Abstract
Purpose
Molecular markers could aid PSA, biopsy Gleason sum and clinical stage in providing accurate information about prostate cancer (CaP) progression. HYAL-1 hyaluronidase and hyaluronic acid (HA) staining in prostatectomy specimens predicts biochemical recurrence. We examined whether HA and HYAL-1 staining in biopsy specimens predicts biochemical recurrence and correlates with the staining in matched prostatectomy specimens.
Materials and Methods
Biopsy and prostatectomy specimens were obtained from patients with clinically localized CaP (n = 61; mean follow-up = 103.1 months) from multiple centers; Gr. 1: patients with biochemical recurrence (n = 23); Gr. 2: patients without recurrence (n = 38). A biotinylated HA-binding protein and an anti-HYAL-1 antibody were used for HA and HYAL-1 staining. The staining was graded between 0 – 300 depending upon staining intensity and the area.
Results
HYAL-1 and HA were expressed in tumor cells and stroma, respectively. In biopsy specimens, HYAL-1 and HA expression was higher in Gr.1 (203.9 and 182.1) when compared to Gr. 2 (48.8 and 87.0; P < 0.0001). In univariate analysis, HA, HYAL-1, biopsy Gleason and PSA significantly predicted biochemical recurrence (P < 0.001). In multivariate analysis only HYAL-1 staining was an independent predictor of recurrence (P < 0.001; accuracy: 81.8%). In prostatectomy specimens only HYAL-1 staining correlated with the staining in biopsy specimens (Spearman ρ= 0.72; P = 0.0002), and predicted biochemical recurrence.
Conclusion
This is the first report that demonstrates that in biopsy specimens HYAL-1 staining is an independent predictor of biochemical recurrence and may be useful in selecting treatment.
Keywords: Prostate cancer, Hyaluronic acid, Hyaluronidase, HYAL-1, Prognostic Markers, biopsy specimens
INTRODUCTION
Patients with clinically localized prostate cancer (CaP) are often treated with radical prostatectomy or radiation with curative intent. However, the disease recurs in a substantial number of the patients and becomes hormone refractory1,2. However, it is often difficult to identify those patients who will progress/recur and may need additional treatment. Currently available pre-operative parameters such as, biopsy Gleason sum, pre-operative prostate specific antigen (PSA), clinical stage are well investigated in terms of providing prognostic information biochemical recurrence. However, these parameters are often similar among patients who experience disease progression and those who do not. Molecular evaluation of biopsy specimens to identify tumors with invasive potential may allow individualization of treatment, including early adjuvant therapy for patients with aggressive CaP.
Hyaluronic acid is a glycosaminoglycan made up of repeating disaccharide units, D-glucuronic acid and N-acetyl-D-glucosamine. HA regulates several cellular functions, including adhesion, migration and proliferation. HA concentration is elevated in a variety of tumors3,4. HA promotes tumor growth and metastasis, however, the prognostic potential of HA appears to depend upon the tissue origin of the tumor3–6. For example, we have shown that the HA test (i.e., measurement of urinary HA levels) is an accurate marker for diagnosing bladder cancer, regardless of tumor grade7. However, HA is not an independent prognostic marker for predicting muscle invasion by a bladder tumor and for predicting biochemical recurrence following radical prostatectomy8–10. Contrarily, high level HA expression in tumor cells correlates with poor prognosis in terms of disease progression, and shortened overall and disease-specific survival in the carcinomas of the gastrointestinal tract, breast and ovary (reviewed in ref 3). There is some evidence that HA may promote progression of CaP to become androgen independent11. We have previously shown that in radical prostatectomy specimens, HA expression is elevated in CaP tissues, but it is not an independent predictor of biochemical recurrence 9,10.
Hyaluronidase (HAase) is an endoglycosidase that degrades the HA polymer. HYAL-1 is the major tumor-derived HAase and its expression in tumor cells correlates with aggressiveness of the tumor3,12. Elevated urinary HYAL-1 level (i.e., measured as the HAase test) is an accurate marker for detecting high-grade bladder cancer. Therefore, when combined with the HA test, the HA-HAase test has about 90% sensitivity and ~ 85% accuracy in detecting bladder cancer7,13. HYAL-1 is also an independent prognostic predictor of muscle invasive bladder cancer8. We have previously shown that silencing HYAL-1 expression in bladder and CaP cells inhibits tumor growth, lymph node, vascular and lymphatic invasion and angiogenesis14,15. We have shown that HYAL-1 expression in radical prostatectomy specimens is an independent prognostic indicator for predicting biochemical recurrence8,9.
In this study, we evaluated HA and HYAL-1 expression in prostate biopsy specimens and correlated in with biochemical recurrence. Tissue fixation and preservation differs in various institutions, academic or community hospitals, which can affect a marker’s performance. Therefore, in this study, the biopsy specimens were obtained from different hospitals. Tumor heterogeneity is inherent in CaP specimens, and therefore, we also determined HA and HYAL-1 expression in matched radical prostatectomy specimens.
