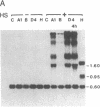
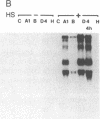
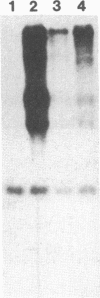
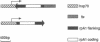Abstract
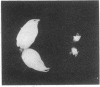
To assess the functional importance of ribosomal protein rpA1 gene expression during development of Drosophila melanogaster, we have transformed into the fly's genome an antisense rpA1 gene driven by a heat shock promoter. Antisense rpA1 expression severely disrupted oogenesis and produced a "small egg" female-sterile phenotype. The severities of these defects were proportional to the level of antisense rpA1 expression. Anti-rpA1 expression did not affect larval or pupal development. Quantitative RNA analysis suggested that high anti-rpA1 expression results in a general decrease of mRNA in the ovary.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Atia G. R., Fruscoloni P., Jacobs-Lorena M. Translational regulation of mRNAs for ribosomal proteins during early Drosophila development. Biochemistry. 1985 Oct 8;24(21):5798–5803. doi: 10.1021/bi00342a017. [DOI] [PubMed] [Google Scholar]
- Bass B. L., Weintraub H. A developmentally regulated activity that unwinds RNA duplexes. Cell. 1987 Feb 27;48(4):607–613. doi: 10.1016/0092-8674(87)90239-x. [DOI] [PubMed] [Google Scholar]
- Bonner J. J., Parks C., Parker-Thornburg J., Mortin M. A., Pelham H. R. The use of promoter fusions in Drosophila genetics: isolation of mutations affecting the heat shock response. Cell. 1984 Jul;37(3):979–991. doi: 10.1016/0092-8674(84)90432-x. [DOI] [PubMed] [Google Scholar]
- Burns D. K., Stark B. C., Macklin M. D., Chooi W. Y. Isolation and characterization of cloned DNA sequences containing ribosomal protein genes of Drosophila melanogaster. Mol Cell Biol. 1984 Dec;4(12):2643–2652. doi: 10.1128/mcb.4.12.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera C. V., Alonso M. C., Johnston P., Phillips R. G., Lawrence P. A. Phenocopies induced with antisense RNA identify the wingless gene. Cell. 1987 Aug 14;50(4):659–663. doi: 10.1016/0092-8674(87)90039-0. [DOI] [PubMed] [Google Scholar]
- Crowley T. E., Nellen W., Gomer R. H., Firtel R. A. Phenocopy of discoidin I-minus mutants by antisense transformation in Dictyostelium. Cell. 1985 Dec;43(3 Pt 2):633–641. doi: 10.1016/0092-8674(85)90235-1. [DOI] [PubMed] [Google Scholar]
- Farnsworth M W. Effects of Homozygous First, Second and Third Chromosome Minutes on the Development of Drosophila Melanogaster. Genetics. 1957 Jan;42(1):19–27. doi: 10.1093/genetics/42.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hough-Evans B. R., Jacobs-Lorena M., Cummings M. R., Britten R. J., Davidson E. H. Complexity of RNA in eggs of Drosophila melanogaster and Musca domestica. Genetics. 1980 May;95(1):81–94. doi: 10.1093/genetics/95.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuki M., Sato M., Kimura M., Yokoyama M., Kobayashi K., Nomura T. Conversion of normal behavior to shiverer by myelin basic protein antisense cDNA in transgenic mice. Science. 1988 Jul 29;241(4865):593–595. doi: 10.1126/science.2456614. [DOI] [PubMed] [Google Scholar]
- Kay M. A., Jacobs-Lorena M. Selective translational regulation of ribosomal protein gene expression during early development of Drosophila melanogaster. Mol Cell Biol. 1985 Dec;5(12):3583–3592. doi: 10.1128/mcb.5.12.3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht D. A., Loomis W. F. Antisense RNA inactivation of myosin heavy chain gene expression in Dictyostelium discoideum. Science. 1987 May 29;236(4805):1081–1086. doi: 10.1126/science.3576221. [DOI] [PubMed] [Google Scholar]
