured ulcer damage scores (DS), myeloperoxidase (MPO) activity, generation of prostaglandin E2 (PGE2) and NO, along with a gene expression study.
Key results:
Resveratrol significantly aggravated damage from indomethacin-induced gastric ulcers, and delayed healing, as shown by increased DS and MPO activity. The mRNA for cyclooxygenase (COX)-1, but not that for COX-2, was inhibited by resveratrol treatment, with reduced synthesis of PGE2 by gastric tissue. However, resveratrol treatment induced eNOS gene expression and shifted the eNOS/iNOS balance. l-Arginine given before resveratrol in mice with indomethacin-induced ulcers significantly increased tissue NO synthesis and improved ulcer healing.
Conclusions and implications:
Exogenous l-arginine increased NO formation via raised levels of eNOS induced by resveratrol and protected against the pro-ulcer effects of resveratrol. Therefore, l-arginine might be useful for alleviation of the pro-ulcer side effects of resveratrol in patients.
Keywords: resveratrol, gastric ulcer, NSAID, l-arginine, l-NAME, eNOS
Introduction
In plants, trans-resveratrol functions as a phytoalexin that protects against fungal infections (Hain et al., 1990). Because of its high concentration in grape skin, significant amounts of resveratrol are present in wine (Siemann and Creasy, 1992; Goldberg et al., 1995), and it has been proposed to explain, at least in part, the apparent ability of a moderate consumption of red wine to reduce the risk of cardiovascular disease (Frankel et al., 1993; Bertelli et al., 1995). Resveratrol has also been reported to have cancer chemopreventive activity (Jang et al., 1997). The wide acceptance of resveratrol stems from its lower toxicity and a lack of contra-indicatons. But, recently, it had been demonstrated (Brzozowski et al., 1999; 2001;) that resveratrol treatment delayed the healing of gastric ulcers by suppressing cyclooxygenase (COX)-1 expression. Later, resveratrol was also found to suppress the phorbol ester-induced COX-2 mRNA expression (Subbaramaiah et al., 1998). However, Martín et al. (2006) showed that resveratrol reduced the damage in chronic experimentally induced colitis by inducing prostaglandin E2 (PGE2) production to basal levels without exerting any inhibitory effects on any of the COX isozymes. Hence, the exact effects of resveratrol on the COX isozymes in ulcerated gastric tissue are not yet clear. Other reports claimed anti-inflammatory effects of resveratrol (Das and Maulik, 2006) as it reduced levels of inducible NO synthase (iNOS), but simultaneously induced endothelial NOS (eNOS) expression. However, both the isoforms of NOS play very important roles in gastric ulcer healing (Ma and Wallace, 2000). The balance of eNOS/iNOS is critical to the production of adequate amounts of NO, which could augment gastric ulcer healing through vasodilatation and increased blood flow (Ma and Wallace, 2000). Nevertheless, the fact that resveratrol treatment could significantly repress COX-1 expression in mice with NSAID-induced ulcers could, ultimately, overshadow the beneficial effects of eNOS (Dey et al., 2009). Conversely, treatment with resveratrol directly could reduce iNOS expression in the pre-ulcerated gastric tissue (Guha et al., 2009). Thus, resveratrol could restrict tissue NO production, resulting in delayed healing of gastric ulcers in mice. We therefore attempted to improve the healing of ulcers by treatment with l-arginine, the endogenous substrate of NOS, thus increasing the tissue production of NO, in ulcer-bearing mice treated with resveratrol.
Methods
Animals
All animal care and experimental procedures were conducted in accordance with the guidelines of the animal ethics committee of the Post Graduate Institute of Basic Medical Sciences, Kolkata, Animal Ethical Committee 507/CPCSEA, Sanction No. IAEC/SB-2/2004/UCM-16, dated 15 June 2004, and were handled following the International Animal Ethics Committee Guidelines, ensuring minimum animal suffering. Male Swiss albino mice (6–8 weeks, 25–30 g) bred in-house with free access to food and water were used for all of the experiments. The mice were kept in 12 h light/dark cycles, and housed at 25°C.
Drug treatment
Resveratrol (Sigma, St Louis, MO, USA) was dissolved in water containing 1.0% (v/v) dimethyl sulphoxide (DMSO), and then added to 2% gum acacia in distilled water as the vehicle. Resveratrol was administered orally (0.5 mL per mouse) at 10 mg·kg−1. Misoprostol (Sigma) (5 µg·kg−1 once daily) in the same vehicle was used as the positive control. In some cases, after ulcer induction, the mice were additionally treated intraperitoneally with Nω-nitro-l-arginine methyl ester hydrochloride (l-NAME) or l-arginine in different doses once daily (Sigma).
Protocol for gastric ulceration and assessment of healing
Acute gastric ulceration in mice was induced by oral administration of indomethacin (18 mg·kg−1, single dose) dissolved in distilled water and suspended in 2% gum acacia as vehicle (Banerjee et al., 2008). The animals were deprived of food but had free access to tap water 24 h before ulcer induction. The normal and untreated control groups received the vehicle only throughout the course of the experiments. The treatment groups received resveratrol (10 mg·kg−1, once daily) or misoprostol (5 µg·kg−1, p.o., once daily) as positive control for different periods, giving the first dose 6 h after indomethacin administration. Misoprostol had been used as positive control because it is a synthetic prostaglandin E1 analogue and has been used for the healing of NSAID-induced gastric ulcer in humans. On the first, second, third, fourth, seventh and fifteenth days, the mice were killed by cervical dislocation under anaesthesia (ketamine, 12 mg·kg−1; Sigma). The stomach was removed rapidly, opened along the greater curvature and thoroughly rinsed with normal saline. The ulcerated gastric mucosal areas were visualized using a transparent sheet and a dissecting microscope. The damage score (DS) was assessed (Dokmeci et al., 2005) by grading the gastric injury on a 0–4 scale, based on the severity of hyperaemia and haemorrhagic erosions: 0, almost normal mucosa; 0.5, hyperemia; 1, one or two lesions; 2, severe lesions; 3, very severe lesions; 4, mucosa full of lesions. The sum of the total scores divided by the number of animals is expressed as the mean DS. The scores were assessed by two investigators unaware of the treatment of animals.
