Abstract
Nicotinic acetylcholine (nACh) and 5-hydroxytryptamine type 3 (5-HT3) receptors are cation-selective ion channels of the pentameric ligand-gated ion channel (pLGIC) superfamily. Multiple lines of evidence adduced over the last 30 years indicate that the lining of the channel of such receptors is formed by the α-helical second transmembrane (TM2) domain and flanking sequences contributed by each of the five subunits present within the receptor complex. Specific amino acid residues within, and adjacent to, the TM2 domain influence single channel conductance, ion selectivity, and other aspects of receptor function that include gating and desensitization. However, more recent work has revealed important structural determinants of single channel conductance and ion selectivity that are not associated with the TM2 domain. Direct experimental evidence indicates that the intracellular domain of eukaryotic pLGICs, in particular a region of the loop linking TM3 and TM4 termed the membrane-associated (MA) stretch, exerts a strong influence upon ion channel biophysics. Moreover, recent computational approaches, complemented by experimentation, implicate the extracellular domain as an additional important determinant of ion conduction. This brief review describes how our knowledge of ion conduction and selectivity in cation-selective pLGICs has evolved beyond TM2.

John Peters and Jeremy Lambert work at the Centre for Neuroscience at the University of Dundee. They have a particular interest in the physiological and pharmacological properties of ligand-gated ion channels of the ‘Cys-loop receptor’ family. Much of their work focuses on how the structure of such receptors relates to their function. Tim Hales, their long-term collaborator in this enterprise has recently moved from George Washington University to join them in Dundee.
Introduction
5-Hydroxytryptamine type 3 (5-HT3) and nicotinic acetylcholine (nACh) receptors, along with a much less extensively studied zinc-activated channel (ZAC), form the excitatory, cation selective, members of the pentameric ligand-gated ion channel (pLGIC) superfamily in eukaryotes (Barnes et al. 2009; Millar & Gotti, 2009). The functionally opposing inhibitory, anion selective channels are the γ-aminobutyric acid type A (GABAA) and strychnine-sensitive glycine receptors (Olsen & Sieghart, 2008; Lynch, 2009). Each of the five subunits of the eukaryotic pLGICs consists of three functional modules: (i) an extracellular domain (ECD) harbouring the ligand binding site, (ii) four α-helical transmembrane (TM) domains, the second of which forms the lining of the ion channel, and (iii) an intracellular domain (ICD), composed of a large loop between the TM3 and TM4 domains and a short sequence linking the TM1 and TM2 domains (Fig. 1). The large loop is essentially absent from the prokaryotic pLGICs of Gloeobacter violaceus (i.e. GLIC) and Erwinia chrysanthemi (i.e. ELIC) (Hilf & Dutzler, 2009).
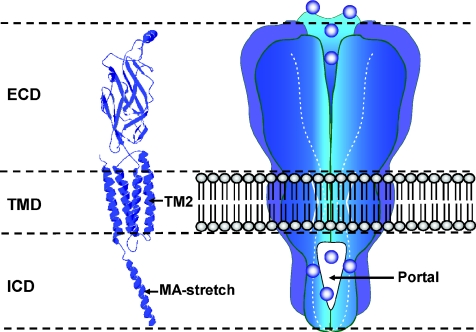
Figure 1. The architecture of the eukaryotic members of the pentameric ligand-gated ion channel superfamily.
Left, a homology model of a human 5-HT3A receptor subunit constructed by Hales et al. (2006) from the 4 Å resolution data of the Torpedo nACh receptor (Unwin, 2005). The locations of the channel lining TM2 domain and MA stretch that frames the lateral aspects of the portals are indicted. Right, schematic illustration of the gross organisation of such receptors indicating the intracellular location of the portals that have recently been shown to contribute to the permeation pathway (Kelley et al. 2003) and which form an obligate route for ion flux. Five such portals are located at the interfaces of the subunits comprising the receptor. Abbreviations: ECD, extracellular domain; TMD, transmembrane domain; ICD, intracellular domain; MA, membrane-associated.
A detailed picture of the three dimensional structure of the pLGIC subunits has been revealed by a number of advances. Firstly, the crystal structure of the molluscan acetylcholine binding protein (AChBP), which is a water soluble pentameric homologue of the ECD of the nACh receptor, in complex with several nicotinic receptor ligands, has been determined (Rucktooa et al. 2009). The latter has permitted homology modelling of the occupied ligand binding domain of the major families of eukaryotic pLGICs. Secondly, the structural organisation of almost the entirety of the nACh receptor of Torpedo marmorata has been solved, at 4 Å resolution, through progressive improvements in cryo-electron microscopy and image reconstruction (Unwin, 2005). Importantly, this structure adds details of the organisation of the TM domains to the information on the ECD gleaned from AChBP. Unfortunately, a substantial portion of ICD of the nACh receptor, which is predicted to be mostly unfolded (Kukhtina et al. 2006), remains to be characterised structurally. Nonetheless, α-helical structures are predicted to occur within two segments of the loop linking TM3 and TM4 (Kukhtina et al. 2006) and one, dubbed the MA (or HA) stretch (Finer-Moore & Stroud, 1984), is resolved in the refined model of the Torpedo nACh receptor (Unwin, 2005). The influence of the MA stretch upon receptor biophysics will be emphasised in this review. Finally, the X-ray structures of GLIC and ELIC have provided the first high resolution structures of pLGIC superfamily members. Importantly, a comparison of the structures of GLIC, obtained in the conducting state, and ELIC, observed in the non-conducting state, provides insight into the gating process (Hilf & Dutzler, 2009).