MATERIALS AND METHODS
Specimens and patients
We analyzed 61 matched biopsy and radical prostatectomy specimens from CaP patients who underwent radical prostatectomy with bilateral lymphadenectomy between 1992 and 2001. This study was approved by University of Miami’s Institutional Review Board. During the nine year period, 911 patients underwent radical prostatectomy, of which 695 did not receive neoadjuvant androgen deprivation therapy. Out of these 695 patients, well preserved tissue blocks for preparing unstained slides could be retrieved for 250 patients. A single surgeon (MSS) had performed radical prostatectomy on all of these patients at University of Miami. Since these 250 patients had undergone prostate biopsy at various hospitals (n = 11) in South Florida, we requested unstained tumor positive biopsy specimen slides from these hospitals. We were able to obtain 69 specimens, out of which 8 could not be evaluated either due to the loss of tissue during staining procedure. Of the 61 patients with stainable biopsy specimens, 23 patients experienced biochemical recurrence following radical prostatectomy and 38 were free of recurrence. The median follow-up on all patients was 99 months (mean follow-up 103.1 months, 95% confidence interval (CI) 85.6 – 109.7 months). The median time to recur was 36 months (mean 40.6 months, 95% CI 11 – 65 months).
Biochemical recurrence was defined as a PSA ≥ 0.4 ng/dL in 2 successive measurements following radical prostatectomy; the date of the first measurement was taken as the date of recurrence. Pre-operative patient characteristics are shown in Table 1.
Table 1. Patient characteristics.
The information on the pre- and post-biopsy parameters was assessed by pathologic examination of the surgical specimens (Gleason sum (biopsy or radical prostatectomy, margin status, extra prostatic extension (EPE), seminal vesicle invasion and lymph node status) and the available clinical information (age, PSA and clinical stage). In this study all patients were node negative. Therefore, these parameters are considered as baseline covariates. Mean ± SD staining scores for HA and HYAL1 are shown.
| Characteristics | Recurred | Non-Recurred |
|---|---|---|
| Number of patients | 23 | 38 |
| Age | Mean: 62.4 | Mean: 61.1 |
| Median: 65; 95% CI: 57 – 66 | Median: 63; 95% CI: 59 – 65 | |
| Parameters available at biopsy stage | ||
| PSA | Mean: 12.8 | Mean: 8.3 |
| Median: 13; 95% CI: 6.7 – 15.7 | Median: 7.3; 95% CI: 6 – 8.4 | |
| Gleason sum | GL6=10 | GL6=28 |
| GL7=5 | GL7=9 | |
| GL8=4 | GL9=1 | |
| GL9=4 | ||
| Clinical Stagea | T1C=14 | T1C=20 |
| T2A=5 | T2A=6 | |
| T2B=3 | T2B=10 | |
| T3=1 | T3=2 | |
| HA staining | 182.1 ± 94.1 | 87 ± 75.8 |
| HYAL1 staining | 203.9 ± 88.6 | 48.8 ± 75.7 |
| Parameters available after prostatectomy | ||
| Gleason sum | GL5= 0 | GL5=2 |
| GL6= 4 | GL6=14 | |
| GL7= 8 | GL7=15 | |
| GL8= 6 | GL8=6 | |
| GL9= 5 | GL9=1 | |
| EPE | (+) 14 | (+) 4 |
| (−) 9 | (−) 34 | |
| Pathologic Stage | T2 = 6 | T2 = 34 |
| T3a = 7 | T3a = 1 | |
| T3b = 10 | T3b = 3 | |
| Seminal vesicle invasion | (+) 10 | (+) 3 |
| (−) 13 | (−) 35 | |
| Margin | +) 14 | (+) 8 |
| (−) 9 | (−) 30 | |
| HA staining | 236 ± 81.1 | 125 ± 96.4 |
| HYAL1 staining | 266.3 ±53 | 86.7 ± 89.5 |
Immunohistochemistry
Each biopsy and radical prostatectomy specimen was evaluated to ascertain the presence of tumor in the specimen and the Gleason sum. Biopsy and radical prostatectomy specimen slides were deparaffinized, rehydrated and treated with an antigen retrieval solution (Dako, Glostrup, Denmark). HA was localized in these specimens using a biotinylated HA-binding protein (1 μg/ml; room temperature for 2 hours), purified from bovine nasal cartilage16. HYAL-1 was localized using a rabbit polyclonal antiHYAL-1 IgG (1 μg/ml; 37°C). We have previously described the nature and specificity of the HA-binding protein and antiHYAL-1 IgG9,17. Following incubation with primary reagents, the slides were incubated with LASB solution (Dako) and 3, 3′-diamonobenzidine staining. The sides were counterstained with hematoxylin. To determine the reproducibility of staining, all biopsy slides and 25% of prostatectomy slides were stained twice and scored independently.