Histological analysis
After scoring the DS, the fundic stomach was prepared for histological studies. The tissue samples were fixed in 10% formalin and embedded in paraffin. The sections (5 µm) were cut with a microtome, stained with haematoxylin and eosin (Dokmeci et al., 2005), and examined under an Olympus microscope (BX41, Hamburg, Germany).
Myeloperoxidase (MPO) assay
The MPO activity was determined following a reported method (Suzuki et al., 1983) with slight modifications. Gastric tissues (100–150 mg) were homogenized in a 50 mM phosphate buffer (pH 6.0) containing 0.5% hexadecyltrimethylammonium bromide (Sigma). This was followed by three cycles of freezing and thawing. The homogenate was centrifuged at 12 000×g for 20 min at 4°C. The supernatant was collected for MPO assay, and a 50 µL sample added to 80 mM phosphate buffer, pH 5.4, 0.03 M tetramethyl benzidine TMB) in dimethyl formamide (Acros, Geel, Belgium) and 0.3 M H2O2 (35%, Lancaster, Morecambe, UK), to make a final reaction volume of 500 µL. After incubating the mixture at 25°C for 25 min, the reaction was terminated by adding 0.5 M H2SO4, and change in the absorbance was measured at 450 nm. Results were expressed as total number of neutrophils by comparing the OD of tissue supernatant with the OD of mice peritoneal neutrophils processed in the same way. A standard curve relating neutrophil numbers and absorbance was obtained by processing purified neutrophils, and assaying the MPO activity with 0.0005% hydrogen peroxide as the substrate. The correlation between the number of neutrophils and units of MPO was determined using a reported technique (Bradley et al., 1982). One unit of MPO activity is defined as that converting 1 µmol of hydrogen peroxide to water min−1 at 22°C.
Real-time PCR
Four days after ulcer induction, the tissue samples of the normal, ulcerated untreated and ulcerated resveratrol treated, mice were immediately immersed into RNA later solution (Sigma). After extracting the total RNA using Gen Elute Mammalian Total RNA miniprep kits (Sigma), and checking its integrity by electrophoresis, the cDNA was synthesized from 5 µg of purified total RNA using Revert Aid H minus first-strand cDNA synthesis kit (Fermentas Life Sciences, Ontario, Canada). Expressions of COX-1, COX-2, eNOS and iNOS were detected using suitably designed primers (Sigma) (Primer Express program, Applied Biosystems, CA, USA) shown in Table 1. The expressions of the designated enzymes were normalized against glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the internal reference. The experiments were performed (real-time PCR Systems 7500, Applied Biosystems) in triplicate by using Dynamo SYBR green qPCR kit (Finnzymes, Oy-keilaranta, Finland). The samples were quantified for all the above genes using the comparative Ct (ΔΔCt) method, as described in the Assays-on-Demand Users Manual (Applied Biosystems). The fold values (x) were calculated using the formula: x= 2(−ΔΔCt), where the data for the sample and sham-treated tissue (here, calibrator being the sham-treated tissues) were first normalized against variations of sample quality and quantity. The ΔΔCt was determined using the formula: ΔΔC(t) =ΔC(t)sample – ΔC(t)calibrator, where ΔC(t)sample =C(t)target gene of sample – C(t)reference, and ΔC(t)calibrator =C(t)target gene of calibrator −C(t)reference. The expression of the target genes was normalized to the reference gene and relative to the calibrator = 2−ΔΔC(t).
Table 1.
Primers for real-time PCR study
| Primer pairs | Database access number | Sequence |
|---|---|---|
| COX-1 (forward) | NM_008969 | 5′-ccggattggtggaggtaggaactttgac-3′ |
| COX-1 (reverse) | 5′-ggcgcatctctcgggactccttg-3′ | |
| COX-2 (forward) | NM_011198 | 5′-accccctgctgcccgacacct-3′ |
| COX-2 (reverse) | 5′-ccagcaacccggccagcaatc-3′ | |
| eNOS (forward) | NM_008713 | 5′-ccggcgctacgaagaatggaagtg-3′ |
| eNOS (reverse) | 5′-gggcgctgggtgctgaactgac-3′ | |
| iNOS (forward) | NM_010927 | 5′-gccttggctccagcatgtaccctcag-3′ |
| iNOS (reverse) | 5′-cctgcccactgagttcgtccccttc-3′ | |
| GAPDH (forward) | NM_008084 | 5′-ctgccacccagaagactgtg-3′ |
| GAPDH (reverse) | 5′-ggtcctcagtgtagcccaag-3′ |
PGE2 assay
Following harvesting of the stomach, the corpus (full thickness) was excised, weighed (100 mg) and suspended in 10 mM sodium phosphate buffer, pH 7.4 (1 mL). The tissues were finely minced and incubated at 37°C for 20 min. After centrifugation (9000×g), the PGE2 levels in the supernatant were measured by elisa, following the prostaglandin E2 EIA kit (Cayman Chemical, Ann Arbor, MI, USA) method.
