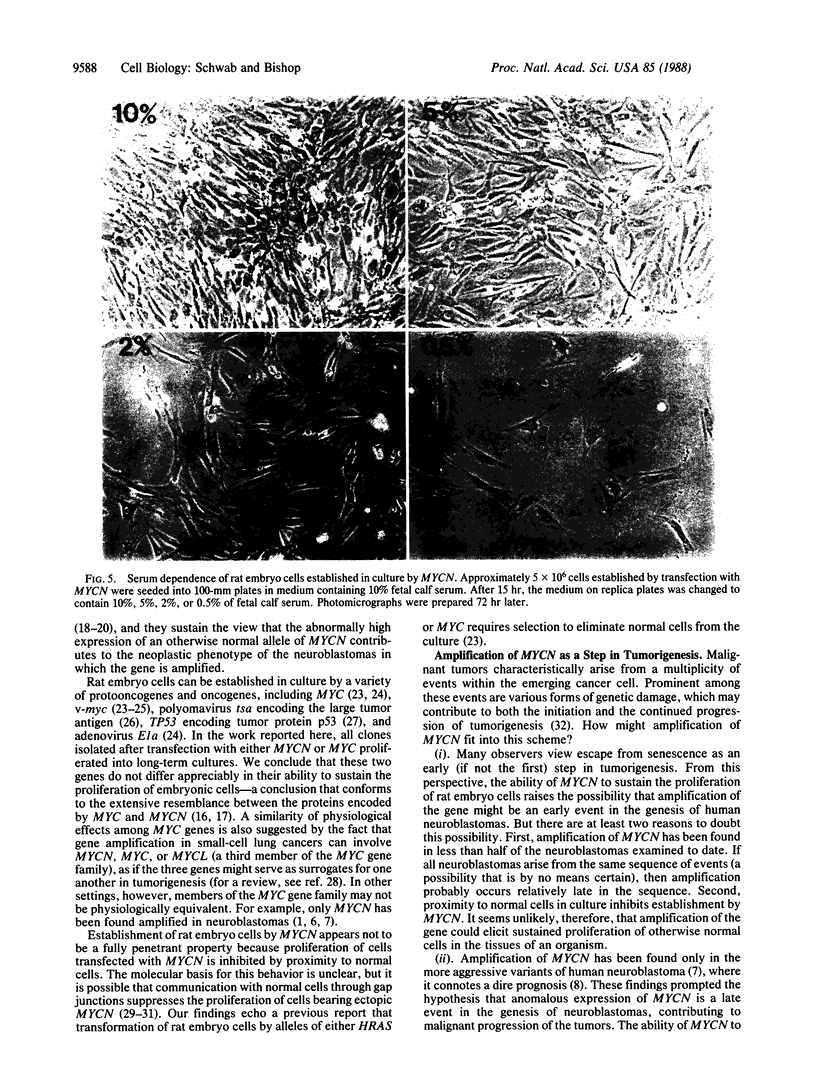
Abstract
Amplification of the human gene MYCN may play a role in the malignant progression of human neuroblastomas. In pursuit of this possibility, previous studies have shown that the abundant expression of MYCN in cultured cells can elicit several aspects of the transformed phenotype. We now extend those findings by demonstrating that rat embryo cells transfected with MYCN can proliferate for at least 200 generations. Isolation of established cells was dependent on high expression of MYCN and on biological selection to eliminate untransfected cells. The established cells were not tumorigenic in syngeneic rats or athymic mice, failed to grow in soft agar, and required relatively high concentrations of serum for proliferation in culture. Our results show that enhanced expression of MYCN can rescue normal cells from senescence, add to the credentials of MYCN as an authentic protooncogene, and identify an additional biological activity that can be used in the characterization of MYCN.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bignami M., Rosa S., Falcone G., Tató F., Katoh F., Yamasaki H. Specific viral oncogenes cause differential effects on cell-to-cell communication, relevant to the suppression of the transformed phenotype by normal cells. Mol Carcinog. 1988;1(1):67–75. doi: 10.1002/mc.2940010113. [DOI] [PubMed] [Google Scholar]
- Bignami M., Rosa S., La Rocca S. A., Falcone G., Tatò F. Differential influence of adjacent normal cells on the proliferation of mammalian cells transformed by the viral oncogenes myc, ras and src. Oncogene. 1988 May;2(5):509–514. [PubMed] [Google Scholar]
- Bishop J. M. The molecular genetics of cancer. Science. 1987 Jan 16;235(4786):305–311. doi: 10.1126/science.3541204. [DOI] [PubMed] [Google Scholar]
- Brodeur G. M., Seeger R. C., Schwab M., Varmus H. E., Bishop J. M. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science. 1984 Jun 8;224(4653):1121–1124. doi: 10.1126/science.6719137. [DOI] [PubMed] [Google Scholar]
- Garson J. A., McIntyre P. G., Kemshead J. T. N-myc amplification in malignant astrocytoma. Lancet. 1985 Sep 28;2(8457):718–719. doi: 10.1016/s0140-6736(85)92950-2. [DOI] [PubMed] [Google Scholar]
- Grady E. F., Schwab M., Rosenau W. Expression of N-myc and c-src during the development of fetal human brain. Cancer Res. 1987 Jun 1;47(11):2931–2936. [PubMed] [Google Scholar]
- Jakobovits A., Schwab M., Bishop J. M., Martin G. R. Expression of N-myc in teratocarcinoma stem cells and mouse embryos. Nature. 1985 Nov 14;318(6042):188–191. doi: 10.1038/318188a0. [DOI] [PubMed] [Google Scholar]
- Jenkins J. R., Rudge K., Currie G. A. Cellular immortalization by a cDNA clone encoding the transformation-associated phosphoprotein p53. Nature. 1984 Dec 13;312(5995):651–654. doi: 10.1038/312651a0. [DOI] [PubMed] [Google Scholar]