The conduction pathway
The most complete description of the journey of an ion from the extracellular to intracellular media via eukaryotic pLGICs is provided by the Torpedo nACh receptor (Miyazawa et al. 2003; Unwin, 2005). In outline, ions enter the conduction pathway through the extracellular vestibule, which has an inner diameter of approximately 20 Å and is approximately 60 Å long. Thereafter they translocate to the narrower transmembrane pore that is lined by the five TM2 α-helices and which contains the gate of the channel. At the intracellular aspect of the membrane, the ions emerge into a vestibule perforated by five narrow fenestrations (‘portals’) that form an obligate pathway through which ions must flux. Such portals are framed laterally by α-helices corresponding to the MA stretch of adjacent subunits and at their roof by the TM1–TM2 loop. The α-helices project interiorly into the cytoplasm and converge on the central axis of the receptor in the manner of an inverted tepee (Miyazawa et al. 2003; Unwin, 2005). Thus, in this model of eukaryotic pLGIC structure, the cytoplasmic vestibule presents five parallel conduction pathways, rather than a single, central conduit. Evidently, the prokaryotic pLGICs lack this structural sophistication raising questions about its purpose in the evolutionarily more advanced channels.
A seminal mutagenesis study of the nACh receptor of Torpedo californica identified three clusters of negatively charged residues that bracket the channel-lining TM2 segment as crucial determinants of single channel conductance (γ), namely the extracellular (20′), inner (−1′) and cytoplasmic (−4′) rings of charge (Imoto et al. 1988; see Fig. 2). Subsequently, the importance of TM2 and the flanking rings of charge in ion conduction and selectivity has become axiomatic for all eukaryotic pLGICs, including the anion selective GABAA and glycine receptors (in which the charged rings occur at the 0′ and 19′ locations and are net positive) (Keramidas et al. 2004; Jensen et al. 2005; Peters et al. 2005). However, more recent studies have revealed that important determinants of γ and ion selectivity also reside within the ICD and the ECD of 5-HT3 and nACh receptors (Kelley et al. 2003; Hales et al. 2006; Deeb et al. 2007; Hansen et al. 2008; Livesey et al. 2008). The influence of specific amino acid residues within these domains upon the biophysical properties of nACh and 5-HT3 receptors is discussed below.
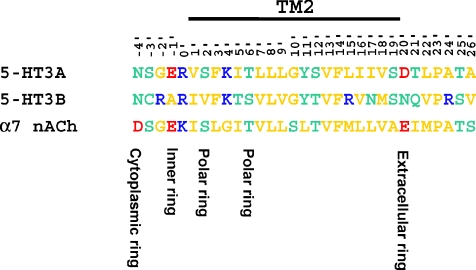
Figure 2. Sequence alignment of the human 5-HT3A, 5-HT3B and α7 nACh subunit across the TM2 domain and flanking sequences.
Note that structural elements identified by Imoto et al. (1988) as important determinants of γ (i.e. the extracellular and inner rings of charge) are conserved between 5-HT3A and α7 nACh subunits, but are absent from the 5-HT3B subunit. Within the alignment basic, acidic, polar and non-polar residues are colour coded blue, red, green and yellow, respectively.
The differential single channel conductance (γ) of homomeric 5-HT3A and heteromeric 5-HT3AB receptors led to the localization of a major determinant of γ to the ICD
Genes encoding five 5-HT3 receptor subunits (5-HT3A–E) have been identified in humans (Karnovsky et al. 2003; Niesler et al. 2003), but only homomeric 5-HT3A (Brown et al. 1998) and heteromeric 5-HT3AB (Davies et al. 1999) receptors have been studied in detail. Note that the receptor and subunit nomenclature employed in this article conforms to recent recommendations issued by NC-IUPHAR (Collingridge et al. 2009). Human 5-HT3A and 5-HT3AB receptors display only modest differences in their ligand binding profile when probed by conventional 5-HT3 receptor ligands (Brady et al. 2001) but differ markedly in their biophysical properties including γ, relative permeability to divalent cations and rectification at the single channel level (Brown et al. 1998; Davies et al. 1999; Mochizuki et al. 1999; Kelley et al. 2003). Most notably, the γ (∼16 pS) of the 5-HT3AB receptor in outside-out membrane patches excised from HEK-293 cells transfected with cDNAs encoding the 5-HT3A and 5-HT3B subunits is much greater than that of the recombinant 5-HT3A receptor (0.4–1.0 pS) inferred by fluctuation analysis of macroscopic current responses (Brown et al. 1998; Davies et al. 1999; Kelley et al. 2003; Deeb et al. 2007). The structural basis of the anomalously low γ of the 5-HT3A receptor does not reside within the TM2 domain, which exhibits a high degree of conservation to that of the high conductance α7 nACh receptor (reviewed by Peters et al. 2005; see also Fig. 2). There is no doubt that TM2 domains form the lining of the 5-HT3A receptor ion channel: the mutation E-1′A, thus neutralising the inner ring of charge, results in a channel that does not discriminate between monovalent cations and anions (PNa/PCl= 0.89) and when combined with the mutation S19′R produces a channel with mild anion selectivity (PNa/PCl= 0.37) (Thompson & Lummis, 2003). Similarly, as found for the α7 nACh receptor (Galzi et al. 1992), the mutations V13′T and E-1′A, coupled with the insertion of proline into the TM1–TM2 loop, generate an anion selective channel (PNa/PCl= 0.08) (Gunthorpe & Lummis, 2001). More recently, we found that neutralisation of the extracellular ring of charge by the mutation D20′A markedly suppressed the relative permeability of Ca2+, but had no influence upon monovalent cation versus anion selectivity (Livesey et al. 2008).
