Abstract
Lysyl oxidase (LOX) is a copper-dependent enzyme which initiates covalent crosslinking of elastin precursors by oxidizing peptidyl lysine to aminoadipic semialdehydes. Previous studies have shown LOX deficiency to affect crosslinking of elastin and collagen in vivo, resulting in disorganized connective tissue formation. In this study, we investigated the utility of exogenously supplemented LOX peptides (50–100 μL/well) to elastin synthesis, crosslinking efficiency and matrix deposition in adult rat aortic smooth muscle cell (RASMC) cultures. Additionally, we also examined the role of LOX peptides on SMC proliferation and matrix metalloproteinases (MMPs) synthesis in these cultures. Highly purified bovine aorta LOX peptide was found to increase matrix elastin synthesis by 40–80% to that in control cultures in a dose-dependent manner, while the crosslinking efficiency significantly (as measured by the ratio of matrix elastin protein to the total elastin protein synthesized) improved to 45–55% of total elastin synthesized under these conditions. However, LOX peptides neither affected SMC proliferation relative to controls, nor elastin precursor (tropoelastin) synthesis or the total elastin synthesis on a per cell basis. In general, LOX peptides also did not affect MMPs-2, 9 activities relative to control cultures, expect for MMP-9 activity suppression at higher LOX dose, suggesting that these LOX peptide cues could be safely used to enhance tropoelastin crosslinking into matrix structures and elastin matrix yield, within tissue engineered constructs, a major challenge in the field.
Keywords: elastin synthesis, extracellular matrix, smooth muscle cells
1. Introduction
The extracellular matrix (ECM) of vascular and other connective tissues consists of a network of elastic fibers, which provides them resilience and the ability to recoil after transient stretch1. Elastic fibers are typically composed of a core of amorphous, crosslinked elastin protein surrounded by peripheral fibrillin microfibrils, besides numerous other components. They are deposited and crosslinked via a highly regulated, multi-step, hierarchical assembly process1. Besides contributing to tissue elasticity, elastic fibers also maintain SMCs quiescent2 and regulate their migration3. Thus, inflammation- or injury-induced elastin breakdown into elastin peptides (EPs) can interrupt normal elastin-SMC signaling pathways3, incite matrix metalloproteinase (MMP) release by recruited macrophages and thus initiate and propagate the pathology of atherosclerotic plaques and aneurysms4. In such cases, vascular elastin must be restored or regenerated as a priority to restore vascular homeostasis.
Efforts to regenerate lost elastin structures in vivo and within tissue-engineered vascular constructs are limited by the progressive destabilization of tropoelastin mRNA expression in adult vascular cells5, 6, and the current unavailability of appropriate cellular cues that upregulate elastin precursor production and assembly into structural matrix protein which are faithful mimics of native elastin networks. In this study, we seek to test the hypothesis that providing biochemical cues that vitally facilitate and regulate elastogenesis in vivo may upregulate cellular synthesis, deposition and maturation of mimics of native elastin matrix protein in vitro. Among these factors are lysyl oxidase (LOX) and LOX-like proteins (LOXL)7, endogenous enzymes that crosslink soluble ECM precursor molecules such as the elastin precursor, tropoelastin8, 9. LOX juxtaposes with tropoelastin on fibulin-5 microfibrils, activates it by deaminating its lysine residues, and allows it to coacervate onto the cellular substrate and spontaneously crosslink1. LOX has also been implicated to play an important role in the morphogenesis and repair of connective tissues of the cardiovascular, respiratory, skeletal, and other systems of the body10.
Despite involvement of endogenous LOX in physiologic elastin synthesis and organization, and our prior findings that the active form of the enzyme is synthesized by cultured SMCs11–13, elastin synthesis and elastin matrix yield [defined as matrix elastin amount/(tropoelastin + matrix elastin)] within SMC cultures and tissue-engineered constructs remains rather poor (15–20%). In this study, we investigate for the first time, if supplementation of SMC cultures with exogenous LOX peptides can improve elastin matrix deposition and/or yields and perhaps even enhance tropoelastin precursor output by vascular cells. If this is so, exogenous LOX peptides may be usefully employed alone or together with other identified elastogenic cues13 (e.g., HA oligomers, TGF-β1) to enhance elastin precursor synthesis itself, and improve elastin matrix yield and maturation (i.e., crosslinking). Our findings are expected to greatly benefit efforts to regenerate elastin matrix in situ within diseased or injured vessels, to stabilize them, and also to augment synthesis and assembly of close mimics of vascular elastin matrix protein using tissue-engineering principles.