Tissue nitric oxide measurement
As most of the NO is oxidized to nitrite and nitrate, the concentrations of these anions have been used as a quantitative measure of NO production. Here, we used nitrate/nitrite fluorometric assay kit (Cayman Chemical) to measure the production of NO by tissue samples. In brief, tissue samples were homogenized in PBS (pH 7.4), and centrifuged at 10 000×g for 20 mins. Then, the supernatant was filtered through 0.45 micron filter (Millipore, Leiden, The Netherlands), and the filtrate was ultrafiltered through a 10 kDa molecular weight cutoff filter (Millipore). Then, 10 µL of ultrapure samples was assayed spectrofluorimetrically by using the fluorescent dye 2,3-diaminonaphthalene (DAN) for nitrite and nitrate using the kit protocol at an excitation of 365 nm and emission of 430 nm.
Nomenclature
The nomenclature of drug and molecular targets in this article follows the recommendations of the BJP's Guide to Receptors and Channels (Alexander et al., 2008).
Statistical analyses
Data are expressed as mean ± SD unless mentioned. Comparisons were made between different treatments (anova) using the software GraphPad InStat. Tukey–Kramer multiple comparison tests was applied for the analysis of significance of all the data.
Results
Resveratrol aggravated ulcer scores and delayed ulcer healing process
The mice receiving vehicle only showed no lesions in the gastric mucosa. Indomethacin (18 mg·kg−1) produced typical time-dependent acute mucosal lesions in mice, as assessed by histology (Figure 1), and quantified in terms of DS (Figure 2). This treatment also produced extensive haemorrhagic damage in the stomach. Analysis of the DS values indicated that peak ulceration was evident at day 4 after ulcer induction, but with noticeable healing at day 7 (Figure 2). We chose the dose of resveratrol (10 mg·kg−1), based on previous reports (Dey et al., 2009; Guha et al., 2009). Treatment with resveratrol progressively enchanced the ulcerative damage up to the fourth day, followed by a gradual decline. However, mice receiving resveratrol showed higher (21%, P < 0.001 and 52% P < 0.001) DS values on 4 and 7 days, compared to the corresponding untreated mice with ulcers, suggesting that resveratrol treatment aggravated ulcer formation and also delayed healing. In resveratrol-treated mice, healing was almost complete at day 15.
Figure 1.
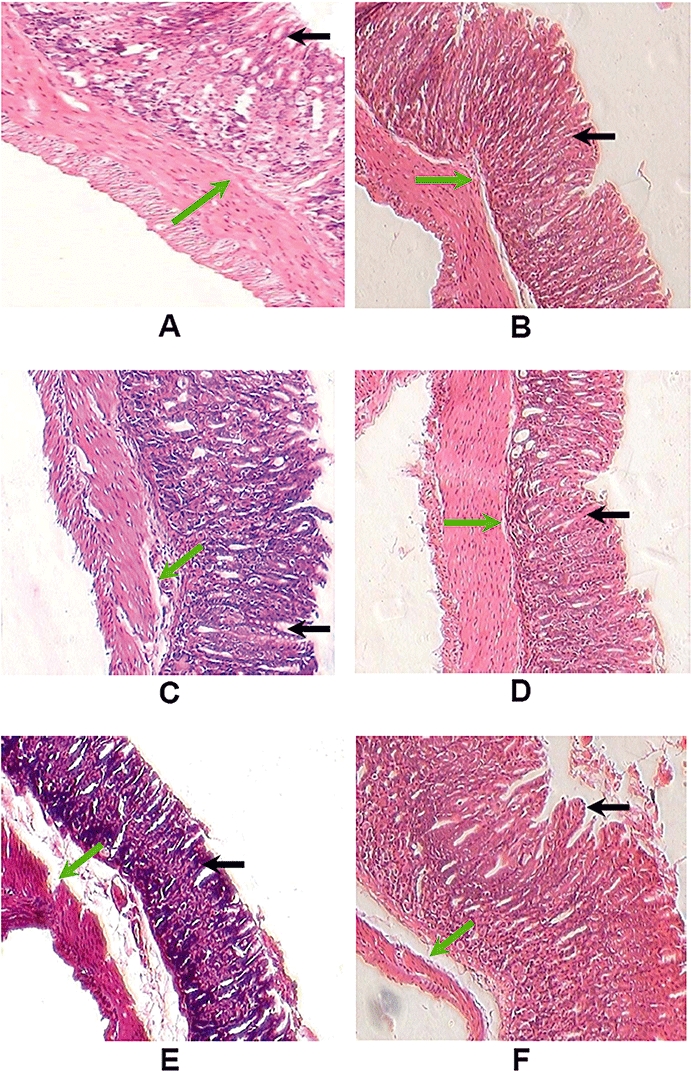
Histology of gastric tissues after induction of ulcers by indomethacin and the effects of resveratrol and l-arginine. Ulceration in mice was induced by indomethacin (18 mg·kg−1, p.o.). Resveratrol (10 mg·kg−1) and l-arginine (75 mg·kg−1) were administered 6 h after ulcer induction. On the fourth and seventh days of ulceration, the mice were killed and the gastric tissue was examined histologically. Histological photograph of Sham-treated (A), l-arginine treated on the seventh day (B), ulcerated untreated on the fourth day (C) and on the seventh day (D), ulcerated + resveratrol treated on the fourth day (E), and on the seventh day (F) of ulceration. Gastric tissue sections were photographed at ×10 magnifications (H&E stain). Mucosal and sub-mucosal layers are shown by black and green arrows respectively.
Figure 2.