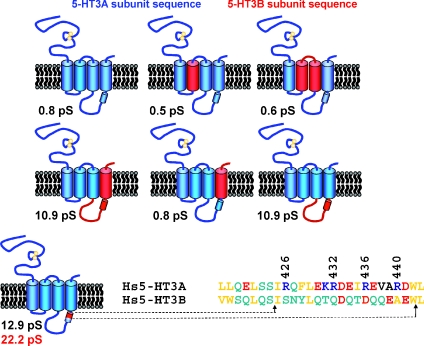
To identify the domain of the 5-HT3B receptor subunit that confers greatly enhanced γ on 5-HT3AB over 5-HT3A receptors, we constructed a series of human 5-HT3A/5-HT3B subunit chimaeras, expressed them in tsA-201 cells either in isolation or in combination with wild-type 5-HT3A subunits, and analysed γ initially by fluctuation analysis (Kelley et al. 2003). This approach, schematically illustrated in Fig. 3, identified the intracellular TM3–TM4 loop as harbouring a crucial determinant of γ. Finer inspection of the loop by less extensive substitution of 5-HT3A by 5-HT3B subunit sequence within homomeric receptors revealed that replacement of an 18 amino acid sequence within the amphipathic MA stretch increased γ by approximately 29-fold (Kelley et al. 2003). A striking feature of all orthologous 5-HT3A subunit sequences is the presence of three arginine residues (R432, R436 and R440 of the human sequence) with a periodicity that places them on the same face of an α-helical structure (Fig. 3). Such a motif is absent from any other vertebrate cation selective pLGIC subunit. The collective mutation of these residues to their aligned counterparts within the 5-HT3B subunit (i.e. R432Q, R436D, R440A) enhanced γ by a factor comparable to that caused by the 18 amino acid insertion of 5-HT3B subunit sequence described above. An analysis of single amino acid substitutions revealed the R436D mutation to have the most pronounced impact upon γ (Kelley et al. 2003). We substantiated the results of fluctuation analysis by single channel recordings performed on outside-out membrane patches and note that whilst the former method consistently underestimates γ determined directly, there is nonetheless an excellent correlation between the data obtained by the two methods (Hales et al. 2006). For convenience of description, we will henceforth refer to the 5-HT3A R432Q, R436D, R440A mutant as the 5-HT3A (QDA) receptor. Under standard recording conditions, the γ of the human 5-HT3A (QDA) receptor is 22 pS, or 36 pS, when determined by fluctuation analysis of whole cell currents, or single channel recording from outside-out membrane patches, respectively (Kelley et al. 2003; Hales et al. 2006).
Figure 3. Summary of the experimental approach that resulted in the identification of the MA stretch as a component of the ion permeation pathway.
Representative 5-HT3A/5-HT3B subunit chimaeras used by Kelley et al. (2003) are shown schematically with human 5-HT3A and 5-HT3B sequences depicted in blue and red, respectively. Single channel conductances (γ, estimated by fluctuation analysis) for the chimaeric constructs co-expressed with the wild-type 5-HT3A subunit in tsA-201 cells are given in each panel. Note that a second γ value (in red) indicates that the construct functioned as a homoligomer obviating the need for co-expression with the wild-type 5-HT3A subunit. The final chimaera examined (bottom row) identified an 18 amino acid sequence within the α-helical MA stretch as a crucial determinant of γ. A sequence alignment across this region indicates the location of the R432, R436 and R440 residues of the human 5-HT3A subunit and their human 5-HT3B subunit counterparts. The collective substitution of the latter into the 5-HT3A sequence increased γ by approximately 40-fold in comparison to the wild-type 5-HT3A receptor (Hales et al. 2006). Within the alignment basic, acidic, polar and non-polar residues are colour coded blue, red, green and yellow, respectively.
