Abstract
Background and Purpose
We aimed to determine if ischemia involving Broca’s area predicts Broca’s aphasia more reliably in acute or chronic stroke.
Methods
We included consecutive right-handed patients with left hemisphere ischemic stroke (<48 hours from onset for acute stroke or >6 months post- stroke for chronic stroke). MRI scans were analyzed for ischemic lesions and/ or hypoperfusion in Broca’s area (Brodmann’s areas 44 and 45). Patients were scored on the Western Aphasia Battery to classify aphasia syndromes. Chi- squared tests were used to identify significant associations.
Results
The presence of infarct involving any part of Broca’s area and the presence of Broca’s or Global aphasia was much stronger in acute (χ2 = 38.1; df1; p<0.0001) than chronic stroke (χ2 = 0.54; df1; p=0.46; ns). The association between infarct and/or hypoperfusion covering all of Broca’s area and the presence of Broca’s or Global aphasia was much stronger in acute (χ2 = 35.8; df1; p<0.0001) than chronic stroke (χ2 = 1.2; df1; p=0.27; ns). In a subset of 20 patients studied longitudinally, the associations were significant only acutely not chronically: χ2 = 20; df1; p<0.0001 versus χ2 = 0; df1; p=1; ns for ischemia involving part of Broca’s area, and χ2 = 16.4; df1; p<0.0001 versus χ2 = 3.2; df1; p=0.08; ns for ischemia covering all of Broca’s area.
Conclusions
Broca’s aphasia is more reliably associated with infarct/ hypoperfusion of Broca’s area in acute stroke. Many chronic patients with damage to part or all of Broca’s area had neither Broca’s nor Global aphasia. Broca’s or Global aphasia was sometimes present initially in these patients, but resolved by 6 months. Our results indicate that the acute aphasia syndrome may allow the clinician to predict the compromised vascular territory , even when structural imaging shows only a small (or no) infarct.
Keywords: Acute stroke, Aphasia, Brain Imaging, Cognitive Impairment, Ischemia, Magnetic Resonance
Introduction
It is commonly taught that aphasia syndromes are not reliably associated with particular lesion locations in acute stroke, as patients’ performance on tasks can vary from day to day in the acute period. However, these fluctuations in language performance may reflect important changes in blood flow1, and the aphasia syndrome at a given point of time may actually be an excellent predictor of the vascular territory that is ischemic (hypoperfused or infarcted). If this is the case, then rapid assessment of language might help direct clinical decision-making in acute stroke. For example, consider two patients who have acute left subcortical infarcts on DWI, but do not have CT or MR perfusion imaging due to relative contraindications for contrast (severe renal failure). One has no aphasia; the second has Wernicke’s aphasia. If the aphasia syndrome reliably predicts the vascular territory that is ischemic in the acute stage of stroke, then the clinician can assume that in patient two only the inferior division of the left middle cerebral artery (MCA) is at risk, requiring aggressive intervention.
It is commonly believed that damage to Broca’s area underlies Broca’s aphasia. However, the two are not always paired exclusively2,3. Many patients with damage to Broca’s area have language deficits immediately after stroke, but recover over time. This recovery may be due to reorganization, in which other brain areas assume the functions of lost tissue, or achieved through rehabilitation, which allows alternative strategies to be employed when confronting language difficulties. In both circumstances, language tasks that previously depended on Broca’s area are now accomplished using different brain regions or different cognitive mechanisms (despite the lesion in Broca's area).4 These transformations require time; they do not occur in the first few days of stroke. Rather, recovery in the first few days requires restoration of blood flow.5 Thus, the association between damage to Broca’s area and Broca’s aphasia may be higher in acute than chronic stroke, due to structure/function reorganization in the chronic stage.