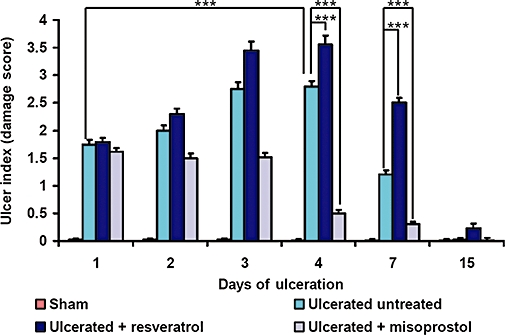
Resveratrol aggravated ulcer condition and delayed healing. Stomach ulceration in mice was induced by oral administration of indomethacin (18 mg·kg−1). Time-dependent activity of resveratrol (10 mg·kg−1) or misoprostol (5 µg·kg−1) against indomethacin-induced gastric ulcer was measured by the DS. The control group (sham) was treated with vehicle (2% gum acacia in distilled water). The values are mean (n= 6), and vertical error bars represent standard deviations. ***P < 0.001, for differences between the groups indicated.
MPO activity was induced by resveratrol treatment
MPO activity, a marker of granulocyte infiltration at the site of ulceration, also indicated that compared to the normal mice, there was an immediate accumulation of inflammatory cells in the ulcerated untreated mice (data not shown), reaching its maximum on the fourth day, but declining by the seventh day (Figure 3). The MPO levels in the resveratrol-treated mice were significantly higher (P < 0.001) at both day 4 and day 7, compared to the ulcerated untreated mice, again implying that resveratrol treatment aggravated the ulceration and also delayed healing.
Figure 3.
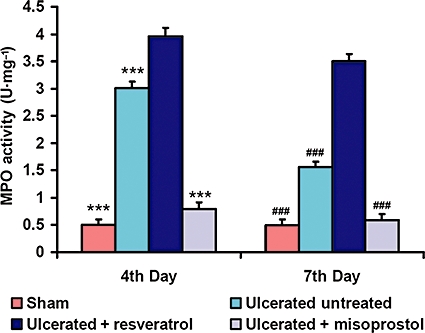
MPO activity was induced by resveratrol treatment. Effects of resveratrol (10 mg·kg−1) and misoprostol (5 µg·kg−1) on the MPO activity in the indomethacin-induced ulcerated mice were examined on the fourth and seventh days. The values are mean (n= 6), and vertical error bars represent standard deviations. ***P < 0.001 versus resveratrol-treated mice on the fourth day and ###P < 0.001 on the seventh day of ulcer induction. Misoprostol-treated mice (5 µg·kg−1) were considered as positive control.
Effects of resveratrol treatment on COX mRNA expressions
The real-time PCR assays showed that both COX-1 and COX-2 mRNA were suppressed on the fourth day after ulceration in the mucosa of the ulcerated untreated mice. At this time, resveratrol suppressed expression of mRNA for COX-1 (Figure 4A and B) (P < 0.001), without affecting the mRNA for COX-2. In contrast, at day 7, expression levels of both COX-1 and 2 increased by 2.7- and 2.6-fold, respectively, in ulcerated untreated mice, but in resveratrol-treated mice, only COX-1 mRNA expression was still significantly suppressed (P < 0.001) compared to the ulcerated untreated mice. However, misoprostol induced both COX-1 and COX-2 on both fourth and seventh days, respectively, compared to resveratrol-treated mice.
Figure 4.
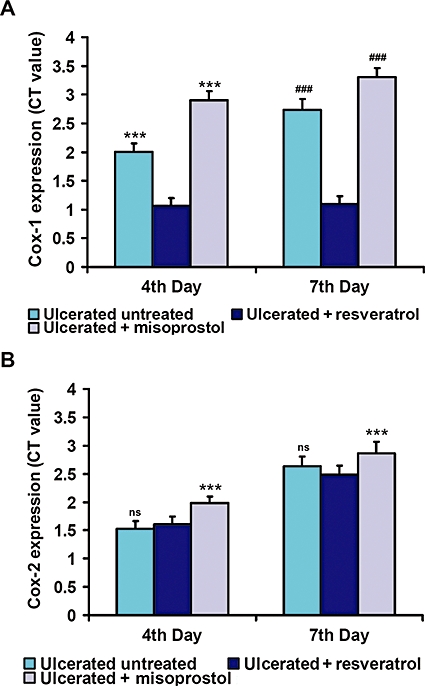
Effects of resveratrol treatment on COX mRNA expressions. COX-1 and COX-2 gene expressions were studied by real-time PCR (RT–PCR) on the fourth and seventh days of ulceration. Relative mRNA expression was calculated according to comparative ΔΔC(t) method. Quantitative mRNA expression of COX-1 (A) and COX-2 (B) were analysed respectively. The expression ratio values are mean (n= 6); vertical error bars represent standard deviations. ***P < 0.001 versus resveratrol-treated mice on the fourth day and ###P < 0.001 on the seventh day of ulcer induction; ns, not significant.
Effects of resveratrol treatment on PGE2 synthesis
The synthesis of mucosal PGE2 was markedly suppressed on the fourth day of ulceration in untreated mice (Figure 5; P < 0.001) and after resveratrol treatment (P < 0.001), compared to the sham value. At day 7, PGE2 synthesis increased in ulcerated untreated mice, and it was higher than that in the resveratrol-treated group. However, misoprostol treatment significantly (P < 0.001) increased PGE2 synthesis at days 4 and 7.
Figure 5.
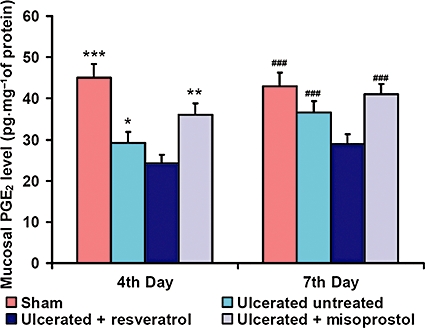
Effects of resveratrol treatment on PGE2 synthesis. The ability of resveratrol (10 mg·kg−1) to regulate PGE2 synthesis in gastric tissue from ulcerated mice was examined on the fourth and seventh days of ulcer induction. The PGE2 levels were measured using elisa. Data are expressed as mean (n= 6), and vertical error bars represent standard deviations. *P < 0.05, ***P < 0.001 versus resveratrol-treated mice on the fourth day and ###P < 0.001 on the seventh day of ulcer induction.