Slide grading
Each slide was graded by three readers (CG, PG and VBL) independently, and in a blinded fashion. In each slide, the tumor areas stained for HA and HYAL-1 were graded for staining intensity (0 – 3+) and then multiplied by the area of staining. Thus, each specimen could receive a score between 0 and 300 for HYAL-1 and HA staining. The scores of the three readers were average to get the final score. Overall there was 90% agreement between the scores of the readers. Any discrepancy was resolved by two readers reading the slide simultaneously. The slides were also evaluated using an IP image analysis software and there was significant correlation between the average scores of the readers and IP image analysis scores (Spearman ρ = 0.851; 95% CI: 0.723 ± 0.927; P < 0.001).
Statistical analyses
To determine the correlation of each clinical/pathologic parameter and HA and HYAL-1 staining scores (analyzed as continuous variables) with biochemical recurrence, we performed logistic regression single parameter analysis. Since this is a retrospective cohort study, we performed the Cox proportional hazard analysis, by including all clinical/pathologic parameters and HA and HYAL-1 staining scores (biopsy or radical prostatectomy) in the model, to determine which parameters jointly predict biochemical recurrence. Kaplan-Meier plots were generated to determine whether HA and HYAL-1 could stratify the patient cohort as recurred versus non-recurred. Correlation between biopsy and radical prostatectomy specimens with respect to HA and HYAL-1 staining scores was evaluated by Pearson’s correlation analysis. Receiver operating characteristic curves were generated to determine the association between staining scores and biochemical recurrence. The cut-off values that yielded highest sensitivity (1-specificity) values, were used to define high and low expression of HA (cut-off: 120) and HYAL-1 (cut-off: 185). These cut-off values were calculated using the JMP 6 software (SAS Institute, Carey NC USA). The sensitivity and specificity for HA and HYAL-1 staining inferences were calculated and cross-validated to obtain mean ± SD and 95% CI, as described before18.
RESULTS
Correlation of HA and HYAL-1 staining in biopsy specimens with biochemical recurrence
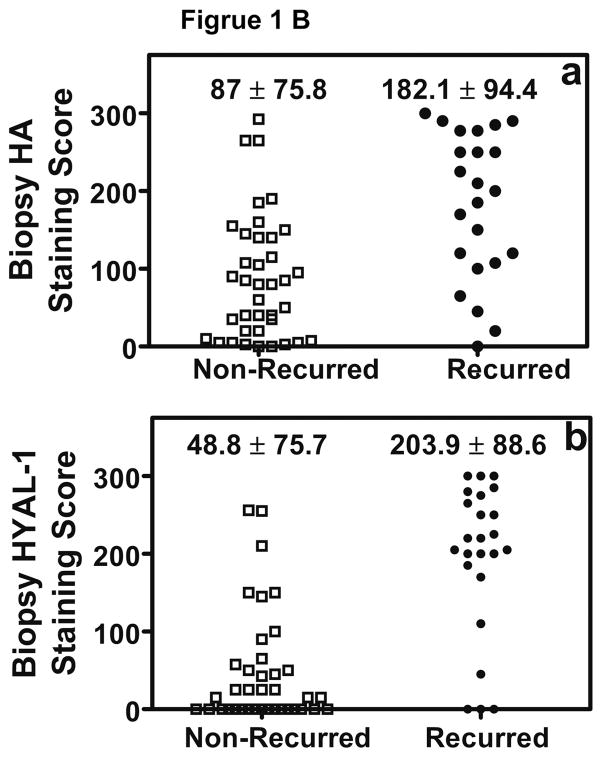
We stained 61 biopsy specimens to evaluate HA and HYAL-1 expression. Figure 1, shows HA and HYAL-1 staining in biopsy specimens from patients with Gleason 7 CaP. HA staining is high in the specimen from a patient who experienced biochemical recurrence following radical prostatectomy, when compared to the patient who did not experience biochemical recurrence (Figure 1A). HA staining is localized primarily in tumor-associated stroma. While there is minimal HYAL-1 expression in prostate tumor in the biopsy specimen from patient who did not experience biochemical recurrence, the HYAL-1 staining is high in the specimen from a patient who had biochemical recurrence (Figure 1A). As we have reported previously, HYAL-1 expression is exclusively in tumor cells9,10 The differences in the mean ± SD intensity scores for both HA (P = 0.0003) and HYAL-1 (P < 0.0001), among patients who experienced biochemical recurrence and those who did not, are statistically significant (Kruscal-Wallis test; Figure 1B).
Figure 1. Localization of HA and HYAL-1 in CaP specimens.