The dramatic increase in γ exhibited by the 5-HT3A (QDA) receptor has been confirmed by at least two other groups who performed mutagenesis upon the mouse, rather than human, 5-HT3A subunit. (Reeves et al. 2005; Bouzat et al. 2008). Moreover, the replacement of the entire intracellular loop linking TM3 and TM4 of the mouse 5-HT3A subunit by the short heptapeptide linker of GLIC results in a channel (termed 5-HT3A-glvM3M4) that is apparently fully functional and has a γ (i.e. 43 pS; Jansen et al. 2008) remarkably similar to that of the human 5-HT3A (QDA) receptor (i.e. 38–42 pS; Reeves et al. 2005; Livesey et al. 2008) under approximately equivalent ionic conditions. Jansen et al. (2008) therefore speculate ‘… that no additional features in the whole M3M4 loop besides the three arginines are involved in limiting the 5-HT3A single channel conductance …’. An alternative, and in our view more likely, interpretation is that the similar values of γ found for the 5-HT3A (QDA) and 5-HT3A-glvM3M4 receptor constructs reflect a common rate limiting step in ion conduction that emerges when the constraint imposed by the whole TM3–TM4 loop, or the triple arginine motif, is removed.
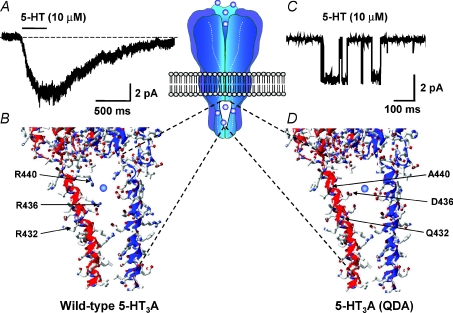
A homology model of the human 5-HT3A receptor (constructed from the atomic scale model of the Torpedo nACh receptor) provides a qualitative glimpse of how the MA stretch R432, R436 and R440 residues might collectively influence γ so profoundly (Hales et al. 2006). Inspection of the model depicted in Fig. 4 reveals the three arginine residues to be positioned within the intracellular portals through which ions must pass to enter, or leave, the cytoplasm. The dimensions of the portals are such that the positively charged and voluminous R432, R440 and particularly R436 residues could act as both steric and electrostatic impediments to cation flux (Fig. 4). It must be acknowledged that the orientations of the residue side chains are not known precisely and that the model is based on the Torpedo nACh receptor in the closed state. However, the following section reviews additional data that support the model.
Figure 4. The influence of amino acid residues within the MA stretch upon the γ of the human 5-HT3A receptor.
A, inward current response evoked by 5-HT applied to an outside-out membrane patch excised from a HEK-293 cell expressing the human homomeric 5-HT3A receptor. The patch was voltage-clamped at a holding potential of −60 mV. Note the absence of discernable single channel current events. B, homology model, constructed as described by Hales et al. (2006) from the 4 Å resolution structure of the Torpedo nACh receptor, of an intracellular portal of the homomeric 5-HT3A receptor illustrating the arginine residues that collectively influence γ. Note that the side chains of R436 and R440 are predicted to face into the portal to impede cation ion flux. C, single channel currents evoked by 5-HT applied to an outside-out membrane patch excised from a tsA-201 cell expressing the human 5-HT3A (QDA) receptor construct. The patch was voltage-clamped at a holding potential of −80 mV. D, homology model of an intracellular portal subsequent to the mutations R432Q, R436D and R440A (i.e. 5-HT3A (QDA)). Note that reduced steric hindrance to ion flux is predicted, together with a local electrostatic potential more conducive to ion permeation. The central inset schematically illustrates the location of the portals within the receptor.
R436 of the human 5-HT3A receptor is a key residue in determining γ and divalent versus monovalent cation selectivity
The solitary substitution R436D within the MA stretch of the human 5-HT3A receptor increases γ from a value of 0.4–1 pS to approximately 9 pS. Similarly, the mutation R440A has a substantial effect upon γ, which increases to approximately 5 pS, but the substitution R432Q has little influence (Hales et al. 2006). The pronounced influence of R436 provided an impetus to evaluate this locus more comprehensively by the use of a cysteine residue that replaced the arginine to form a substrate for covalent modification by reactive methanethiosulphonate (MTS) regents (Deeb et al. 2007). The R436C mutation elevated γ (to approximately 8 pS), but a superior window for evaluating reduction, or enhancement, of γ by MTS reagents was provided by the 5-HT3A R3432Q, R436C, R440A (5-HT3A (QCA)) receptor construct which was found to have a γ of approximately 18 pS (Deeb et al. 2007). MTS reagents were selected on the basis of charge and size in an attempt to determine the contribution of electrostatic and steric factors to the detrimental effect of R436 upon γ. In brief, both volume and charge at the 436 position influence γ, since an increase in volume, but no addition of charge, provided by modification of the cysteine residue by neutral propyl-MTS reduces γ, but not to the same extent as 2-aminoethyl-MTS, which adds similar volume, but additionally positive charge. Negatively charged 2-carboxyethyl-MTS and 2-sulphonatoethyl-MTS both increase γ, despite increasing side chain volume at the 436 position (Deeb et al. 2007). The data are consistent with the side chain orientation of R436 predicted in the homology model of the 5-HT3A receptor in which the positively charged guanidinium group projects into the portal.