Broca’s area itself is controversial, as it is defined using different neuroanatomical boundaries. It is most often defined as Brodmann’s areas 44 and 45, areas of cytoarchitecture commonly found in the left posterior inferior frontal cortex.6 Broca’s area has been implicated in a variety of language functions, including grammatical speech production, verb naming, comprehending syntactically complex sentences (e.g. passive voice), phonological working memory, and orchestrating speech articulation. 7–10
The complete vascular syndrome of Broca’s aphasia (with impaired fluency, word- finding, articulation, repetition and comprehending and producing complex grammatical structures) is thought to require not just damage to Broca’s area, but also infarct of the insula and adjacent cerebrum.10,11 Damage to Broca’s area alone may produce an isolated impairment of motor speech, sometimes known as apraxia of speech, and not the full syndrome of Broca’s aphasia.10,12 This observation may not be true in the first day of stroke, however, when tissue dysfunction relatively restricted to Broca’s area results in the full clinical (vascular) syndrome of Broca’s aphasia12.
We aimed to test the hypothesis that ischemia involving Broca’s area is more strongly associated with Broca’s aphasia in acute stroke than chronic stroke. If confirmed, results would indicate that the vascular aphasia syndromes, including Broca’s aphasia, may be more reliably associated with the area of ischemia (hypoperfusion or infarct) in the acute stage, and that day to day behavioral variations reflect changes in blood flow in the acute period that can be monitored using perfusion-weighted imaging.
Methods
Participants
We included consecutive patients who were right-handed with left hemisphere ischemic stroke (<48 hours from onset of symptoms or >6 months post-stroke) and native speakers of English. Exclusion criteria included: inability to complete language testing or magnetic resonance imaging (MRI) within 24 hours of hospital admission, previous symptomatic stroke or other neurological disease; hemorrhage on initial CT (computed tomography scan) or MRI, impaired level of consciousness, intravenous sedation, uncorrected hearing or visual loss, or inability to provide informed consent or indicate a family member to provide consent. We attempted to follow up all patients who were studied acutely. However, only 20/50 returned for retesting at 6 months. An additional 10 patients were studied only chronically.
Imaging
MRI scans, including T2, Diffusion- and Perfusion- Weighted Images (DWI and PWI) were obtained on a 3 Tesla Philips magnet (in a few cases scans were obtained on a 1.5 Tesla GE or Siemens magnet; but the variability across patients’ brains far exceeds the variability between scanners). For PWI, 20 cc GdDTPA (Gadolinium) was power-injected at 5 cc/sec. Voxel size for PWI and DWI was 4.4 mm3. Slices were 5 mm, with whole brain coverage. Images were analyzed by technicians blinded to the language data and aphasia classification for the presence of dense ischemia (bright on DWI, dark on ADC) in the acute stage or infarct (on T2 in the chronic stage) and/or hypoperfusion (on time to peak – TTP – maps derived from PWI) involving Broca’s area, with Broca’s area defined as Brodmann’s area 44 and/ or 45 using a probabilistic cytoarchitectural map.6 TTP maps were registered to T2 images, which have better spatial resolution, to provide anatomical landmarks. Each patient's scans were classified according to the presence or absence of ischemia (on DWI) or infarct (on T2) and/or hypoperfusion, defined as > 4 sec delay in TTP relative to the homologous region in the right hemisphere (1) in any part of Broca's area; or (2) covering the entire region of Broca’s area.
Language Assessment
The Western Aphasia Battery- Revised13 was administered to all patients. WAB-R results were used to classify patients as having Broca’s aphasia, Wernicke's Aphasia, Global aphasia, Anomic aphasia, Conduction aphasia, Transcortical Motor aphasia, Transcortical Sensory aphasia, Isolation aphasia, or non- aphasic or unclassifiable, by a research assistant blinded to the imaging results.
Statistical Analysis
As dysfunction in Broca's area is classically associated with both Broca's aphasia and Global aphasia, we evaluated this combined aphasia syndrome/lesion association at the acute and chronic stages of stroke. We identified the relationship between the dichotomous lesion classifications (presence vs. absence of ischemia anywhere in/covering all of Broca's area) and dichotomous aphasia classifications (presence vs. absence of Broca's or Global aphasia) with chi square tests. We used an alpha level of p<0.05 after correction for four comparisons (p<0.0125).