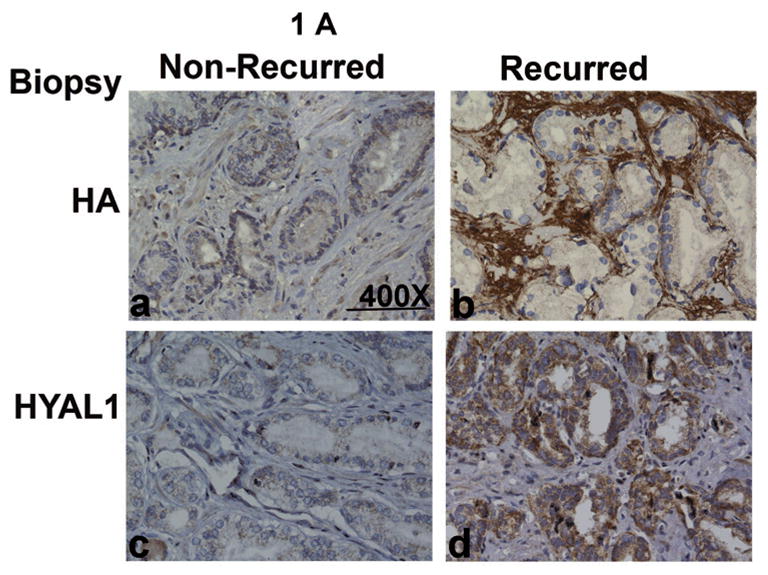
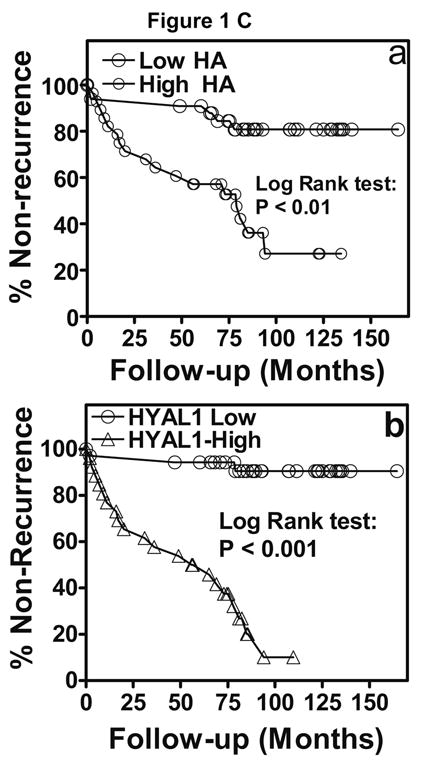
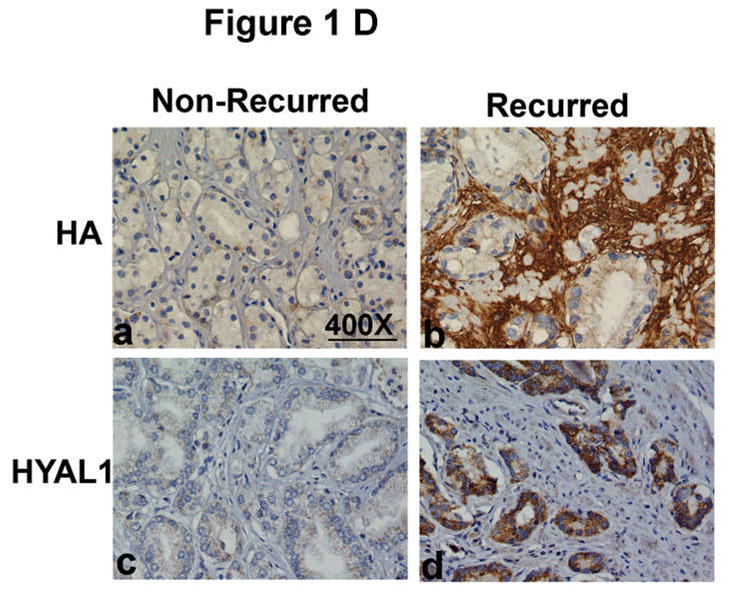
A. HA and HYAL-1 expression in biopsy specimens from patients with Gleason 7 CaP who either had biochemical recurrence or did not recur. a, b: HA staining; c, d: HYAL-1 staining. a, c: non-recurred; b, c: recurred. Magnification: 400X. B: Scatter diagram of HA and HYAL-1 staining scores in CaP specimens, from patients who either had biochemical recurrence or did not recur. The mean ±SD scores for HA (a) and HYAL-1 (b) staining intensity are indicated. Each specimen could receive a score between 0 and 300. C: Kaplan-Meier plots were created to stratify patients with respect to biochemical recurrence based on HA (a) or HYAL-1 (b) staining intensity scores in biopsy specimens. D: HA and HYAL-1 expression in radical prostatectomy specimens from patients with Gleason 7 CaP who either had biochemical recurrence or did not recur. a, b: HA staining; c,d : HYAL-1 staining. a, c: non-recurred; b, c: recurred. Magnification: 400X.