Resveratrol treatment significantly induced eNOS expression
Ulceration led to increased expressions of eNOS (1.5-fold) and iNOS (2.5-fold) (Figure 6A and B, respectively) in the ulcerated untreated mice, while in the resveratrol-treated mice the increase of the corresponding expressions were 2.5- and 1.9-fold on the fourth day after ulceration. At day 7, eNOS expression in ulcerated untreated mice increased (3.2-fold), and iNOS expression level was 1.7-fold, whereas resveratrol treatment increased the eNOS expression markedly, compared to ulcerated untreated mice (6.3-fold) (P < 0.001). Hence, the eNOS/iNOS ratio (Figure 6C) was relatively high in resveratrol-treated group at day 4, and it became significantly higher compared to the ulcerated untreated mice at day 7. Misoprostol treatment also induced eNOS expression at days 4 and 7 respectively. However, expression levels of iNOS in misoprostol-treated group were higher compared to resveratrol on the fourth day, but were almost equal on the seventh day after treatment.
Figure 6.
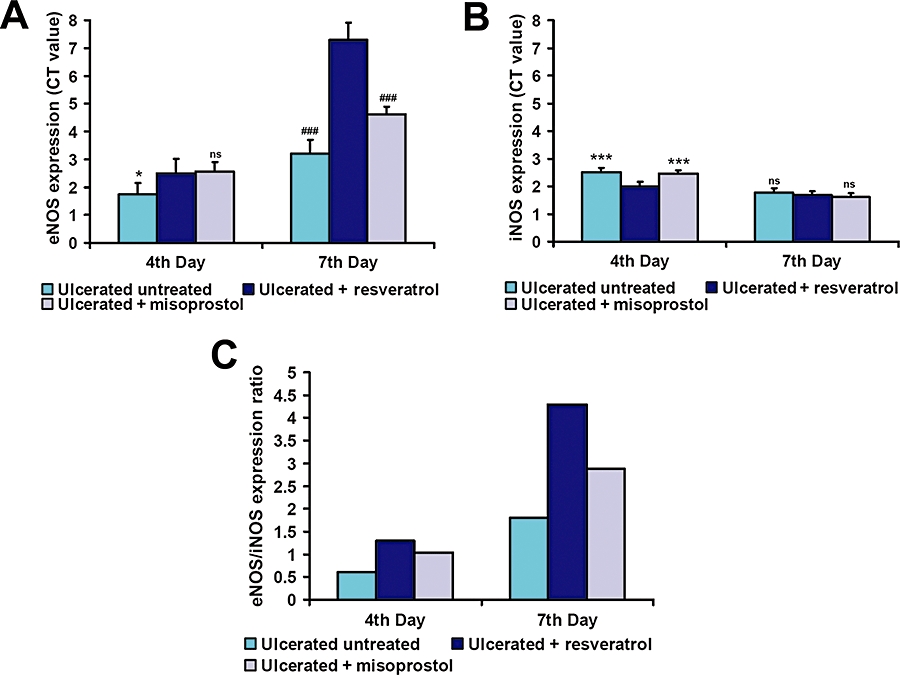
Resveratrol treatment significantly induced eNOS expression. The eNOS and iNOS gene expressions were studied by real-time PCR (RT–PCR), and on the fourth and seventh days of ulceration. Relative mRNA expression was calculated according to comparative ΔΔC(t) method. Quantitative mRNA expression of eNOS (A) and iNOS (B) was analysed. The expression ratio values are mean (n= 6), and vertical error bars represent standard deviations. *P < 0.05, ***P < 0.001 versus resveratrol-treated mice on the fourth day and ###P < 0.001 on the seventh day of ulcer induction; ns, not significant. (C) Ratios of eNOS/iNOS expression in gastric tissue.
l-Arginine, the substrate of NOS, dose dependently induced ulcer healing, which was reversed by l-NAME
To take advantage of the increased eNOS expression for accelerating ulcer healing, l-arginine was injected (i.p.) in resveratrol-treated mice. l-Arginine dose dependently reversed the pro-ulcer effects of resveratrol, and accelerated healing. The DS values and MPO assays showed that l-arginine reduced both these variables significantly (Figure 7A) (P < 0.001), compared to the resveratrol-treated mice, and accelerated healing on the seventh day after ulcer induction.
Figure 7.