Results
A consecutive series of patients who met inclusion and exclusion criteria participated in this study. There were a total of 50 patients studied acutely (<48 hours post-onset) patients and 30 studied chronically (>6 months post-onset). A subset of 20 patients was studied at both time periods. There were no significant differences between acute and chronic patients regarding demographics. Acute patients were 47% female, age 31–83 years, with 7–24 years of education. Chronic patients were 53% female, age 24–85 years, with between 12–20 years of education. Mean age for acute patients was 59.6 years (SD=12.1) and 58.8 years (SD=15.4) for chronic patients. Mean education was 14.0 years (SD=3.7) for acute patients versus 15.3 years (SD= 2.6) for chronic patients. Acute stroke patients, not surprisingly, had more severe aphasia. Mean WAB-R Aphasia Quotient was 70.0 (SD=30.7) for acute versus 82.1 (SD=22.6) for chronic stroke patients; the Aphasia Quotient ranges from 0–100, with 100 being non-aphasic. The subset of 20 patients studied at both time periods was similar to the entire group: age 39–80 (mean 56.6; SD=11.9); education 12–20 years (mean 15.4; SD 2.8); 55% female. Their acute AQ range was 2 to 97.6 (mean 41.5; SD 36.1); chronic AQ range was 1.6 to 100 (mean 84.5; SD 22.3).
Ischemia involving any Part of Broca's Area
The association between infarct and hypoperfusion anywhere within Broca’s area and the presence of Broca’s or Global aphasia was much stronger in acute (χ2 = 38.1; df1; p<0.0001) than in chronic stroke (χ2 = 0.54; df1; p=0.46; ns). All acute stroke patients with Broca’s aphasia or Global aphasia had infarct/ hypoperfusion that included at least part of Broca’s area. In contrast, one chronic stroke patient with Broca’s aphasia had an infarct that did not include Broca’s area. Moreover, 80% of acute and 16.6% of chronic stroke patients with infarction/ hypoperfusion involving Broca’s area had either Broca’s aphasia or Global aphasia. Only two acute patients with infarct/ hypoperfusion involving Broca’s area did not have Broca’s or Global aphasia. These patients were both classified as having Transcortical Motor Aphasia on the WAB-R (see Figure 1). However, the other acute patients with Transcortical Motor Aphasia (n=3) had more classical lesions in the watershed area between the anterior cerebral artery and middle cerebral artery territories (see example in Figure 2).
Figure 1.
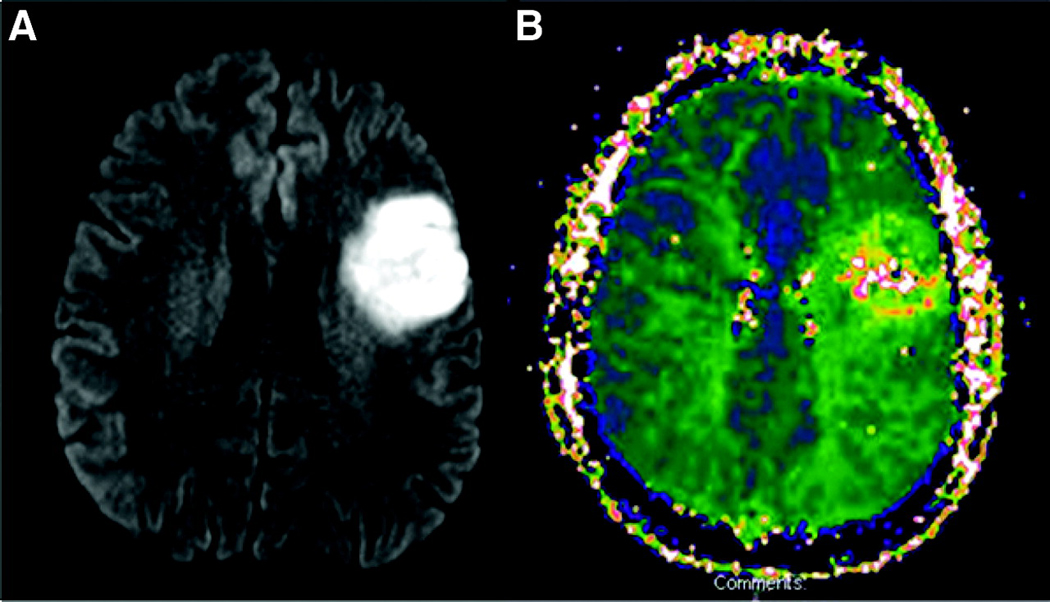
Scans (A. DWI and B. PWI) of an acute patient with infarct/ hypoperfusion involving Broca’s area who did not have Broca’s or Global aphasia. This patient was classified as having Transcortical Motor Aphasia on the WAB-R.