2. Materials and Methods
2.1 Bovine LOX Synthesis
Bovine LOX was kindly supplied by Dr. Herbert Kagan at Boston University. LOX protein was isolated from bovine aorta as per methods previously described14, 15. All chemicals for isolation and purification of LOX were purchased from Sigma Aldrich (St. Louis, MO). Briefly, bovine aortae from 2–6 week old calves were coarsely ground and extracted twice with buffer (0.4 M NaCl, 16 mM potassium phosphate, pH 7.8, 4°C). The extracted pellets in 4 M urea (with 16 mM potassium phosphate, pH 7.8) were mixed with hydroxyapatite, stirred for 10 min at 4°C, allowed to settle for 30 min, and decanted. The supernatant was centrifuged at 10000 g for 10 min, concentrated, and dialyzed against buffer containing 16 mM potassium phosphate, pH 7.8. The crude enzyme was precipitated by adding equal volume of 1 M potassium phosphate, pH 7.8, and was resolved by chromatography through a column eluting with 16 mM potassium phosphate, 6 M urea, pH 7.8. The enzymatically active fractions from the chromatography column were pooled and the urea concentration was adjusted from 6 to 2 M by dilution with 16 mM potassium phosphate, pH 7.8. The column was washed with buffer until optical density of the effluent < 0.002, and further washed with 16 mM potassium phosphate, pH 7.8, followed by 0.4 M NaCl, 16 mM potassium phosphate, pH 7.8. LOX was then eluted using a gradient of urea concentration (from 0 urea, 0.4 M NaCl, 16mM potassium phosphate, pH 7.8, to 6 M urea 0.4 M NaCl, 16 mM potassium phosphate, pH 7.8) at a flow rate of 1 mL/min. The enzymatically active fractions were pooled, concentrated, dialyzed against 16 mM potassium phosphate, pH 7.8, buffer and stored in aliquots at 280°C. SDS PAGE revealed the presence of a 32-kDa (90%) and a low-molecular-weight (24-kDa) band (10%) in the purified sample. The enzyme was stored in a 4 M urea, 16 mM potassium phosphate, pH 7.8 buffer. Prior to use, the enzyme was dialyzed with water overnight, lyophilized, reconstituted in 1 mL of distilled water, and stored at −20°C. The yield of enzyme was 0.4 mg from 350 g of bovine aorta. The purified enzyme aliquot, as used, contained 68 μg of LOX protein/mL and exhibited specific activity of 0.5 nMoles of H2O2 release/min/μg, as measured using a fluorometric assay (Amplex Red Assay; Invitrogen, Eugene, OR) based on detection of H2O2, generated when LOX acts on a synthetic substrate.
2.2 Cell Culture
Rat aortic smooth muscle cells (RASMCs; passage 3) were seeded onto 6-well tissue culture plates (A = 10 cm2) at a seeding density of 4 × 104 cells/well and cultured in 3 mL of DMEM-F12 medium (Invitrogen, Grand Island, NY) containing 10% v/v of fetal bovine serum, and 1% v/v of Penstrep. LOX was supplemented exogenously to the culture wells on day 1, at final doses of either 50μg/well (LOX-1) or 100μg/well (LOX-2), except in control cultures which received no LOX (n = 3 wells/dose/time point). These concentrations correspond to initial LOX doses of 1.25 ng and 2.5 ng, respectively on a per cell basis. Since a prior study29 showed that 10 μg/mL dose of LOX pro-peptides (not LOX itself) influences SMC behavior, we selected the enzyme concentrations in this dose range for the current study. A 1-mL culture medium was replaced regularly, and the spent medium from each well pooled at the end of 21 day culture and frozen for further biochemical analysis.
2.3 DNA Assay
The DNA content of cell layers was quantified at 1 and 21 days of culture to assess the proliferation of SMCs and to normalize the measured amounts of synthesized matrix to cell count. Briefly, cell layers were detached with 0.25% v/v trypsin-EDTA, homogenized, pelleted by centrifugation, re-suspended in NaCl/Pi buffer, sonicated, and assayed using a fluorometric assay16. The cell density was calculated on the basis of an estimated 6 pg DNA/cell16.
2.4 Fastin Assay for Elastin
The amount of elastin deposited within the cellular matrix (alkali-soluble and insoluble fractions) or released as soluble tropoelastin (in pooled spent medium) were quantified using a Fastin assay (Accurate Scientific Corp, Westbury, NY), as detailed previously17. The cell layers were homogenized in distilled water, pelleted by centrifugation (10000 g, 10 min) and digested with 1 mL of 0.1 N NaOH (1 h, 98°C). The digestate was then centrifuged to isolate a mass of insoluble, crosslinked elastin. The supernatant containing uncrosslinked matrix elastin was neutralized with an equal volume of 12 N HCl, and hydrolyzed at 110 °C for 16 h, dried overnight, and reconstituted in 0.5-mL of water. Since the Fastin assay quantifies only soluble α-elastin, the insoluble elastin was first reduced to a soluble form by digesting with 0.25 N oxalic acid (1 h, 95°C), and the pooled digestate filtered within microcentrifuge tubes fitted with 10 kDa cut-off membranes. Spent fractions of medium pooled at weekly intervals over the 3-week culture period were lyophilized and processed for tropoelastin using the Fastin assay. The measured elastin amounts were normalized to corresponding DNA amounts to provide a reliable basis of comparison between samples.