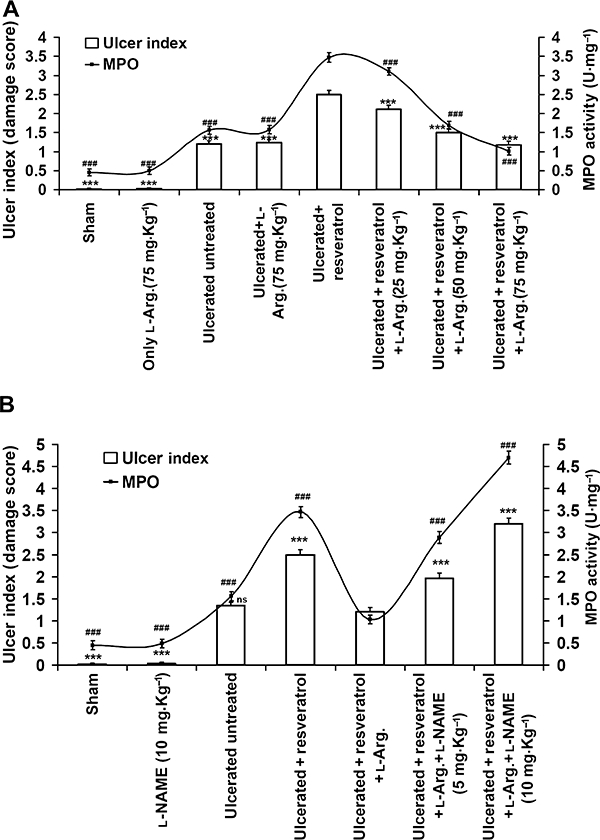
l-Arginine, the substrate of NOS, dose-dependently induced ulcer healing, which was reversed by l-NAME. (A) Ulcerated mice were treated with resveratrol (10 mg·kg−1) alone or in conjunction with l-arginine in a range of doses (25, 50, 75 mg·kg−1, once daily, i.p.). Ulcer healing was assessed by the DS values and MPO activity on the seventh day. The values are mean (n= 6), and vertical error bars represent standard deviations. ***P < 0.001 represents DS and ###P < 0.001 represents MPO activity versus resveratrol-treated mice. (B) To confirm that the healing of ulcers was due to the increased NO output from eNOS after l-arginine treatment, the mice were further treated, 15 min before l-arginine, with l-NAME, a non-selective inhibitor of NOS at two doses (5 and 10 mg·kg−1 i.p.). Data are expressed as mean (n= 6), and vertical error bars represent standard deviations. ***P < 0.001 represents DS and ###P < 0.001 represents MPO activity versus resveratrol +l-arginine- (75 mg·kg−1) treated mice.
The protective effect of l-arginine was prevented in a dose-dependent manner by l-NAME (Figure 7B). l-NAME reversed the beneficial effect of l-arginine in a dose-dependent manner by increasing the MPO activity and DS value, and severely delaying ulcer healing.
l-Arginine treatment induced tissue nitric oxide (NO) synthesis in resveratrol-treated ulcerated mice
Spectrofluorimetric data revealed that l-arginine treatment (75 mg·kg−1) significantly (P < 0.001) creased the synthesis of total NO in tissues from resveratrol-treated mice compared to l-arginine untreated, resveratrol-treated and ulcerated untreated mice on the fourth and seventh days, respectively (Figure 8). In the misoprostol-treated group, NO synthesis was significantly (P < 0.001) increased on the fourth day, but became almost equal on the seventh day to that in the ulcerated untreated group. However, l-NAME (10 mg·kg−1) reversed the beneficial effect of l-arginine, and significantly reduced the NO synthesis.
Figure 8.
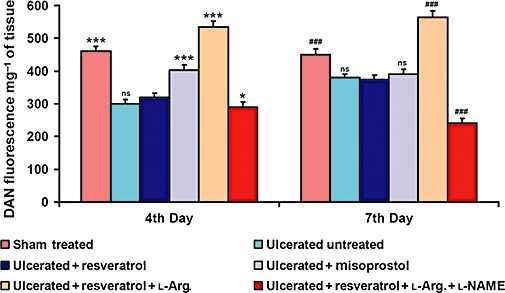
l-Arginine treatment induced tissue nitric oxide (NO) synthesis in resveratrol-treated ulcerated mice. Ability of l-arginine (75 mg·kg−1) and l-NAME (10 mg·kg−1) in regulating tissue NO synthesis in ulcerated + resveratrol-treated mice was examined on the fourth and seventh days of ulcer induction. The NO levels were measured using the fluorescence dye DAN, and assayed by spectrofluorimeter. Data are expressed as mean (n= 6), and vertical error bars represent standard deviations. *P < 0.05, ***P < 0.001 versus resveratrol-treated mice on the fourth day and ###P < 0.001 on the seventh day of ulcer induction.
Discussion and conclusion
Delayed healing of ulcers or pro-ulcer effects of resveratrol are major side effects overshadowing its otherwise beneficial therapeutic properties. It is well known that red wine contains a high percentage of resveratrol, and the antioxidant property of resveratrol is of benefit against different disease processes, including neurodegeneration and cardiac dysfunction (Hao and He, 2004), etc. We have already shown that resveratrol at a higher dose aggravated ulcers, but at a lower dose, it helped in healing ulcers (Dey et al., 2009), indicating a dose-dependent shift from antioxidant to pro-oxidant effects. Excessive intake of red wine can be ulcerogenic, but moderate wine consumption may be beneficial for health. Resveratrol given alone does not induce ulcers (data not shown), but given chronically it delays healing of pre-existing ulcers. Thus, elimination of its pro-ulcer effects is an essential prerequisite for any clinical use of resveratrol. This is the first report demonstrating that l-arginine treatment could effectively alleviate the pro-ulcer effects of resveratrol.
Our macroscopic examinations revealed that administration of indomethacin caused marked haemorrhagic damage in stomach within 6 h. Maximum ulcerative damage was observed on the fourth day after administration of indomethacin (Figure 1). However, on the seventh day, healing of the ulcers was evident. MPO activity, a marker of neutrophil accumulation at the site of inflammation, is frequently increased in ulcerated conditions, and is reduced as the healing progresses (Souza et al., 2004). Consistent with this, we found that ulceration increased the MPO activity up to the fourth day, followed by its gradual reduction on the seventh day (Figure 3). Resveratrol (10 mg·kg−1) aggravated ulceration (Figure 1) and also delayed healing, as observed earlier (Dey et al., 2009; Guha et al., 2009). The DS results were well supported by the MPO activity data, with a similar time course (Figure 2). This established that resveratrol exhibited pro-ulcer effects in our model of gastric ulceration induced by indomethacin in mice.