Figure 2.

DWI of a patient with Transcortical Motor Aphasia and the more typical lesion location.
In the 20 patients studied longitudinally, the association between ischemia involving part of Broca’s area and Broca’s or Global Aphasia was significant only acutely (χ2 = 20; df1; p<0.0001), not chronically (χ2 = 0; df1; p=1; ns). Eleven of these patients had ischemia in Broca’s area acutely and chronically; all 11 had Broca’s or Global aphasia acutely, and only one had Global aphasia (and none had Broca’s aphasia) at 6 months.
Ischemia Covering all of Broca's Area
The association between infarct and hypoperfusion covering all of Broca’s area and the presence of Broca’s or Global aphasia was much stronger in acute (χ2 = 35.8; df1; p<0.0001) than in chronic stroke (χ2 = 1.2; df1; p=0.27; ns). All of the acute stroke patients with dysfunction of all of Broca’s area were classified as either Broca’s or Global aphasics. Among chronic stroke patients, 83.3% with infarct covering all of Broca’s area did not have either Broca’s or Global aphasia.
In the 20 patients studied longitudinally, the association between ischemia covering all of Broca’s area and Broca’s or Global aphasia was significant only acutely (χ2 = 16.4; df1; p<0.0001) not chronically (χ2 = 3.2; df1; p=0.08; ns).
Classical Vascular Aphasia Syndrome/Lesion Associations
In acute stroke, six patients had infarct (or dense ischemia) on DWI covering only part of Broca’s area, and three had infarct or dense ischemia covering all of Broca’s area. Of these, six had matched perfusion defects, one had no perfusion defect, and two with infarcts involving part of Broca’s area had hypoperfusion of all of Broca’s area. Another two patients had no infarct in Broca’s area but hypoperfusion of part of Broca’s area or all of Broca’s area (see Figure 3). The patient with hypoperfusion only in Broca’s area had Broca’s aphasia; the patient with hypoperfusion of the entire MCA territory had Global aphasia. That is, the area of hypoperfusion, but not the infarct in these two cases, predicted the classical vascular aphasia syndrome in the acute phase after stroke.
Figure 3.

Panel A. DWI or T2 (top) and PWI (bottom) at Day 1 (left) and follow-up (right) in a patient who had Broca’s aphasia at Day 1 when he had hypoperfusion of Broca’s area, which resolved with reperfusion the Broca’s area (before Day 2; he remained non-aphasic at six month follow-up). Panel B. DWI or T2 (top) and PWI (bottom) at Day 1 (left) and follow-up (right) in a patient who had Global aphasia at Day 1 when she had hypoperfusion of the entire left MCA and ACA territories, which resolved with reperfusion (at Day 10; she remained non-aphasic at six month follow-up).
Most of the chronic stroke patients (selected for having had a left hemisphere stroke, not for having aphasia) showed anomic aphasia or no aphasia. However, one chronic stroke patient showed a classic lesion-deficit association: Global aphasia associated with infarct involving both Broca's area and Wernicke's area (Figure 4). Another chronic stroke patient had Broca's aphasia with a lesion involving both Broca's and Wernicke's areas. This patient initially had Global aphasia, but had recovered comprehension of words and sentences with simple syntax. Nevertheless, speech remained halting with impaired articulatory planning.
Figure 4.

The one chronic stroke patient who showed a classic lesion- deficit association. This patient had Global aphasia, associated with infarct involving both Broca’s area and Wernicke’s area.