2.4 ELISA for Desmosine Crosslinks
The desmosine crosslink densities within elastin matrix protein were quantified using ELISA to determine if any of the provided cues enhanced elastin crosslinking11. The 21-day old cell layers were digested with collagenase (Type II, Worthington Scientific, Lakewood, NJ; 12 h, 37°C) and elastase (Worthington Scientific; 12 h, 37°C), the digestates acid-hydrolyzed (6 N HCl, 110°C, 16 h), and desmosine content in the reconstituted dried residue determined by ELISA, and compared to corresponding trends in insoluble matrix elastin production.
2.5 Gel Zymography for MMP activity
MMPs-2, 9 activity in test and control RASMC cultures in response to potential activation by bovine LOX, were compared using gelatin zymography18. Briefly, aliquots of spent culture medium were assayed for protein content using the BCA assay, and all lanes were loaded in triplicate with 15 μg of protein from each extract alongside with pre-stained molecular weight standards (Bio-Rad). After development and staining, densities of MMP-2 and 9 bands, visible on a dark background of stained gelatin were measured using Gel Pro Analysis software (Media Cybernetics, Bethesda, MD), and reported as relative density units (RDU).
2.6 Statistical Analysis
All experiments were performed in triplicate and quantitative results reported as mean ± SD. Statistical significance between and within groups was determined using 2-way ANOVA, which is appropriate since the data followed a near-Gaussian distribution. Results were deemed significantly different from controls for p < 0.05.
3. Results
3.1 Cell Proliferation
RASMCs cultured with LOX-1 or LOX-2 appeared morphologically similar to control cells. All cultures achieved confluence between 2 and 3 weeks after seeding. Figure 1 shows the DNA content of control RASMCs and those cultured with bovine LOX. Over 21 days, RASMCs in control cultures proliferated 11.7 ± 2.35 –fold over the cell count at day 1, which corresponded to a DNA content of 264 ± 0.1 ng/well. Test cultures that received the lower dose of LOX (LOX-1) exhibited a moderate increase in cell proliferation (1.45 ± 0.34-fold increase over controls; p = 0.067 vs. controls), while those that received the higher dose (LOX-2) did not differ from control cultures (1.23 ± 0.23–fold vs. controls; p = 0.8). Differences in cell proliferation ratios between cultures that received LOX-1 and LOX-2 were deemed statistically insignificant (p = 0.13 for LOX-1 vs. LOX-2).
FIG 1.
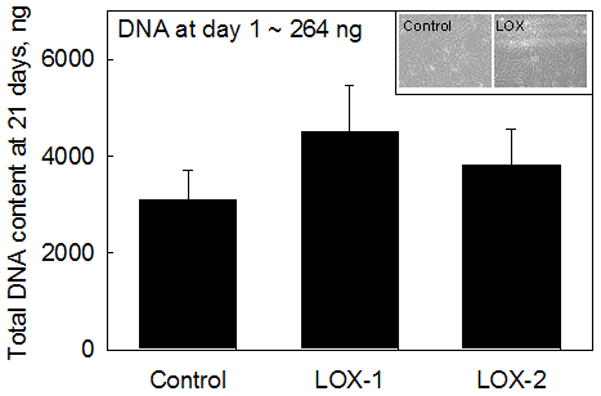
Total DNA content in RASMC cultures supplemented with bovine LOX (LOX-1: 50 μL/mL or 1.25 ng/cell, LOX-2: 100 μL/mL or 2.5 ng/cell) were not significantly different from additive-free control cultures. Data shown represent mean ± SD of DNA content measured in n = 3 cell layers/treatment after 21 days of culture. Significance of differences relative to controls was deemed for p<0.05. Cell proliferation ratios in each case may be calculated as the respective ratios of DNA content at 21 days to that at 1 day after seeding. Control and LOX-treated (both doses) cell layers, shown at 21 days of culture did not exhibit any differences in cell morphology or density.
3.2 Elastin Protein Synthesis
Table 1 compares the absolute amounts of elastin production within cultures receiving the different treatments. Over 21 days, SMCs cultured in presence of LOX-1 generated less tropoelastin (29.0 ± 8.3 mg/well), than in control cultures (46.5 ± 5.0 mg/well), while those cultured in presence of LOX-2 generated almost identical amounts (50.5 ± 0.8 mg/well). When normalized to DNA content, tropoelastin synthesis in the presence of LOX-1 was likewise much lower (6441 ± 1834 ng/ng DNA) relative to controls (14990 ± 1618 ng/ng of DNA) while that in presence of LOX-2 (13235 ± 225 ng/ng DNA) was similar to controls (p = 0.001 and 0.11 respectively vs. controls; Figure 2A). The increase in tropoelastin synthesis with the corresponding doubling of supplemented LOX dose was deemed to be statistically significant (p = 0.022 for LOX-1 vs. LOX-2).