The electrostatic environment provided by the triplet arginine motif could potentially impact upon the ion selectivity of the 5-HT3A receptor. However, the 5-HT3A (QDA) receptor retains perfect selectivity for monovalent cations over anions (Livesey et al. 2008). Clearly, the residues identified by Thompson & Lummis (2003; see above) dominate over any influence of the portal region, a conclusion reinforced by the observation that the 5-HT3A-glvM3M4 construct that lacks the large intracellular loop is also strongly cation selective (Jansen et al. 2008). However, divalent cations, such as Ca2+, would experience a particularly strong repulsive Coulombic force emanating from the arginine triplet and consistent with this we found the 5-HT3A (QDA) construct to have a much greater relative permeability to Ca2+ (PCa/PCs= 3.7) than the wild-type receptor (PCa/PCs= 1.4) (Livesey et al. 2008). The influence of the individual arginine residues upon relative permeability to Ca2+ parallels the findings for γ, since the R436D and R440A mutations increase PCa/PCs to 3.1 and 2.3, respectively, whereas the R432Q substitution is essentially without effect (Livesey et al. 2008). However, such increases in relative permeability to Ca2+ do not appear to result in an enhanced fractional current carried by the divalent (Pf %). Noam et al. (2008) found that the Pf % value for the wild-type 5-HT3A receptor (4.1) was in fact higher than that for the 5-HT3A (QDA) receptor construct (1.8). The latter value approximates to that found for the heteromeric 5-HT3AB receptor (i.e. 2.1; Noam et al. 2008).
Residues within the MA stretch influence voltage-dependent block of the human 5-HT3A receptor by extracellular Ca2+
Intracellular dialysis with a variety of phosphate-containing compounds renders the human 5-HT3A receptor susceptible to voltage-dependent blockade by extracellular Ca2+, independent of phosphorylation (Noam et al. 2008). Specifically, cytoplasmic phosph[on]ate compounds including ATP, GTP, CTP, UTP PPPi and particularly PPi and Pi induce a region of negative slope conductance in the macroscopic current–voltage (I–V) relationship to 5-HT at negative holding potentials, conditional upon the presence of extracellular Ca2+ (Noam et al. 2008). In the absence of intracellular phosph[on]ates the I–V relationship is inwardly rectifying, irrespective of the presence or absence of Ca2+ in the extracellular medium (Noam et al. 2008). This finding apparently reconciles divergent reports in the literature where Ca2+-induced blockade of macroscopic currents mediated by 5-HT3 receptors has been shown to be either voltage independent (e.g. Brown et al. 1998; Hu & Lovinger, 2005), or voltage dependent (e.g. Kawa, 1994; van Hooft & Wadman, 2003), the latter studies having been conducted upon cells dialysed with ATP. Significantly, intracellular phosphosph[on]ates do not confer voltage-dependent block by Ca2+ upon the 5-HT3A(QDA) receptor (Noam et al. 2008), although we have shown that the γ of this construct is reduced by extracellular Ca2+ in a concentration-dependent and voltage-independent manner (Livesey et al. 2008). Hydrogen-bonded and salt bridged complexes between the guanidinium head group of arginine and phosph[on]ates, at concentrations that are biologically relevant, are extensively documented (Schug & Lindner, 2005). Whether such an interaction is involved in the genesis of voltage-dependent block by Ca2+ that is induced by intracellular phosph[on]ates is unknown.
The MA stretch also influences the γ of nACh α4β2 receptors
To assess whether residues within the MA stretch exert an influence upon the γ of other cation-selective pLGICs, we studied the nACh α4β2 receptor (Hales et al. 2006), which has been well characterised at the single channel level (e.g. Nelson et al. 2003). Figure 2 gives a sequence alignment of relevant segments of 5-HT3 and nACh receptor subunits and highlights residues homologous to R432, R436 and R440 of the human 5-HT3A receptor. In the rat nicotinic α4 subunit these are E584, F588 and E592 and in the rat β2 subunit E439, Q443 and E447, respectively. Notably, these residues are either neutral or opposite in sign to those of the 5-HT3A receptor. Co-expression of mutant α4 E584R, or F588R, subunits with wild-type β2 subunits yielded functional channels with γ values that were modestly, but significantly, reduced relative to wild-type α4β2 receptors. In the case of the mutant β2 E439R subunit co-expressed with wild-type α4, γ was effectively halved, whereas similarly expressed β2 Q443R demonstrated only a small decrement in γ. As anticipated, when mutations were simultaneously present in both α4 and β2 subunits (i.e. α4 E584R/β2 E439R or α4 F588R/β2 Q443R), γ was markedly reduced. Unfortunately, the influence of the mutations α4 E592R and β2 E447R could not be evaluated due to limited functional expression (Hales et al. 2006). Collectively, such data indicate that the γ of a representative nACh receptor is, like the 5-HT3A receptor, influenced by select residues within the MA stretch.