- Kongsuwan K., Yu Q., Vincent A., Frisardi M. C., Rosbash M., Lengyel J. A., Merriam J. A Drosophila Minute gene encodes a ribosomal protein. Nature. 1985 Oct 10;317(6037):555–558. doi: 10.1038/317555a0. [DOI] [PubMed] [Google Scholar]
- Kuroiwa A., Hafen E., Gehring W. J. Cloning and transcriptional analysis of the segmentation gene fushi tarazu of Drosophila. Cell. 1984 Jul;37(3):825–831. doi: 10.1016/0092-8674(84)90417-3. [DOI] [PubMed] [Google Scholar]
- Li J C. Development in DROSOPHILA MELANOGASTER. Genetics. 1927 Jan;12(1):1–58. doi: 10.1093/genetics/12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley D. L., Sandler L., Baker B. S., Carpenter A. T., Denell R. E., Hall J. C., Jacobs P. A., Miklos G. L., Davis B. K., Gethmann R. C. Segmental aneuploidy and the genetic gross structure of the Drosophila genome. Genetics. 1972 May;71(1):157–184. doi: 10.1093/genetics/71.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark G. E., MacIntyre R. J., Digan M. E., Ambrosio L., Perrimon N. Drosophila melanogaster homologs of the raf oncogene. Mol Cell Biol. 1987 Jun;7(6):2134–2140. doi: 10.1128/mcb.7.6.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermod J. J., Jacobs-Lorena M., Crippa M. Changes in rate of RNA synthesis and ribosomal gene number during oogenesis of Drosophila melanogaster. Dev Biol. 1977 Jun;57(2):393–402. doi: 10.1016/0012-1606(77)90224-x. [DOI] [PubMed] [Google Scholar]
- Mismer D., Rubin G. M. Analysis of the promoter of the ninaE opsin gene in Drosophila melanogaster. Genetics. 1987 Aug;116(4):565–578. doi: 10.1093/genetics/116.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikura K., Murray J. M. Antisense RNA of proto-oncogene c-fos blocks renewed growth of quiescent 3T3 cells. Mol Cell Biol. 1987 Feb;7(2):639–649. doi: 10.1128/mcb.7.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian S., Zhang J. Y., Kay M. A., Jacobs-Lorena M. Structural analysis of the Drosophila rpA1 gene, a member of the eucaryotic 'A' type ribosomal protein family. Nucleic Acids Res. 1987 Feb 11;15(3):987–1003. doi: 10.1093/nar/15.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebagliati M. R., Melton D. A. Antisense RNA injections in fertilized frog eggs reveal an RNA duplex unwinding activity. Cell. 1987 Feb 27;48(4):599–605. doi: 10.1016/0092-8674(87)90238-8. [DOI] [PubMed] [Google Scholar]
- Rosenberg U. B., Preiss A., Seifert E., Jäckle H., Knipple D. C. Production of phenocopies by Krüppel antisense RNA injection into Drosophila embryos. Nature. 1985 Feb 21;313(6004):703–706. doi: 10.1038/313703a0. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982 Oct 22;218(4570):348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Ruddell A., Jacobs-Lorena M. Preferential expression of actin genes during oogenesis of Drosophila. Dev Biol. 1984 Sep;105(1):115–120. doi: 10.1016/0012-1606(84)90266-5. [DOI] [PubMed] [Google Scholar]
- Vaslet C. A., O'Connell P., Izquierdo M., Rosbash M. Isolation and mapping of a cloned ribosomal protein gene of Drosophila melanogaster. Nature. 1980 Jun 26;285(5767):674–676. doi: 10.1038/285674a0. [DOI] [PubMed] [Google Scholar]
- Wool I. G. The structure and function of eukaryotic ribosomes. Annu Rev Biochem. 1979;48:719–754. doi: 10.1146/annurev.bi.48.070179.003443. [DOI] [PubMed] [Google Scholar]
- Wormington W. M. Stable repression of ribosomal protein L1 synthesis in Xenopus oocytes by microinjection of antisense RNA. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8639–8643. doi: 10.1073/pnas.83.22.8639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost H. J., Lindquist S. RNA splicing is interrupted by heat shock and is rescued by heat shock protein synthesis. Cell. 1986 Apr 25;45(2):185–193. doi: 10.1016/0092-8674(86)90382-x. [DOI] [PubMed] [Google Scholar]