Exceptions to the Classical Vascular Aphasia Lesion Associations
Scans of the only two acute stroke patients who had ischemia in Broca's area but not the classically associated syndrome (i.e. had Transcortical Motor Aphasia rather than Broca's aphasia) are shown in Figure 1. Several patients with chronic stroke with lesions involving or covering all of Broca's area had anomic aphasia or no aphasia (see examples in Figure 5).
Figure 5.
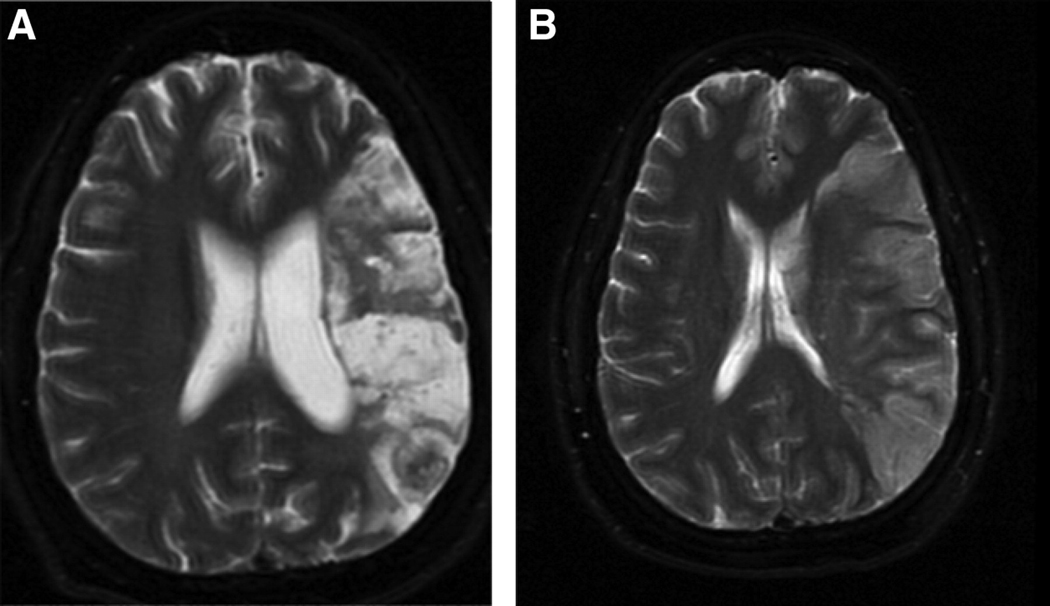
Exceptions to the classical vascular aphasia lesion associations in chronic stroke. A. Patient with Anomic Aphasia but infarct involving both Broca’s and Wernicke's area. B. Patient with no aphasia, but infarct involving both Broca’s and Wernicke's area.
Discussion
In our patient sample, Broca’s aphasia was more consistently associated with infarction/ hypoperfusion of Broca’s area in acute stroke than in chronic stroke. Broca’s aphasia is a vascular syndrome. Many patients with damage to all of Broca’s area at the chronic stage had neither Broca’s nor Global aphasia. These patients may have had Broca’s aphasia initially, but it may have resolved over time due to reorganization of structure/ function relationships (as confirmed in the longitudinal study of 20 patients).
Our results differ from those reported in early CT studies of classical aphasia in two ways. First, we selected patients on the basis of having a first symptomatic ischemic stroke in the left hemisphere, either in the past 48 hours or at least 6 months previously. Earlier studies selected patients who had chronic aphasia only. Consistent with the majority of our patients, it is highly likely that those patients with chronic Broca's aphasia do have lesions involving Broca's area, at least in most cases.14,15 Secondly, by including perfusion-weighted imaging, we were able to identify dysfunctional brain tissue that would not have been visible on acute CT or structural MRI that likely contributed to the patients' language impairments in the acute stage (see Figure 3). Our results do not address the issue of whether dysfunctional tissue restricted to Broca's area more commonly results in the full syndrome of Broca's aphasia12 or a more restricted motor speech disorder.10
Non-fluent aphasias including Broca’s aphasia are reported in higher frequencies in younger patients. In the acute phase, this has been attributed to a higher proportion of anterior lesions in younger patients and higher proportion of posterior lesions in older patients.16 The age influence on location of infarct may reflect stroke mechanism, with more frequent cardioembolic strokes (which are associated with inferior division MCA strokes)17 in older individuals, and more frequent carotid dissection (which often causes infarcts that include superior division MCA territory, including Broca's area) in younger individuals. Irrespective of the explanation for the age effect on lesion location, the effect can account for the different frequency of aphasic vascular syndromes at different ages.18 This conclusion augments our finding that infarct location may be more closely related to the aphasia syndrome in acute than chronic stroke.