The role of the MA stretch in anion-selective pLGICs
As noted by Unwin (2005), the net charge over the MA stretch of the majority subunits of cation-selective pLGICs is negative whereas for anion-selective subunits it is positive. This suggests that the MA stretch of GABAA and glycine receptors may also influence γ. For the human homomeric α1 glycine receptor there is direct experimental evidence to support this since Carland et al. (2009) found that charge reversal (i.e. lysine to glutamate mutations) performed collectively at four locations within the MA stretch reduced inwardly directed γ from approximately 92 to 60 pS. However, outwardly directed single channel currents were largely unaffected by such mutations and selectivity between monovalent anions and cations was unperturbed (Carland et al. 2009). The influence of the MA stretch in GABAA receptors has not been addressed experimentally (but see Mokrab et al. 2007 for a theoretical treatment), other than in a very recent study that reported the application of a synthetic peptide mimicking the MA stretch helix of the GABAA receptor γ2 subunit to inside-out patches excised from hippocampal neurones to reduce γ. Such an effect was interpreted to result from reduced receptor clustering mediated by self-association between MA stretch elements of adjacent GABAA receptors (Everitt et al. 2009).
The influence of the ECD upon γ
Amino acid residues within the ECD may also strongly influence γ and ion selectivity. The extracellular vestibule of the nAChR of Torpedo marmorata is approximately 20 Å wide and is abundant in glutamate and aspartate residues that are distributed throughout its 60 Å length creating a strongly electronegative environment that locally concentrates cations (Unwin, 2005; Corry, 2006; Song & Corry, 2009). By contrast, positive charges predominate over negative in the lining of the extracellular vestibule of the (α1)2(β2)2γ2 GABAA receptor (O’Mara et al. 2005). The theoretical effects of an electronegative ECD in cation-selective channels were considered over 20 years ago (Dani, 1986; Jordan, 1987) and can be summarised as: (i) an elevation of cation concentration within the vestibule relative to bulk solution and enhanced γ, particularly at low electrolyte concentration; (ii) selection for cations over anions and divalent cations over monovalent cations, and (iii) binding (stabilization) of cations within the vestibule.
Molecular dynamics (MD) simulations of ion transport performed upon a homology model of the adult nAChR of the neuromuscular junction (i.e. (α1)2β1δɛ) suggest that rings of charged and polar residues within the ECD participate in determining γ and ion selectivity. Specifically, 16 ns duration MD simulations of Na+ ions in flux reveal them to be stabilised at positions corresponding to N47, E83 and D97 of the human α1 subunit sequence (Wang et al. 2008). The importance of the nACh α1 D97 and the homologous 5-HT3A D127 subunit residue has recently been proven experimentally. Mutation of the nACh α1 D97 residue and homologous residues of the β1, δ and ɛ subunits to lysine produced graded decreases in γ up to a maximum of approximately 80% when all five subunits were mutated (Hansen et al. 2008). We found that mere neutralisation of the 5-HT3A (QDA) receptor D127 residue by mutation to asparagine reduced γ from approximately 41 to 8 pS. Moreover, relative permeability to Ca2+ was greatly depressed (PCa/PCs reduced from 3.55 to 0.12) (Livesey et al. 2009). Clearly, the ECD exerts an important control over not only γ, but selectivity between mono- and divalent cations also.
Concluding remarks
Data obtained over the last six years from cation-selective pLGICs strongly support the concept of an extended ion permeation pathway within pLGICs where the ICD and ECD participate with the TM2 domain in determining γ and ion selectivity. Recent findings suggest that a similar scenario might pertain in the anion selective pLGICs. Clearly, there is much scope for further experimentation in this field of fundamental research.
Acknowledgments
This work was supported by grants from the Wellcome Trust to J.A.P. and J.J.L., from the Anonymous Trust and Tenovus Scotland to J.A.P. and from the National Science Foundation to T.G.H. M.R.L. was supported by a BBSRC-CASE award in association with Eli Lilly.