To determine whether age, pre-operative PSA, biopsy Gleason sum, clinical stage and/or the staining scores HA/HYAL-1 predict biochemical recurrence, we performed logistic regression analysis (univariate analysis). As shown in Table 2, pre-operative PSA, Gleason sum (overall and stratified), and HA and HYAL-1 staining intensity scores correlate with biochemical recurrence.
Table 2. Univariate Analysis of pre- and post-biopsy parameters and HA and HYAL-1 staining inferences.
Logistic regression single parameter analysis was used to determine the association of clinical (age, pre-operative PSA, and clinical stage) parameters and biopsy Gleason sum and HA and HYAL-1 staining inferences with biochemical recurrence.
| Parameter | Chi-Square | P-value | Odds Ratio | 95% CI |
|---|---|---|---|---|
| Age | 0.89 | 0.33 | 0.97 | 0.89 – 1.06 |
| a Gleason sum (GS) | 7.49 | 0.0062b | 2.02 | 1.28 – 3.55 |
| GS ≥ 7 | 6.36 | 0.012b | 2.05 | 1.19 – 3.64 |
| GS ≥ 8 | 7.3 | 0.007b | 4.44 | 1.8 – 19.6 |
| Clinical Stage | 0.114 | 0.736 | 1.11 | 0.604 – 2.11 |
| HA | 14.86 | 0.001b | 1.01c | 1.00 – 1.03 |
| HYAL-1 | 31.99 | 0.0001b | 1.02c | 1.01 – 1.03 |
| PSA | 7.6 | 0.006b | 1.12c | 1.03 – 1.23 |
Gleason sum was either continuous or categorized.
Statistically significant;
Change in odds ratio per unit change in the parameter.
HA and HYAL-1 staining intensity scores in biopsy specimens independently correlate with biochemical recurrence
To determine which of the clinical and pathologic parameters and/or HA and HYAL-1 staining inferences predict biochemical recurrence, we performed the Cox proportional hazards analysis. When only clinical and pathologic parameters (i.e., age, biopsy Gleason, pre-operative PSA and clinical stage) were included in the model, biopsy Gleason sum reached statistical significance (χ2: 5.21; P = 0.025 Risk ratio 1.48 95% CI: 1.06 – 2.06). As shown in Table 2, when HA staining score is included in the model, along with all clinical and pathologic parameters described above, HA staining is the only parameter that reached statistical significance in predicting biochemical recurrence. However, when biopsy Gleason sum is categorized as ≥ 7 and < 7, both HA and Gleason sum reach statistical significance (data not shown). When HYAL-1 is included in the model, only HYAL-1 staining reaches statistical significance, regardless of whether Gleason sum is included as a continuous or a categorized variable. When both HA and HYAL-1 are included in the model, again, only HYAL-1 reaches statistical significance (Table 3). These results show that HYAL-1 staining in biopsy specimens (and HA staining if HYLA-1 staining scores are absent) is potentially an independent predictor of biochemical recurrence.
Table 3. Multivariate analyses of pre- and post-biopsy parameters and HA and HYAL-1 inferences.
Cox proportional hazard analysis was performed by including either HA (a), HYAL-1 (b) or HA and HYAL-1 (c: as individual continuous variables) staining inferences together with clinical parameters (i.e., age, PSA, and clinical stage) and biopsy Gleason sum.
| Parameter | Chi-Square | P-value | Risk Ratio | 95% CI |
|---|---|---|---|---|
| HAa | ||||
| HA | 6.93 | 0.009 | 1.00* | 1.00 – 1.01 |
| HYAL-1b | ||||
| HYAL-1 | 26.93 | < 0.001 | 1.01* | 1.00 – 1.02 |
| HA and HYAL-1c | ||||
| HYAL-1 | 20.11 | < 0.001 | 1.01* | 1.00 – 1.02 |
Change in risk ratio per unit change in HA or HYAL-1 staining score.
We next determined whether HA and HYAL-1 staining inferences stratify patients as those who recurred and those who did not. The Kaplan-Meier plot in Figure 1C shows that patients with high HA staining (≥ 120) recurred faster (50% within 73 months; log rank test: χ2: 12.75; p value = 0.0004) than with low HA staining (20% after 78 months). Similarly, patients with high HYAL-1 staining (≥ 185) recurred faster (50% within 49 months; log rank test: χ2: 34.56; p value < 0.0001) than with low HYAL-1 staining (10% after 80 months).
We next evaluated whether HA, and more importantly, HYAL-1 can accurately predict biochemical recurrence. As shown in Table 4, HA staining has reasonable sensitivity but low specificity to predict biochemical recurrence, whereas, HYAL-1 staining has both high sensitivity and specificity to predict biochemical recurrence. Although, the sample size in this study is relatively small, and the sensitivity and specificity of HA or HYAL-1 staining to predict biochemical recurrence will require independent confirmation, the cross-validation results presented in Table 4, strengthen the results.