- Kohl N. E., Kanda N., Schreck R. R., Bruns G., Latt S. A., Gilbert F., Alt F. W. Transposition and amplification of oncogene-related sequences in human neuroblastomas. Cell. 1983 Dec;35(2 Pt 1):359–367. doi: 10.1016/0092-8674(83)90169-1. [DOI] [PubMed] [Google Scholar]
- Kohl N. E., Legouy E., DePinho R. A., Nisen P. D., Smith R. K., Gee C. E., Alt F. W. Human N-myc is closely related in organization and nucleotide sequence to c-myc. Nature. 1986 Jan 2;319(6048):73–77. doi: 10.1038/319073a0. [DOI] [PubMed] [Google Scholar]
- Land H., Chen A. C., Morgenstern J. P., Parada L. F., Weinberg R. A. Behavior of myc and ras oncogenes in transformation of rat embryo fibroblasts. Mol Cell Biol. 1986 Jun;6(6):1917–1925. doi: 10.1128/mcb.6.6.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. H., Murphree A. L., Benedict W. F. Expression and amplification of the N-myc gene in primary retinoblastoma. 1984 May 31-Jun 6Nature. 309(5967):458–460. doi: 10.1038/309458a0. [DOI] [PubMed] [Google Scholar]
- Lee W. M., Schwab M., Westaway D., Varmus H. E. Augmented expression of normal c-myc is sufficient for cotransformation of rat embryo cells with a mutant ras gene. Mol Cell Biol. 1985 Dec;5(12):3345–3356. doi: 10.1128/mcb.5.12.3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein W. R. Junctional intercellular communication and the control of growth. Biochim Biophys Acta. 1979 Feb 4;560(1):1–65. doi: 10.1016/0304-419x(79)90002-7. [DOI] [PubMed] [Google Scholar]
- Mougneau E., Lemieux L., Rassoulzadegan M., Cuzin F. Biological activities of v-myc and rearranged c-myc oncogenes in rat fibroblast cells in culture. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5758–5762. doi: 10.1073/pnas.81.18.5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nau M. M., Brooks B. J., Battey J., Sausville E., Gazdar A. F., Kirsch I. R., McBride O. W., Bertness V., Hollis G. F., Minna J. D. L-myc, a new myc-related gene amplified and expressed in human small cell lung cancer. Nature. 1985 Nov 7;318(6041):69–73. doi: 10.1038/318069a0. [DOI] [PubMed] [Google Scholar]
- Ramsay G., Stanton L., Schwab M., Bishop J. M. Human proto-oncogene N-myc encodes nuclear proteins that bind DNA. Mol Cell Biol. 1986 Dec;6(12):4450–4457. doi: 10.1128/mcb.6.12.4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassoulzadegan M., Naghashfar Z., Cowie A., Carr A., Grisoni M., Kamen R., Cuzin F. Expression of the large T protein of polyoma virus promotes the establishment in culture of "normal" rodent fibroblast cell lines. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4354–4358. doi: 10.1073/pnas.80.14.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab M., Alitalo K., Klempnauer K. H., Varmus H. E., Bishop J. M., Gilbert F., Brodeur G., Goldstein M., Trent J. Amplified DNA with limited homology to myc cellular oncogene is shared by human neuroblastoma cell lines and a neuroblastoma tumour. Nature. 1983 Sep 15;305(5931):245–248. doi: 10.1038/305245a0. [DOI] [PubMed] [Google Scholar]