Glossary
Abbreviations
- 5-HT
5-hydroxytryptamine
- ACh
acetylcholine
- AChBP
acetylcholine binding protein
- ECD
extracellular domain
- ICD
intracellular domain
- MA stretch
membrane-associated stretch
- MD
molecular dynamics
- MTS
methanethiosulphonate
- pLGIC
pentameric ligand-gated ion channel
- TM
transmembrane
- γ
single channel conductance
Author's present address
J. E. Carland: School of Medical Sciences, University of New South Wales, Sydney, 2052, Australia.
References
- Barnes NM, Hales TG, Lummis SCR, Peters JA. The 5-HT3 receptor – the relationship between structure and function. Neuropharmacology. 2009;56:273–284. doi: 10.1016/j.neuropharm.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzat C, Bartos M, Corradi J, Sine SM. The interface between extracellular and transmembrane domains of homomeric Cys-loop receptors governs open-channel lifetime and rate of desensitization. J Neurosci. 2008;28:7808–7819. doi: 10.1523/JNEUROSCI.0448-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady CA, Stanford IM, Ali I, Lin L, Williams JM, Dubin AE, Hope AG, Barnes NM. Pharmacological comparison of human homomeric 5-HT3A receptors versus heteromeric 5-HT3A/3B receptors. Neuropharmacology. 2001;41:282–284. doi: 10.1016/s0028-3908(01)00074-0. [DOI] [PubMed] [Google Scholar]
- Brown AM, Hope AG, Lambert JJ, Peters JA. Ion permeation and conduction in a human recombinant 5-HT3 receptor subunit (h5-HT3A. J Physiol. 1998;507:653–665. doi: 10.1111/j.1469-7793.1998.653bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carland JE, Cooper MA, Sugiharto S, Jeong HJ, Lewis TM, Barry PH, Peters JA, Lambert JJ, Moorhouse AJ. Characterization of the effects of charged residues in the intracellular loop on ion permeation inα1 glycine receptor channels. J Biol Chem. 2009;284:2023–2030. doi: 10.1074/jbc.M806618200. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Olsen RW, Peters J, Spedding M. A nomenclature for ligand-gated ion channels. Neuropharmacology. 2009;56:2–5. doi: 10.1016/j.neuropharm.2008.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corry B. An energy-efficient gating mechanism of the acetylcholine receptor channel suggested by molecular and Brownian dynamics. Biophys J. 2006;90:799–810. doi: 10.1529/biophysj.105.067868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA. Ion-channel entrances influence permeation.Net charge, size, shape, and binding considerations. Biophys J. 1986;49:607–618. doi: 10.1016/S0006-3495(86)83688-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PA, Pistis M, Hanna MC, Peters JA, Lambert JJ, Hales TG, Kirkness EF. The 5-HT3B subunit is a major determinant of serotonin receptor function. Nature. 1999;397:359–363. doi: 10.1038/16941. [DOI] [PubMed] [Google Scholar]
- Deeb TZ, Carland JE, Cooper MA, Livesey MR, Lambert JJ, Peters JA, Hales TG. Dynamic modification of a mutant cytoplasmic cysteine residue modulates the conductance of the human 5-HT3A receptor. J Biol Chem. 2007;282:6172–6182. doi: 10.1074/jbc.M607698200. [DOI] [PubMed] [Google Scholar]
- Everitt AB, Seymour VA, Curmi J, Laver DR, Gage PW, Tierney ML. Protein interactions involving theγ2 large cytoplasmic loop of GABAA receptors modulate conductance. FASEB J. 2009;23:4361–4369. doi: 10.1096/fj.09-137042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finer-Moore J, Stroud RM. Amphipathic analysis and possible formation of the ion channel in an acetylcholine receptor. Proc Natl Acad Sci U S A. 1984;81:155–159. doi: 10.1073/pnas.81.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galzi J-L, Devillers-Thiéry A, Hussy N, Bertrand S, Changeux JP, Bertrand D. Mutations in the channel domain of a neuronal nicotinic receptor convert ion selectivity from cationic to anionic. Nature. 1992;359:500–505. doi: 10.1038/359500a0. [DOI] [PubMed] [Google Scholar]
- Gunthorpe MJ, Lummis SCR. Conversion of the ion selectivity of the 5-HT3A receptor from cationic to anionic reveals a conserved feature of the ligand-gated ion channel superfamily. J Biol Chem. 2001;276:10977–10983. [PubMed] [Google Scholar]
- Hales TG, Dunlop JI, Deeb TZ, Carland JE, Kelley SP, Lambert JJ, Peters JA. Common determinants of single channel conductance within the large cytoplasmic loop of 5-hydroxytryptamine type 3 andα4β2 nicotinic acetylcholine receptors. J Biol Chem. 2006;281:8062–8071. doi: 10.1074/jbc.M513222200. [DOI] [PubMed] [Google Scholar]
- Hansen SB, Wang HL, Taylor P, Sine SM. An ion selectivity filter in the extracellular domain of Cys-loop receptors reveals determinants for ion conductance. J Biol Chem. 2008;283:36066–36070. doi: 10.1074/jbc.C800194200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilf RJ, Dutzler R. A prokaryotic perspective on pentameric ligand-gated ion channel structure. Curr Opin Struct Biol. 2009;19:418–424. doi: 10.1016/j.sbi.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Hu XQ, Lovinger DM. Role of aspartate 298 in mouse 5-HT3A receptor gating and modulation by extracellular Ca2+ J Physiol. 2005;568:381–396. doi: 10.1113/jphysiol.2005.092866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imoto K, Busch C, Sakmann B, Mishina M, Konno T, Nakai J, Bujo H, Mori Y, Fukuda K, Numa S. Rings of negatively charged amino acids determine the acetylcholine receptor channel conductance. Nature. 1988;335:645–648. doi: 10.1038/335645a0. [DOI] [PubMed] [Google Scholar]