Table 4. Determination of sensitivity and specificity of HA and HYAL-1 staining inferences for predicting biochemical recurrence.
The sensitivity, specificity and accuracy for HA and HYAL-1 staining inferences in biopsy and radical prostatectomy specimens were calculated using the cut-off limits determined from the ROC curves and then cross-validated. The data presented in the parentheses are mean values and 95% CI, derived from the cross validation analyses.
| Parameter | Area Under the Curve | Sensitivity | Specificity | Accuracy |
|---|---|---|---|---|
| HA (Biopsy) | 0.77 | 72.7% (71.5%; 95% CI: 60.5 – 76.5) | 63.7% (63.9%; 95% CI: 60.4 – 72.2) | 68.2% (67.7%; 95% CI: 62.9 – 75.3) |
| HA (prostatectomy) | 0.79 | 90.9% (85.8%; 95% CI: 80.6 – 93.5) | 55.2% (56.3%; 95% CI: 51.7 – 63.8) | 73% (71.8%; 95% CI: 67.6 – 77.4) |
| HYAL-1 (Biopsy) | 0.87 | 81.8% (82.3%; 95% CI: 76.6 – 92.3) | 81.8% (81.9%; 95% CI: 76.3 – 86.8) | 81.8% (81.4%; 95% CI: 76.9 – 88.4) |
| HYAL-1 (prostatectomy) | 0.95 | 90.9% (88.9%; 95% CI: (81.3 – 95.6) | 75% (76.6%: 95% CI: 72.3 – 81.4) | 83% (82.6%; 95% CI: 77.1 – 87.5) |
Correlation between HA and HYAL-1 expression, in biopsy and radical prostatectomy specimens
To determine whether the HA and HYAL-1 expression in CaP detected in biopsy specimens was representative of the expression in the final pathology specimens, we stained the radical prostatectomy specimens from the same patients for HA and HYAL-1. Figure 1D, shows HA and HYAL-1 staining in radical prostatectomy specimens from patients with Gleason sum 7 CaP. In these specimens, HA and HYAL-1 staining is high if the patient had biochemical recurrence when compared to the specimens from patients who did not recur. Pearson’s correlation analysis (non-parametric) showed significant correlation between HYAL-1 expression in biopsy and radical prostatectomy specimens (Spearman ρ = 0.719; 95% CI: 0.4052 to 0.8813; p = 0.0002). However, the correlation between HA expression in biopsy and radical prostatectomy specimens was not statistically significant (Spearman ρ = 0.275; 95% CI: −0.167 to 0.625; p = 0.205). In the multivariate analysis that included clinical (age, pre-operative PSA, clinical stage) and pathologic parameters (Gleason sum, EPE, seminal vesicle invasion and margin) and HA and HYAL-1 staining scores, only HYAL-1 staining (χ2: 9.58; P = 0.002 Risk ratio 1.01 95% CI: 1.00 – 1.03). The sensitivity and specificity data presented in Table 4 show that, using the same cut-off value (i.e., 120) as for the biopsy specimens, HA staining in radical prostatectomy specimens has high sensitivity but poor specificity to predict biochemical recurrence. Contrarily, as in the case of biopsy specimens, HYAL-1 staining in radical prostatectomy specimens predicted biochemical recurrence with high sensitivity and reasonable specificity.
DISCUSSION
Although the widespread acceptance of PSA has increased the number of CaP cases detected and cured each year, the majority of the newly detected patients have biopsy Gleason between 6 and 7 and serum PSA between 4 and 10-ng/ml. Thus, individualized treatment may be possible if molecular markers that function in promoting invasion/metastasis are added as adjuncts to the current diagnostic tools19. Consistent with this thesis, in this study we found that HA, but more importantly HYAL-1 expression in biopsy specimens independently predicts biochemical recurrence following radical prostatectomy.
HA due to its ability to promote cell migration and motility supports tumor metastasis, but it is HYAL-1 that often correlates with the aggressiveness of the tumor4. For example, HYAL-1 is necessary for cell cycle progression and invasive activity of CaP cells in vitro and to promote tumor growth and vascular/lymphatic invasion in vivo14. Moreover, it has been consistently shown that in addition to HA, HYAL-1 expression is necessary for CaP metastasis6,20. Consistent with the dependence of HA on HYAL-1 to promote metastasis, in this study we found that when HYAL-1 staining inference is added to the multivariate model, it erases the independent prognostic significance of HA expression in biopsy specimens. Furthermore, in this study, as well as, in two previous studies we found that HA expression in radical prostatectomy specimens is not an independent prognostic predictor of biochemical recurrence and two independent studies have confirmed these findings20,21.