Table 1.
Absolute amounts of elastin produced in cultures treated with LOX-1, LOX-2 and within non-additive controls at the end of 21 days. Data shown here represents the average of n = 3/case.
| Culture Condition | Tropoelastin, mg | Matrix elastin, mg | Total elastin/DNA, mg/mg | |
|---|---|---|---|---|
| Soluble fraction, mg | Crosslinked Insoluble fraction, mg | |||
| Control | 46.5 ± 5 | 16.3 ± 2.2 | 2.3 ± 0.3 | 21.017 ± 2445 |
| LOX-1 | 29 ± 8.3 | 32.5 ± 4.7 | 5.8 ± 0.01 | 14953 ± 2897 |
| LOX-2 | 50.5 ± 0.8 | 34.4 ± 0.5 | 7.1 ± 0.2 | 24111 ± 423 |
FIG 2.
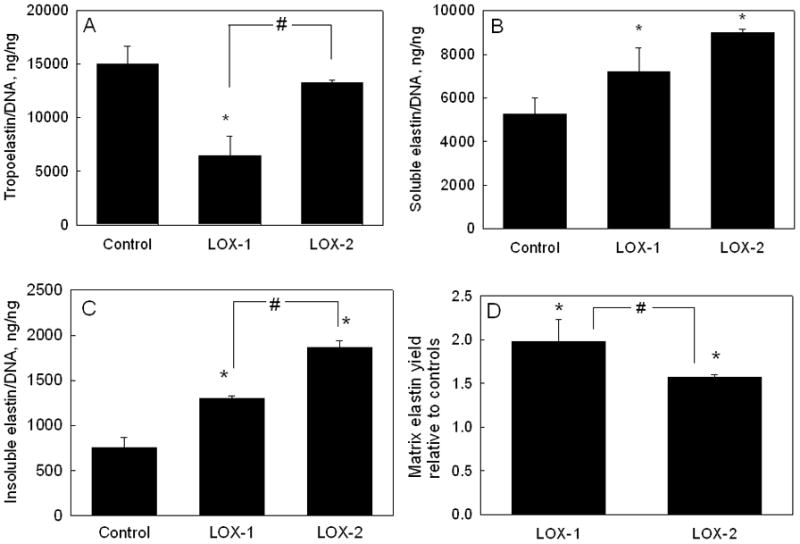
Effects of exogenous LOX additives at doses of 1.25 ng/cell (LOX-1) and 2.5 ng/cell (LOX-2) on tropoelastin (A), alkali-soluble matrix elastin (B), and alkali-insoluble (crosslinked) matrix elastin C) synthesized by adult RASMCs, and on (D) elastin matrix yield. Values (mean ± SD) are shown normalized to the DNA content of the respective cell layers at 21 days of culture (n = 3/case), and in the case of matrix yield, are instead shown normalized to the yield in control cultures. * represents significant differences relative to control cultures, and # represents significant differences between LOX-1 and LOX-2, deemed for p <0.05.
Elastin incorporated into the cell layer (matrix elastin) was quantified as the sum of two individual fractions, i.e., a highly crosslinked, alkali-insoluble elastin pellet, and an alkali-soluble fraction. Absolute amounts of matrix elastin measured in the respective cultures are specified in Table 1. On a DNA-normalized basis, addition of LOX-1 and LOX-2 increased total matrix elastin synthesis by 1.4 ± 0.17–fold and 1.8 ± 0.03–fold, respectively, relative to controls (6027 ± 826 ng/ng DNA; p < 0.01 vs. controls in both cases; Table 1); LOX-1 was found to increase soluble matrix elastin synthesis 1.37 ± 0.2-fold over control cultures (5268 ± 719 ng/ng DNA), and LOX-2 to increase the same by 1.7 ± 0.02–fold (p < 0.01 vs. controls in both cases; p = 0.097 for LOX-1 vs. LOX-2; Figure 2B). Similarly, LOX-1 induced a 1.71 ± 0.04-fold increase in synthesis of the highly crosslinked alkali-insoluble matrix elastin fraction over control cultures (758 ± 106 ng/ng DNA), while LOX-2 induced a 2.46 ± 0.08 fold increase (p < 0.001 vs. controls in both the cases; p = 0.0045 for LOX-1 vs. LOX-2; Figure 2C).
As indicated in Table 1, total elastin output (i.e., tropoelastin + matrix elastin) from LOX-1-supplemented cultures was lower than in control cultures, signifying a corresponding decrease in elastin output on a per cell basis (15 μg/ng of DNA vs. 21 μg/ng of DNA for controls); in comparison, total elastin output in LOX-2 supplemented cultures was greater than in control cultures both on absolute (see Table 1) and DNA-normalized terms (24μg/ng of DNA vs. 21 μg/ng of DNA for controls).