Our patients were relatively young (on average about 59 years), but not atypical for stroke patients admitted to our hospital. In a recent study, we reported data from 226 caucasian/white and 284 African American/black patients admitted to our stroke service19. There was a significant difference in age across racial groups by ANOVA (F=4.2; p = 0.016). Mean age was 63.4±16.1 years for caucasian vs. 59.8±14.4 for African American patients. Slightly more than half of our patients were African American, so it is not surprising that our patients were, on average, closer to 59 years. Also, we excluded patients with previous strokes, dementia, and uncorrected hearing or visual loss, which would have been more common in older stroke patients.
Lesion location has for long been acknowledged as the major determinant of aphasia attributes. However, the methods used to determine the aphasia classification are important. A large percentage (50–60%) of aphasias are not classifiable in either the acute stage18 or the chronic stage20, using classic descriptions or the Boston Diagnostic Aphasia Examination. In contrast, nearly all patients can be classified using the WAB-R, which groups patients into one syndrome classification or another based on scores on fluency, naming, comprehension, repetition, and auditory comprehension. The resulting aphasia classification, however, does not always match the clinical impression. 21 Indeed, in our patient sample, the two patients with acute ischemia in Broca's area who did not have Broca's aphasia by the WAB-R had transcortical motor aphasia on the WAB-R. These patients had relatively high scores on sentence repetition due to partial credit on most items, but had halting, agrammatic sentence repetition, more consistent with the classical description of Broca's aphasia. Nevertheless, we used the WAB-R for classification in this study, because it is relatively objective and reproducible.
Recognizing the limitations of WAB-R classification and the relatively small sample size of our study, our results challenge the commonly held belief that acute aphasia does not reflect classic lesion-deficit associations, at least when "lesion" is defined as ischemic tissue that can be visualized with PWI. This result is clinically important, because it indicates that the acute aphasia syndrome allows the clinician to predict the vascular territory that is compromised, even when structural imaging shows only a small (or no) infarct. For example, if the patients whose scans are shown in Figure 3 had not had PWI, their vascular syndromes would have indicated that there was eloquent brain tissue at risk, indicating a need for aggressive intervention.
Acknowledgments
The research reported in this paper was supported by NIH R01 DC05375 to AH. We gratefully acknowledge this support and the participation of the patients in the study.