Although the prognostic significance of HA to predict biochemical recurrence is limited, HYAL-1 expression in both biopsy and radical prostatectomy specimens appears to be a strong predictor of biochemical recurrence (this study and ref. 8,9). The discrepancy in these two functionally interdependent markers, with respect to prognostic significance, may be because HA expression is mainly stromal, whereas, HYAL-1 is expressed exclusively by invasive tumor cells. In fact, silencing HYAL-1 expression in prostate xenografts decreases HA expression in the tumor-associated stroma11. Thus, HYAL-1 may be a molecular switch that controls the metastatic promotion of CaP by the HA-HYAL-1 system.
Tumor heterogeneity in CaP is a significant factor that impedes the accurate prognostic prediction of tumor behavior based on clinical and pathologic parameters, as well as molecular markers available at the biopsy stage. For example, case of p53 and bcl-2, although the staining in biopsy specimen correlates with biochemical recurrence, the staining in the biopsy specimens for both markers does not correlate with the staining in radical prostatectomy specimens.23 In our study there was upstaging in Gleason sum in 44% of the specimens (27 out of 61). However, it is noteworthy that HYAL-1 staining scores in biopsy specimens significantly correlated with the scores in radical prostatectomy specimens, suggesting that molecular markers that correlate with invasive behavior of the tumor may help in making better prognostic predictions at the biopsy stage.
This is the first study that has evaluated HYAL-1 and HA expression in prostate biopsy specimens. The clinical significance of our study is that current preoperative parameters do not provide adequate information regarding which patients will remain disease free versus those who will experience disease progression despite radical prostatectomy. Our study shows that HYAL-1 staining very likely has the potential to not only predict biochemical recurrence at the radical prostatectomy stage, but also at the biopsy stage. Such information at the biopsy stage may help the physicians to stratify patients into different risk categories for biochemical recurrence and identify those patients who will need additional treatments following radical prostatectomy. The biopsy specimens were obtained from multiple community centers and the same markers were evaluated in the matched biopsy and radical prostatectomy specimens. However, this was a retrospective cohort study and the sample size was limited. Taking into consideration some of these caveats, a prospective multicenter center will need to be conducted to fully assess the prognostic potential of HYAL-1.
Conclusions
HYAL-1 staining in biopsy specimen has potential to aid existing clinical and pathologic parameters to stratify patients developing biochemical recurrence following radical prostatectomy. If these results can be confirmed in prospective multicenter study, it could help in individualizing treatment options.
Acknowledgments
Funding: R01 CA 123063-01 (VBL), CURED (Department of Urology, University of Miami); Judith Knapp: Supported by the International Academy of Life Sciences, Biomedical Science Exchange Program fellowship
Abbreviations used
- CaP
Prostate cancer
- HA
Hyaluronic acid
- HAase
Hyaluronidase
- PSA
prostate specific antigen
References
- 1.Heidenreich A, Aus G, Bolla M, Joniau S, Matveev VB, Schmid HP, et al. EAU guidelines on prostate cancer. Eur Urol. 2008;53:68. doi: 10.1016/j.eururo.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Hadaschik BA, Gleave ME. Therapeutic options for hormone-refractory prostate cancer in 2007. Urol Oncol. 2007;25:413. doi: 10.1016/j.urolonc.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Tammi RH, Kultti A, Kosma VM, Pirinen R, Auvinen P, Tammi MI. Hyaluronan in human tumors: pathobiological and prognostic messages from cell-associated and stromal hyaluronan. Semin Cancer Biol. 2008;18:288. doi: 10.1016/j.semcancer.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Simpson MA, Lokeshwar VB. Hyaluronan and hyaluronidase in genitourinary tumors. Front Biosci. 2008;13:5664. doi: 10.2741/3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Golshani R, Lopez L, Estrella V, Kramer M, Iida N, Lokeshwar VB. Hyaluronic acid synthase-1 expression regulates bladder cancer growth, invasion, and angiogenesis through CD44. Cancer Res. 2008;68:483. doi: 10.1158/0008-5472.CAN-07-2140. [DOI] [PubMed] [Google Scholar]