Elastin matrix yields [matrix yield = total matrix elastin/(tropoelastin + total matrix elastin)], calculated from the elastin synthesis data are shown in Figure 2D. While elastin matrix yield in control (non-additive) cultures was 29 ± 4%, addition of LOX-1 and LOX-2 increased the yield to 57 ± 7% and 45 ± 1% respectively (p<0.01 vs. controls in both cases, p = 0.001 for LOX-1 vs. LOX-2).
3.3 Desmosine Crosslinking
Desmosine densities in LOX-supplemented cultures [(8.1 ± 0.07) × 10−5 ng/ng of insoluble matrix elastin for LOX-1; and (6.8 ± 0.14) × 10−5 ng/ng for LOX-2] were not significantly different from those in control cultures [(7.7 ± 0.12) × 10−5 ng/ng].
3.4 MMPs-2, 9 Activity
Gel zymography analysis showed that activity of MMPs-2 and 9 were not enhanced by addition of LOX-1, compared to that in control cultures (Figure 3; p = 0.71 vs. controls). LOX-2 did not impact MMP-2 activity either, though it suppressed MMP-9 activity significantly (0.8 ± 0.06-fold; p < 0.01 vs. controls).
FIG 3.
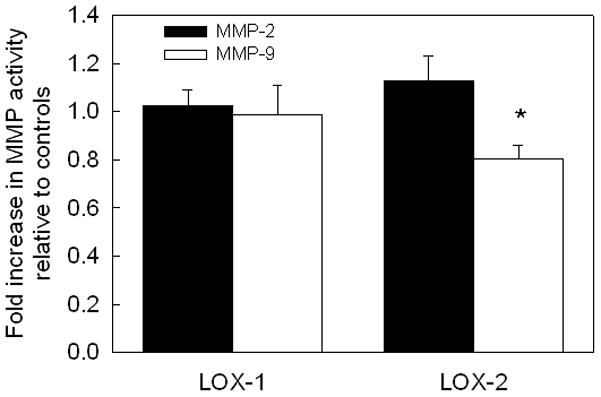
Exogenous LOX effects on endogenous MMPs-2 and 9. LOX-1 (1.25 ng/cell of LOX) did not impact activities of MMPs 2, and 9 released from cultured RASMCs, while LOX-2 (2.5 ng/ml) suppressed MMP-9 activity, as quantified using gel zymography. The values are shown as mean ± SD of n =3/case for each test case, and further normalized to the respective amounts in control cultures. * represents significance in differences relative to controls, for p < 0.05.
4. Discussion
A number of studies have sought to either investigate cues that stimulate cellular elastin synthesis, or alternately, develop methods to circumvent these needs. Elastin “building” strategies have attempted to (a) coax non-elastin producing healthy adult cells in 2-D cultures to crosslink exogenous elastin precursors into matrix protein19, (b) assemble elastomers from natural or synthesized polypeptides20, 21, and (c) develop synthetic scaffolds that will provide cues necessary for robust elastin synthesis in vitro or in vivo22, 23. However, these studies have not progressed sufficiently to indicate if structural and functional mimics of native elastin can be regenerated24. A logical elastin regeneration approach is thus to select elastogenic cues from amongst a subset of ECM molecules (e.g., growth factors, crosslinking enzymes) that participate in and regulate elastic fiber deposition during development25. As mentioned earlier, the stabilization and crosslinking of tropoelastin molecules on micro-fibrillar scaffolds occur initially via copper-dependent LOX amine oxidase, whereby lysine or hydroxylysine residues in the non-helical portions of the molecule are converted into aldehydes, which then condense with hydroxylysine and other residues in adjacent molecules to form intermolecular bonds. Since extracellular LOX availability and activity are dependent on the presence of copper (Cu2+) ions26, we earlier investigated the utility of exogenously supplemented Cu2+ ions to cellular-mediated elastin synthesis, organization and crosslinking12, 27. Though this approach improved synthesis of endogenous LOX and elastin on a per cell basis, matrix yields still remained poor12, 27. In this context, Kagan et al. showed that tropoelastin generated by recombinant DNA techniques can be crosslinked by purified LOX28, which suggests our hypothesis that cell-generated tropoelastin can be similarly assembled and crosslinked by exogenously provided LOX, beyond that possible with endogenous LOX.