- Schwab M., Ellison J., Busch M., Rosenau W., Varmus H. E., Bishop J. M. Enhanced expression of the human gene N-myc consequent to amplification of DNA may contribute to malignant progression of neuroblastoma. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4940–4944. doi: 10.1073/pnas.81.15.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab M., Klempnauer K. H., Alitalo K., Varmus H., Bishop M. Rearrangement at the 5' end of amplified c-myc in human COLO 320 cells is associated with abnormal transcription. Mol Cell Biol. 1986 Jul;6(7):2752–2755. doi: 10.1128/mcb.6.7.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab M., Varmus H. E., Bishop J. M. Human N-myc gene contributes to neoplastic transformation of mammalian cells in culture. Nature. 1985 Jul 11;316(6024):160–162. doi: 10.1038/316160a0. [DOI] [PubMed] [Google Scholar]
- Seeger R. C., Brodeur G. M., Sather H., Dalton A., Siegel S. E., Wong K. Y., Hammond D. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N Engl J Med. 1985 Oct 31;313(18):1111–1116. doi: 10.1056/NEJM198510313131802. [DOI] [PubMed] [Google Scholar]
- Slamon D. J., Boone T. C., Seeger R. C., Keith D. E., Chazin V., Lee H. C., Souza L. M. Identification and characterization of the protein encoded by the human N-myc oncogene. Science. 1986 May 9;232(4751):768–772. doi: 10.1126/science.3008339. [DOI] [PubMed] [Google Scholar]
- Small M. B., Hay N., Schwab M., Bishop J. M. Neoplastic transformation by the human gene N-myc. Mol Cell Biol. 1987 May;7(5):1638–1645. doi: 10.1128/mcb.7.5.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire J., Goddard A. D., Canton M., Becker A., Phillips R. A., Gallie B. L. Tumour induction by the retinoblastoma mutation is independent of N-myc expression. Nature. 1986 Aug 7;322(6079):555–557. doi: 10.1038/322555a0. [DOI] [PubMed] [Google Scholar]
- Stanton L. W., Schwab M., Bishop J. M. Nucleotide sequence of the human N-myc gene. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1772–1776. doi: 10.1073/pnas.83.6.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele C. J., Reynolds C. P., Israel M. A. Decreased expression of N-myc precedes retinoic acid-induced morphological differentiation of human neuroblastoma. 1985 Jan 31-Feb 6Nature. 313(6001):404–406. doi: 10.1038/313404a0. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Nisen P. D., Tesfaye A., Kohl N. E., Goldfarb M. P., Alt F. W. N-myc can cooperate with ras to transform normal cells in culture. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5455–5459. doi: 10.1073/pnas.82.16.5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman K. A., Yancopoulos G. D., Collum R. G., Smith R. K., Kohl N. E., Denis K. A., Nau M. M., Witte O. N., Toran-Allerand D., Gee C. E. Differential expression of myc family genes during murine development. 1986 Feb 27-Mar 5Nature. 319(6056):780–783. doi: 10.1038/319780a0. [DOI] [PubMed] [Google Scholar]