- 6.Bharadwaj AG, Rector K, Simpson MA. Inducible hyaluronan production reveals differential effects on prostate tumor cell growth and tumor angiogenesis. J Biol Chem. 2007;282:20561. doi: 10.1074/jbc.M702964200. [DOI] [PubMed] [Google Scholar]
- 7.Lokeshwar VB, Obek C, Pham HT, Wei D, Young MJ, Duncan RC, Soloway MS, et al. Urinary hyaluronic acid and hyaluronidase: markers for bladder cancer Detection and evaluation of grade. J Urol. 2000;163:348. doi: 10.1016/s0022-5347(05)68050-0. [DOI] [PubMed] [Google Scholar]
- 8.Kramer MW, Golshani R, Merseburger AS, Knapp J, Garcia A, Hennelotter J, et al. HYAL-1 hyaluronidase: A potential prognostic indicator for progression to muscle invasion and recurrence in bladder cancer. Eur Urol. 2009 doi: 10.1016/j.eururo.2009.03.057. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Posey JT, Soloway MS, Ekici S, Sofer M, Civantos F, Duncan RC, et al. Evaluation of the prognostic potential of hyaluronic acid and hyaluronidase (HYAL-1) for prostate cancer. Cancer Res. 2003;63:2638. [PubMed] [Google Scholar]
- 10.Ekici S, Cerwinka WH, Duncan R, Gomez P, Civantos F, Soloway MS, et al. Comparison of the prognostic potential of hyaluronic acid, hyaluronidase (HYAL- 1), CD44v6 and microvessel density for prostate cancer. Int J Cancer. 2004;112:121. doi: 10.1002/ijc.20368. [DOI] [PubMed] [Google Scholar]
- 11.Lin SL, Chang D, Chiang A, Ying SY. Androgen receptor regulates CD168 expression and signaling in prostate cancer. Carcinogenesis. 2008;29:282. doi: 10.1093/carcin/bgm259. [DOI] [PubMed] [Google Scholar]
- 12.Lokeshwar VB, Selzer MG. Hyalurondiase: both a tumor promoter and suppressor. Semin Cancer Biol. 2008;18:281. doi: 10.1016/j.semcancer.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schroeder GL, Lorenzo-Gomez MF, Hautmann SH, Friedrich MG, Ekici S, Huland H, et al. A side by side comparison of cytology and biomarkers for bladder cancer detection. J Urol. 2004;172:1123. doi: 10.1097/01.ju.0000134347.14643.ab. [DOI] [PubMed] [Google Scholar]
- 14.Lokeshwar VB, Cerwinka WH, Isoyama T, Lokeshwar BL. HYAL1 Hyaluronidase in prostate cancer: a tumor promoter and suppressor. Cancer Res. 2005;65:7782. doi: 10.1158/0008-5472.CAN-05-1022. [DOI] [PubMed] [Google Scholar]
- 15.Lokeshwar VB, Cerwinka WH, Lokeshwar BL. HYAL1 hyaluronidase: a molecular determinant of bladder tumor growth and invasion. Cancer Res. 2005;65:2243. doi: 10.1158/0008-5472.CAN-04-2805. [DOI] [PubMed] [Google Scholar]
- 16.Lokeshwar VB, Lokeshwar BL, Pham HT, Block NL. Association of elevated levels of hyaluronidase, a matrix-degrading enzyme, with prostate cancer progression. Cancer Res. 1996;56:651. [PubMed] [Google Scholar]
- 17.Lokeshwar VB, Young MJ, Goudarzi G, Iida N, Yudin AI, Cherr GN, et al. Identification of bladder tumor-derived hyaluronidase: its similarity to HYAL1. Cancer Res. 1999;59:4464. [PubMed] [Google Scholar]
- 18.Caruso DJ, Carmack AJ, Lokeshwar VB, Duncan RC, Soloway MS, Lokeshwar BL. Osteopontin and interleukin-8 expression is independently associated with prostate cancer recurrence. Clin Cancer Res. 2008;14:4111. doi: 10.1158/1078-0432.CCR-08-0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gurel B, Iwata T, Koh CM, Yegnasubramanian S, Nelson WG, De Marzo AM. Molecular alterations in prostate cancer as diagnostic, prognostic, and therapeutic targets. Adv Anat Pathol. 2008;15:319. doi: 10.1097/PAP.0b013e31818a5c19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bharadwaj AG, Kovar JL, Loughman E, Elowsky C, Oakley GG, Simpson MA. Spontaneous metastasis of prostate cancer is promoted by excess hyaluronan synthesis and processing. Am J Pathol. 2009 Feb 13; doi: 10.2353/ajpath.2009.080501. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lipponen P, Aaltomaa S, Tammi R, Tammi M, Ågren U, Kosma VM. High stromal hyaluronan level is associated with poor differentiation and metastasis in prostate cancer. Eur J Cancer. 2001;37:849. doi: 10.1016/s0959-8049(00)00448-2. [DOI] [PubMed] [Google Scholar]
- 22.Aaltomaa S, Lipponen P, Tammi R, Tammi M, Viitanen J, Kankkunen JP, et al. Strong stromal hyaluronan expression is associated with PSA recurrence in local prostate cancer. Urol Int. 2002;69:266. doi: 10.1159/000066123. [DOI] [PubMed] [Google Scholar]
- 23.Stackhouse GB, Sesterhenn IA, Bauer JJ, Mostofi FK, Connelly RR, Srivastava SK, et al. p53 and bcl-2 immunohistochemistry in pretreatment prostate needle biopsies To predict recurrence of prostate cancer after radical prostatectomy. J Urol. 1999;162:2040. doi: 10.1016/S0022-5347(05)68095-0. [DOI] [PubMed] [Google Scholar]