In this study, addition of exogenous LOX peptides at doses less than 2.5 ng/cell (33 μg/ml) appeared to marginally enhance SMC proliferation, though the proliferation ratios within these cultures were not significantly different from control cultures. Differently, in a prior study, Hurtado et al. showed that proliferation of cultured SMCs is suppressed upon exposure to 5–10 μg/mL of LOX pro-peptide (LOX-PP), which represents the N-terminal propeptide region of the LOX protein29. Comparison of these two sets of results suggests that the C-terminal and other domains of the LOX protein may modulate the anti-mitotic effects of LOX-PP. Another study by Gacheru et al30., determined that LOX mRNA expression and activity strongly correlated with SMC proliferation levels, which agrees with our current observations of marginal increases in SMC proliferation in response to addition of exogenous LOX. Interestingly, our earlier studies showed that supplementation of 0.1 M of Cu2+ ions suppressed SMC proliferation to levels that were ~25% of that occurring in control cultures12, while simultaneously promoting endogenous LOX production 2.6-fold. On the basis of these different sets of outcomes, we hypothesize that LOX may influence SMC proliferation in a dose- and delivery-dependent manner. Nevertheless, enhanced SMC proliferation on addition of LOX peptide can be beneficial from a tissue engineering standpoint, to increase overall matrix accumulation, a vital determinant of our ability to regenerate finite-sized constructs for clinical implantation.
Numerous studies have demonstrated the role of MMPs-2, 9 in SMC proliferation and ECM degradation under various pathological conditions31. However, little is known about the role of LOX peptides in stimulating MMP release by SMCs cultured in vitro. Our data shows that LOX peptides (at both doses) did not enhance MMPs-2, 9 activity in RASMC cultures, and in fact suppressed MMP-9 activity at the higher provided dose. These results are in direct agreement with the observations by Hurtado et al.29, where LOX peptides (10 μg/mL) inhibited MMP-9 expression in RASMC cultures, possibly via Erk1/2 MAP kinase pathways. Though more rigorous testing of cell responses to a wider range of LOX doses is warranted, our results thus far suggest that bovine LOX does not activate/inflame rat aortic SMCs (as evidenced from MMPs activity) likely due to the high structural homology (>95%) between bovine and rat LOX enzymes8.
Although LOX is crucial to crosslinking and maturation of elastin, not many studies have focused on elucidating the utility of LOX in tissue engineering elastin matrix protein. Previous tissue engineering approaches attempted to enhance elastin production by stimulating SMCs with growth factors such as TGF-β132 or by applying cyclic mechanical strain conditioning33. However, these approaches do not necessarily upregulate elastin crosslinking and deposition into matrix form, which is important from a tissue engineering standpoint. Elbjeirami et al. demonstrated the benefits of LOX-transfected SMCs to desmosine-mediated elastin matrix crosslinking34 in an engineered vascular-like tissue. Likewise, in a previous study12, we observed exogenously provided 0.1 M of Cu2+ ions to significantly improve elastin matrix synthesis and crosslinking in RASMC cultures, though the matrix yield remained the same relative to controls. In this study, total elastin (tropoelastin + matrix elastin) production, both absolute amounts, and on a per cell basis, either showed a decrease or marginal increase upon culture with LOX-1 and LOX-2 respectively. Thus, as with SMC proliferation, LOX has biphasic effects on elastin synthesis depending on its dose. On the other hand, LOX, irrespective of the dose, increased matrix elastin production (both soluble and crosslinked forms) multi-fold. Relative to controls, matrix elastin deposited on a per cell basis, increased by 41% in the presence of LOX-1 and 80% in the presence of LOX-2; likewise increases were observed in elastin matrix yields. These results attest to the effectiveness of LOX peptides in the recruitment and crosslinking of tropoelastin precursors into matrix structures, although the exact mechanism by which this happens is beyond the scope of this paper. In light of possible dose-dependent suppression of elastin production by LOX, future studies must seek to optimize LOX doses to benefit tropoelastin recruitment and crosslinking into matrix structures without compromising elastin synthesis itself. Regardless, we believe that this exogenous LOX peptide delivery approach can be used in conjunction with other existing tissue engineering strategies that promote cellular-mediated ECM crosslinking and deposition, to thereby enhance the mechanical and biological integrity of tissue engineering grafts.
5. Conclusions
This study has demonstrated the ability of exogenous bovine LOX (2.5 ng/cell) to promote cellular elastin matrix synthesis, deposition, and crosslinking efficiency (yield) in a dose-dependent manner. This was achieved with no significant increases in RASMC proliferation or activity of matrix-degrading MMPs-2, 9 expression relative to control cultures. This novel approach of exogenous LOX peptide supplementation appears to benefit vascular tissue engineering applications where elastin matrix regeneration is still a major challenge, and merits more detailed investigation.
Acknowledgments
This study was funded by the American Heart Association (SDG 0335085N) and the National Institutes of Health (C06RR018823, EB006078-01A1, HL092051-01A1) to Anand Ramamurthi. We thank Dr. Herbert Kagan of Boston University for kindly providing us the LOX enzyme.