The NSAIDs exert both their therapeutic and toxic effects mainly through inhibiting COX and decreasing the levels of PGE2 at the gastric mucosa. There has been accumulating evidence that PGs might contribute to ulcer healing by inducing pro-angiogenic factors (Miller, 1983). Reduced levels of PGE2 cause gastric ulceration and also exacerbate pre-existing gastric ulcers in rodents and humans.
Our gene expression studies revealed reduced expression of both COX-1 and COX-2 after indomethacin. We also found that resveratrol selectively suppressed the expression of COX-1 mRNA, but not of COX-2 (Figure 4A and B). It is believed that gastric injury develops only when both COX-1 and COX-2 are inhibited. Given that resveratrol did not alter the COX-2 status, it should not be ulcerogenic in healthy mice, as we observed in our studies. Our results on the depletion of the mucosal PGE2 level due to ulceration itself, and more by resveratrol (Figure 5), also correlated well with the COX-1 results.
Another essential enzyme that is actively involved in inflammation and healing processes is NOS, which metabolizes l-arginine to l-citrulline and NO. Hence, the level of l-arginine is an important factor determining NO formation by NOS. NOS exists as constitutive and inducible isoforms (iNOS). eNOS and neuronal NOS (nNOS) are expressed constitutively in cells, but iNOS is largely induced under certain pathological conditions. In gastric ulceration, there is a major shift in the eNOS/iNOS balance, associated with vasoconstriction and reduced gastric mucosal blood flow (Hirose et al., 1991). NO constantly formed through eNOS in the endothelium of the gastric tissue dilates blood vessels and increases blood flow in the gastric mucosa, and plays a major role in ulcer healing (Ma et al., 1999).
We also found low eNOS expression associated with significant enhancement of iNOS after indomethacin treatment (Figure 6A and B). This might contribute to stimulating inflammatory situations, contributing to the ulcerogenic property of indomethacin. However, resveratrol reduced iNOS expression substantially, while augmenting eNOS, leading to an increased ratio of eNOS/iNOS (Figure 6C).
Hence, we tried to take advantage of this increased eNOS activity to overcome the pro-ulcer effects of resveratrol, and its effects as a specific inhibitor of COX-1, by giving mice l-arginine simultaneously with resveratrol to induce eNOS activity. Treatment with l-arginine dose dependently reversed the pro-ulcer effects (Figure 7A) of resveratrol, and the effective dose was 75 mg·kg−1 (i.p., once daily). Importantly, in this dosage, l-arginine did not show any healing response in ulcerated untreated mice. This difference would be compatible with the increased levels of eNOS in mice treated with resveratrol, allowing full utilization of exogenous l-arginine to provide higher levels of NO and reversing the pro-ulcer side effects of resveratrol.
To confirm that l-arginine modulated eNOS activity and thus induced healing, the mice were further treated with l-NAME, a non-selective inhibitor of NOS simultaneously with l-arginine, which dose dependently delayed ulcer healing and reversed the healing effects of l-arginine (Figure 7B). This observation thus confirmed that l-arginine enhanced NO production via eNOS, and accelerated the healing process.
Although eNOS expression was increased by resveratrol use of this increased synthetic capacity by providing more substrate (l-arginine) was essential to potentiate ulcer healing. To explore this further, the total NO output from gastric tissue was analysed, and showed that resveratrol-treated ulcerated mice produced significantly (P < 0.001) less NO than l-arginine-treated mice. This fall in NO might be due to the down-regulation of iNOS, counteracting the increased NO production by the induced eNOS, after resveratrol treatment.
In conclusion, resveratrol treatment delayed ulcer healing in mice with gastric ulcers induced by indomethacin, and suppressed COX-1, but not COX-2, expression. Resveratrol simultaneously induced eNOS expression, which, after l-arginine treatment, produced increased levels of NO. This increased utilization of l-arginine by eNOS induced by resveratrol then alleviated the pro-ulcer adverse effects of resveratrol.
Acknowledgments
The authors are grateful to the reviewers for valuable suggestions for the improvement of the manuscript; Professor P.K. Mitra, Director, IPGME&R, Kolkata for providing infrastructural facilities, as well as constant encouragement. The work was financially supported by Life Science Research Board, Defence Research & Development Organisation and Department of Science and Technology, government of India.
Glossary
Abbreviations:
- COX
cyclooxygenase
- DS
damage score
- eNOS
endothelial NOS
- iNOS
inducible NOS
- l-NAME
Nω-nitro-l-arginine methyl ester hydrochloride
- MPO
myeloperoxidase
- NSAID
non-steroidal anti-inflammatory drug
- PGE2
prostaglandin E2
Conflict of interest
None.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Dose-dependent effects of trans-resveratrol on indomethacin-induced ulcer in mice. ***P < 0.001 with respect to trans-resveratrol dose 20 mg·kg−1 respectively. ns, non-significant. The values are mean ± SD (n = 6).
Figure S2 The gene sequences of set of genes taken for RT–PCR and the sites, where the respective primer binds (highlighted in yellow).