References
- 1.Croquelois A, Wintermark M, Reichhart M, Meuli R, Bogousslavsky J. Aphasia in hyperacute stroke: language follows brain penumbra dynamics. Annals of Neurology. 2003;54:321–329. doi: 10.1002/ana.10657. [DOI] [PubMed] [Google Scholar]
- 2.Basso A, Lecours AR, Moraschini S, Vanier M. Anatomical correlations of the aphasia defined through computerized tomography: Exceptions. Brain and Language. 1985;26:201–229. doi: 10.1016/0093-934x(85)90039-2. [DOI] [PubMed] [Google Scholar]
- 3.Dronkers NF, Shapiro JK, Redfern B, Knight RT. The role of Broca's area in Broca's aphasia. Journal of Clinical and Experimental Neuropsychology. 1992;14:52–53. [Google Scholar]
- 4.Kertesz A. Recovery of aphasia. In: Feinberg TE, Farah MJ, editors. Behavioral Neurology and Neuropsychology. New York: McGraw Hill; 1997. pp. 167–182. [Google Scholar]
- 5.Hillis AE, Heidler J. Mechanisms of early aphasia recovery: Evidence from mr perfusion imaging. Aphasiology. 2002;16:885–896. [Google Scholar]
- 6.Amunts K, Schleicher A, Burgel U, Mohlberg H, Uylings HBM, Zilles K. Broca's region revisited: Cytoarchitecture and intersubject variability. Journal of Comparative Neurology. 1999;412:319–941. doi: 10.1002/(sici)1096-9861(19990920)412:2<319::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 7.Grodzinsky Y. The neurology of syntax: Language use without Broca's area. The Behavioral and Brain Sciences. 2000;23:1–71. doi: 10.1017/s0140525x00002399. [DOI] [PubMed] [Google Scholar]
- 8.Paulesu E, Frith DC, Frakowiak RS. The neural correlates of the verbal component of working memory. Nature. 1993;362:342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- 9.Stowe LA. Sentence comprehension and the left inferior frontal gyrus: Storage, not computation. Behavioral and Brain Sciences. 2000;23:51. [Google Scholar]
- 10.Mohr JP, Pessin MS, Finkelstein S, Funkenstein HH, Duncan GW, Davis KR. Broca aphasia: Pathologic and clinical. Neurology. 1978;28:311–324. doi: 10.1212/wnl.28.4.311. [DOI] [PubMed] [Google Scholar]
- 11.Dronkers NF, Plaisant O, Iba-Zizen MT, Cabanis EA. Paul Broca's historic cases: High resolution MR imaging of the brains of leborgne and lelong. Brain. 2007;130:1432–1441. doi: 10.1093/brain/awm042. [DOI] [PubMed] [Google Scholar]
- 12.Davis C, Kleinman JT, Newhart M, Gingis L, Pawlak M, Hillis AE. Speech and language functions that require a functioning Broca's area. Brain and Language. 2008;105:50–58. doi: 10.1016/j.bandl.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Kertesz A. Western aphasia battery- revised. Austin: Pro-Ed.; 2006. [Google Scholar]
- 14.Naeser MA, Hayward RW. Lesion localization in aphasia with cranial computed tomography and Boston Diagnostic Aphasia Examination. Neurology. 1978;28:545–551. doi: 10.1212/wnl.28.6.545. [DOI] [PubMed] [Google Scholar]
- 15.Alexander MP. Aphasia: Clinical and anatomical aspects. In: Feinberg TEFMJ, editor. Behavioral Neurology and Neuropsychology. New York: McGraw Hill; 1997. pp. 133–150. [Google Scholar]
- 16.Ferro JM, Madureira S. Aphasia type, age and cerebral infarct localization. Journal of Neurology. 1997;244:505–509. doi: 10.1007/s004150050133. [DOI] [PubMed] [Google Scholar]
- 17.Knepper LE, Biller J, Tranel D, Adams HP, Jr, Marsh EE., 3rd Etiology of stroke in patients with Wernicke's aphasia. Stroke. 1989;12:1730–1732. doi: 10.1161/01.str.20.12.1730. [DOI] [PubMed] [Google Scholar]
- 18.Godefroy O, Dubois C, Debachy B, Leclerc M, Kreisler A. Vascular aphasias: Main characteristics of patients hospitalized in acute stroke units. Stroke. 2002;33:702–705. doi: 10.1161/hs0302.103653. [DOI] [PubMed] [Google Scholar]
- 19.Gildersleeve KL, Heidler-Gary J, Newhart M, Davis C, Kannan V, Cloutman L, Hillis AE. Racial differences in stroke characteristics: a retrospective cohort and imaging study. Abstract presented at the Annual Meeting of the American Academy of Neurology; Seattle, WA. 2009. Apr, [Google Scholar]
- 20.Goodglass H, Kaplan E. Boston Diagnostic Examination for Aphasia. Philadelphia: Lea & Febiger; 1972. [Google Scholar]
- 21.Swindel CS, Hollan AL, Fromm D. Classification of aphasia: WAB type vs. Clinical impression. In: Brookshire RH, editor. Clinical aphasiology. Minneapolis: BRK Publishers; 1984. pp. 48–54. [Google Scholar]