References
- 1.Kielty CM, Sherratt MJ, Shuttleworth CA. Elastic fibers. J Cell Sci. 2002;115:2817–2828. doi: 10.1242/jcs.115.14.2817. [DOI] [PubMed] [Google Scholar]
- 2.Raines EW, Ross R. Smooth muscle cells and the pathogenesis of the lesions of atherosclerosis. Br Heart J. 1993;69(1 Suppl):S30–37. doi: 10.1136/hrt.69.1_suppl.s30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karnik SK, Brooke BS, Antonio BG, Sorensen L, Wythe JD, Schwartz RS, et al. A critical role for elastin signaling in vascular morphogenesis and disease. Development. 2003;130:411–423. doi: 10.1242/dev.00223. [DOI] [PubMed] [Google Scholar]
- 4.Seyama Y, Wachi H. Atherosclerosis and matrix dystrophy. J Atheroscler Thromb. 2004;11(5):236–245. doi: 10.5551/jat.11.236. [DOI] [PubMed] [Google Scholar]
- 5.Johnson DJ, Robson P, Hew Y, Keeley FW. Decreased elastin synthesis in normal development and in long-term aortic organ and cell cultures is related to rapid and selective destabilization of mRNA for elastin. Circ Res. 1995;77(6):1107–1113. doi: 10.1161/01.res.77.6.1107. [DOI] [PubMed] [Google Scholar]
- 6.McMahon MP, Faris B, Wolfe BL, Brown KE, Pratt CA, Toselli P, Franzblau C. Aging effects on the elastin composition in the extracellular matrix of cultured rat aortic smooth muscle cells. In Vitro Cell Dev Biol. 1985;21(12):674–680. doi: 10.1007/BF02620921. [DOI] [PubMed] [Google Scholar]
- 7.Molnar J, Fong KS, He QP, Hayashi K, Kim Y, Fong SF, Fogelgren B, Szauter KM, Mink M, Csiszar K. Structural and functional diversity of lysyl oxidase and the LOX-like proteins. Biochim Biophys Acta. 2003;1647:220–224. doi: 10.1016/s1570-9639(03)00053-0. [DOI] [PubMed] [Google Scholar]
- 8.Kagan HM, Li W. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem. 2003;88:660–672. doi: 10.1002/jcb.10413. [DOI] [PubMed] [Google Scholar]
- 9.Kagan HM, Trackman PC. Properties and function of lysyl oxidase. Am J Respir Cell Mol Biol. 1991;5:296–310. doi: 10.1165/ajrcmb/5.3.206. [DOI] [PubMed] [Google Scholar]
- 10.Smith-Mungo LI, Kagan HM. Lysyl oxidase: properties, regulation and multiple functions in biology. Matrix Biol. 1998;16(7):387–398. doi: 10.1016/s0945-053x(98)90012-9. [DOI] [PubMed] [Google Scholar]
- 11.Kothapalli CR, Ramamurthi A. Benefits of concurrent delivery of hyaluronan and IGF-1 cues to regeneration of crosslinked elastin matrix protein by adult rat vascular cells. J Tissue Eng Regen Med. 2008;2:106–116. doi: 10.1002/term.70. [DOI] [PubMed] [Google Scholar]
- 12.Kothapalli CR, Ramamurthi A. Biomimetic Regeneration of Elastin Matrix protein Using Hyaluronan and Copper Ion Cues. Tissue Eng. 2009;15(1):103–113. doi: 10.1089/ten.tea.2007.0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kothapalli CR, Taylor PM, Smolenski RT, Yacoub MH, Ramamurthi A. TGF-β1 and Hyaluronan Oligomers Synergistically Enhance Elastin Matrix Regeneration by Vascular Smooth Muscle Cells. Tissue Eng. 2008 doi: 10.1089/ten.tea.2008.0040. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kagan HM, Cai P. Isolation of active site peptides of lysyl oxidase. Methods Enzymol. 1995;258:122–132. doi: 10.1016/0076-6879(95)58041-7. [DOI] [PubMed] [Google Scholar]
- 15.Palamakumbura AH, Trackman PC. A Fluorometric Assay for Detection of Lysyl Oxidase Enzyme Activity in Biological Samples. Anal Biochem. 2002;300:245–251. doi: 10.1006/abio.2001.5464. [DOI] [PubMed] [Google Scholar]
- 16.Labarca C, Paigen K. A simple, rapid, and sensitive DNA assay. Anal Biochem. 1980;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- 17.Joddar B, Ramamurthi A. Fragment size- and dose-specific effects of hyaluronan on matrix synthesis by vascular smooth muscle cells. Biomaterials. 2006;27(15):2994–3004. doi: 10.1016/j.biomaterials.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 18.Simionescu A, Philips K, Vyavahare N. Elastin-derived peptides and TGF-beta1 induce osteogenic responses in smooth muscle cells. Biochem Biophys Res Commun. 2005;334:524–532. doi: 10.1016/j.bbrc.2005.06.119. [DOI] [PubMed] [Google Scholar]