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edn. (2008 revision) Br J Pharmacol. 2008;153(Suppl 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee D, Bauri AK, Guha RK, Bandyopadhyay SK, Chattopadhyay S. Healing properties of malabaricone B and malabaricone C, against indomethacin-induced gastric ulceration and mechanism of action. Eur J Pharmacol. 2008;578:300–312. doi: 10.1016/j.ejphar.2007.09.041. [DOI] [PubMed] [Google Scholar]
- Bertelli AA, Giovannini L, Giannessi D, Migliori M, Bernini W, Fregoni M, et al. Antiplatelet activity of synthetic and natural resveratrol in red wine. Int J Tissue React. 1995;17:1–3. [PubMed] [Google Scholar]
- Bradley PP, Christensen RD, Rothstein G. Cellular and extracellular myeloperoxidase in pyogenic inflammation. Blood. 1982;3:618–622. [PubMed] [Google Scholar]
- Brzozowski T, Konturek PC, Konturek SJ, Sliwowski Z, Drozdowicz D, Stachura J, et al. Cyclooxygenase-1 and cyclooxygenase-2 in healing of ischemia–reperfusion-induced gastric lesions. Eur J Pharmacol. 1999;385:47–61. doi: 10.1016/s0014-2999(99)00681-0. [DOI] [PubMed] [Google Scholar]
- Brzozowski T, Konturek PC, Konturek SJ, Sliwowski Z, Pajdo R, Drozdowicz D, et al. Classic NSAID and selective cyclooxygenase (COX)-1 and COX-2 inhibitors in healing of chronic gastric ulcers. Microsc Res Tech. 2001;53:343–353. doi: 10.1002/jemt.1102. [DOI] [PubMed] [Google Scholar]
- Das DK, Maulik N. Resveratrol in cardioprotection: a therapeutic promise of alternative medicine. Mol Interv. 2006;6:36–47. doi: 10.1124/mi.6.1.7. [DOI] [PubMed] [Google Scholar]
- Dey A, Guha P, Chattopadhyay S, Bandyopadhyay SK. Biphasic activity of resveratrol on indomethacin-induced gastric ulcers. Biochem Biophys Res Commun. 2009;381:90–95. doi: 10.1016/j.bbrc.2009.02.027. [DOI] [PubMed] [Google Scholar]
- Dokmeci D, Akpolat M, Aydogu N, Doganay L, Turan FN. l-Carnitine inhibits ethanol-induced gastric mucosal injury in rats. Pharmacol Rep. 2005;57:481–488. [PubMed] [Google Scholar]
- Frankel EN, Waterhouse AL, Kinsella JE. Inhibition of human LDL oxidation by resveratrol. Lancet. 1993;341:1103–1104. doi: 10.1016/0140-6736(93)92472-6. [DOI] [PubMed] [Google Scholar]
- Goldberg DM, Karumanchiri A, Yan J, Soleas G, Ng E, Waterhouse AL, et al. A global survey of trans-resveratrol concentrations in commercial wines. Am J Enol Vitic. 1995;46:159–165. [Google Scholar]
- Guha P, Dey A, Sarkar B, Dhyani MV, Chattopadhyay S, Bandyopadhyay SK. Improved antiulcer and anticancer properties of a trans-resveratrol analog in mice. J Pharmacol Exp Ther. 2009;328:1–10. doi: 10.1124/jpet.108.145334. [DOI] [PubMed] [Google Scholar]
- Hain R, Bieseler B, Kindl H, Schroder G, Stocker R. Expression of a stilbene synthase gene in Nicotiana tabacum results in synthesis of the phytoalexin resveratrol. Plant Mol Biol. 1990;15:325–335. doi: 10.1007/BF00036918. [DOI] [PubMed] [Google Scholar]
- Hao HD, He LR. Mechanisms of cardiovascular protection by resveratrol. J Med Food. 2004;7:290–298. doi: 10.1089/jmf.2004.7.290. [DOI] [PubMed] [Google Scholar]
- Hirose H, Takeuchi K, Okabe S. Effect of indomethacin on gastric mucosal blood flow around acetic acid-induced gastric ulcers in rats. Gastroenterology. 1991;100:1259–1265. [PubMed] [Google Scholar]
- Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- Ma L, Wallace JL. Endothelial nitric oxide synthase modulates gastric ulcer healing in rats. Am J Physiol Gastrointest Liver Physiol. 2000;279:341–346. doi: 10.1152/ajpgi.2000.279.2.G341. [DOI] [PubMed] [Google Scholar]
- Ma L, Yip J, Chow C, Cho CH. Cigarette smoking delays ulcer healing: role of constitutive nitric oxide synthase in rat stomach. Am J Physiol Gastrointest Liver Physiol. 1999;276:238–248. doi: 10.1152/ajpgi.1999.276.1.G238. [DOI] [PubMed] [Google Scholar]
- Martín AR, Villegas I, Sánchez-Hidalgo M, de la Lastra CA. The effects of resveratrol, a phytoalexin derived from red wines, on chronic inflammation induced in an experimentally induced colitis model. Br J Pharm. 2006;147:873–885. doi: 10.1038/sj.bjp.0706469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TA. Protective effects of prostaglandins against gastric mucosal damage: current knowledge and proposed mechanisms. Am J Physiol. 1983;245:G601–G623. doi: 10.1152/ajpgi.1983.245.5.G601. [DOI] [PubMed] [Google Scholar]
- Siemann EH, Creasy LL. Concentration of the phytoalexin resveratrol in wine. Am J Enol Vitic. 1992;43:49–52. [Google Scholar]
- Souza MHLP, Lemos HP, Oliveira RB, Cunha FQ. Gastric damage and granulocyte infiltration induced by indomethacin in tumour necrosis factor receptor 1 (TNF-R1) or inducible nitric oxide synthase (iNOS) deficient mice. Gut. 2004;53:791–796. doi: 10.1136/gut.2002.012930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaramaiah K, Chung WJ, Michaluart P, Telang N, Tanabe T, Inoue H, et al. Resveratrol inhibits cyclooxygenase-2 transcription and activity in phorbol ester-treated human mammary epithelial cells. J Biol Chem. 1998;273:21875–21882. doi: 10.1074/jbc.273.34.21875. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Ota H, Sasagawa S, Sakatani T, Fujikura T. Assay method for myeloperoxidase in human polymorphonuclear leukocytes. Anal Biochem. 1983;132:345–352. doi: 10.1016/0003-2697(83)90019-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