- 19.Stone PJ, Morris SM, Griffin S, Mithieux S, Weiss AS. Building Elastin. Incorporation of recombinant human tropoelastin into extracellular matrix protein using nonelastogenic rat-1 fibroblasts as a source for lysyl oxidase. Am J Respir Cell Mol Biol. 2001;24(6):733–739. doi: 10.1165/ajrcmb.24.6.4304. [DOI] [PubMed] [Google Scholar]
- 20.Mithieux SM, Rasko JE, Weiss AS. Synthetic elastin hydrogels derived from massive elastic assemblies of self-organized human protein monomers. Biomaterials. 2004;25(20):4921–4927. doi: 10.1016/j.biomaterials.2004.01.055. [DOI] [PubMed] [Google Scholar]
- 21.Wright ER, Conticello VP. Self-assembly of block copolymers derived from elastin-mimetic polypeptide sequences. Adv Drug Deliv Rev. 2002;54(8):1057–1073. doi: 10.1016/s0169-409x(02)00059-5. [DOI] [PubMed] [Google Scholar]
- 22.Stankus JJ, Guan J, Wagner WR. Fabrication of biodegradable elastomeric scaffolds with sub-micron morphologies. J Biomed Mater Res A. 2004;70(4):603–614. doi: 10.1002/jbm.a.30122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee SH, Kim BS, Kim SH, Choi SW, Jeong SI, Kwon IK, Kang SW, Nikolovski J, Mooney DJ, Han YK, Kim YH. Elastic biodegradable poly(glycolide-co-caprolactone) scaffold for tissue engineering. J Biomed Mater Res A. 2003;66(1):29–37. doi: 10.1002/jbm.a.10497. [DOI] [PubMed] [Google Scholar]
- 24.Opitz F, Schenke-Layland K, Cohnert TU, Starcher B, Halbhuber KJ, Martin DP, Stock UA. Tissue engineering of aortic tissue: dire consequence of suboptimal elastic fiber synthesis in vivo. Cardiovasc Res. 2004;63(4):719–730. doi: 10.1016/j.cardiores.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Kielty CM, Stephan S, Sherratt MJ, Williamson M, Shuttleworth CA. Applying elastic fibre biology in vascular tissue engineering. Phil Trans R Soc B. 2007;362:1293–1312. doi: 10.1098/rstb.2007.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rucker R, Kosonen T, Clegg MS, Mitchell AE, Rucker RB, Uriu-Hare JY, Keen CL. Copper, lysyl oxidase, and extracellular matrix protein crosslinking. Am J Clin Nutr. 1998;67:996S–1000S. doi: 10.1093/ajcn/67.5.996S. [DOI] [PubMed] [Google Scholar]
- 27.Kothapalli CR, Ramamurthi A. Copper nanoparticle cues for biomimetic cellular assembly of crosslinked elastin fibers. Acta Biomat. 2009;5(2):541–553. doi: 10.1016/j.actbio.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bedell-Hogan D, Trackman P, Abrams W, Rosenbloom J, Kagan H. Oxidation, cross-linking, and insolubilization of recombinant tropoelastin by purified lysyl oxidase. J Biol Chem. 1993;268(14):10345–10350. [PubMed] [Google Scholar]
- 29.Hurtado PA, Vora S, Sume SS, Yang D, Hilaire CS, Guo Y, Palamakumbura AH, Schreiber BM, Ravid K, Trackman PC. Lysyl oxidase propeptide inhibits smooth muscle cell signaling and proliferation. Biochem Biophys Res Commun. 2008;366(1):156–161. doi: 10.1016/j.bbrc.2007.11.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gacheru SN, Thomas KM, Murray SA, Csiszar K, Smith-Mungo LI, Kagan HM. Transcriptional and post-transcriptional control of lysyl oxidase expression in vascular smooth muscle cells: Effects of TGB-1 and serum deprivation. J Cell Biochem. 1997;65:395–407. doi: 10.1002/(sici)1097-4644(19970601)65:3<395::aid-jcb9>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 31.Newby AC, Zaltsman AB. Molecular mechanisms in intimal hyperplasia. J Pathol. 2000;190:300–309. doi: 10.1002/(SICI)1096-9896(200002)190:3<300::AID-PATH596>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 32.Mann BK, Schmedlen RH, West JL. Tethered-TGF-b increases extracellular matrix production of vascular smooth muscle cells. Biomaterials. 2001;22:439–444. doi: 10.1016/s0142-9612(00)00196-4. [DOI] [PubMed] [Google Scholar]
- 33.Kim BS, Mooney DJ. Scaffolds for engineering smooth muscle under cyclic mechanical strain conditions. J Biomech Eng. 2000;122:210–215. doi: 10.1115/1.429651. [DOI] [PubMed] [Google Scholar]
- 34.Elbjeirami WM, Yonter EO, Starcher BC, West JL. Enhancing mechanical properties of tissue-engineered constructs via lysyl oxidase crosslinking activity. J Biomed Mater Res A. 2003;66:513–521. doi: 10.1002/jbm.a.10021. [DOI] [PubMed] [Google Scholar]