- Jansen M, Bali M, Akabas MH. Modular design of Cys-loop ligand-gated ion channels: functional 5-HT3 and GABAρ1 receptors lacking the large cytoplasmic M3M4 loop. J Gen Physiol. 2008;131:137–146. doi: 10.1085/jgp.200709896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen ML, Schousboe A, Ahring PK. Charge selectivity of the Cys-loop family of ligand-gated ion channels. J Neurochem. 2005;92:217–225. doi: 10.1111/j.1471-4159.2004.02883.x. [DOI] [PubMed] [Google Scholar]
- Jordan PC. How pore mouth charge distributions alter the permeability of transmembrane ionic channels. Biophys J. 1987;51:297–311. doi: 10.1016/S0006-3495(87)83336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnovsky AM, Gotow LF, McKinley DD, Piechan JL, Ruble CL, Mills CJ, Schellin KA, Slightom JL, Fitzgerald LR, Benjamin CW, Roberds SL. A cluster of novel serotonin receptor 3-like genes on human chromosome 3. Gene. 2003;319:137–148. doi: 10.1016/s0378-1119(03)00803-5. [DOI] [PubMed] [Google Scholar]
- Kawa K. Distribution and functional properties of 5-HT3 receptors in the rat hippocampal dentate gyrus: a patch-clamp study. J Neurophysiol. 1994;71:1935–1947. doi: 10.1152/jn.1994.71.5.1935. [DOI] [PubMed] [Google Scholar]
- Kelley SP, Dunlop JI, Kirkness EF, Lambert JJ, Peters JA. A cytoplasmic region determines single-channel conductance in 5-HT3 receptors. Nature. 2003;424:321–324. doi: 10.1038/nature01788. [DOI] [PubMed] [Google Scholar]
- Keramidas A, Moorhouse AJ, Schofield PR, Barry PH. Ligand-gated ion channels: mechanisms underlying ionic selectivity. Prog Biophys Mol Biol. 2004;86:161–204. doi: 10.1016/j.pbiomolbio.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Kukhtina V, Kottwitz D, Strauss H, Heise B, Chebotareva N, Tsetlin V, Hucho F. Intracellular domain of nicotinic acetylcholine receptor: the importance of being unfolded. J Neurochem. 2006;97(Suppl 1):63–67. doi: 10.1111/j.1471-4159.2005.03468.x. [DOI] [PubMed] [Google Scholar]
- Livesey MR, Cooper MA, Deeb TZ, Carland JE, Kozuska J, Hales TG, Lambert JJ, Peters JA. Structural determinants of Ca2+ permeability and conduction in the human 5-hydroxytryptamine type 3A receptor. J Biol Chem. 2008;283:19301–19313. doi: 10.1074/jbc.M802406200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livesey M, Cooper M, Lambert J, Peters J. D127 of the human 5-HT3A receptor is a major determinant of ion permeation. 2009. http://wwwpa2online.org/Vol7Issue2abst037.pdf.
- Lynch JW. Native glycine receptor subtypes and their physiological roles. Neuropharmacology. 2009;56:303–309. doi: 10.1016/j.neuropharm.2008.07.034. [DOI] [PubMed] [Google Scholar]
- Millar NS, Gotti C. Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology. 2009;56:237–246. doi: 10.1016/j.neuropharm.2008.07.041. [DOI] [PubMed] [Google Scholar]
- Miyazawa A, Fujiyoshi Y, Unwin N. Structure and gating mechanism of the acetylcholine receptor pore. Nature. 2003;423:949–955. doi: 10.1038/nature01748. [DOI] [PubMed] [Google Scholar]
- Mochizuki S, Miyake A, Furuichi K. Ion permeation properties of a cloned human 5-HT3 receptor transiently expressed in HEK 293 cells. Amino Acids. 1999;17:243–255. doi: 10.1007/BF01366923. [DOI] [PubMed] [Google Scholar]
- Mokrab Y, Bavro VN, Mizuguchi K, Todorov NP, Martin IL, Dunn SM, Chan SL, Chau PL. Exploring ligand recognition and ion flow in comparative models of the human GABA type A receptor. J Mol Graph Model. 2007;26:760–774. doi: 10.1016/j.jmgm.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Nelson ME, Kuryatov A, Choi CH, Zhou Y, Lindstrom J. Alternate stoichiometries of α4β2 nicotinic acetylcholine receptors. Mol Pharmacol. 2003;63:332–341. doi: 10.1124/mol.63.2.332. [DOI] [PubMed] [Google Scholar]
- Niesler B, Frank B, Kapeller J, Rappold GA. Cloning, physical mapping and expression analysis of the human 5-HT3 serotonin receptor-like genes HTR3C, HTR3D and HTR3E. Gene. 2003;310:101–111. doi: 10.1016/s0378-1119(03)00503-1. [DOI] [PubMed] [Google Scholar]
- Noam Y, Wadman WJ, van Hooft JA. On the voltage-dependent Ca2+ block of serotonin 5-HT3 receptors: a critical role of intracellular phosphates. J Physiol. 2008;586:3629–3638. doi: 10.1113/jphysiol.2008.153486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of γ-aminobutyric acidA receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev. 2008;60:243–260. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Mara M, Cromer B, Parker M, Chung S-H. Homology model of the GABAA receptor examined using Brownian mechanics. Biophys J. 2005;88:3286–3299. doi: 10.1529/biophysj.104.051664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JA, Hales TG, Lambert JJ. Molecular determinants of single-channel conductance and ion selectivity in the Cys-loop family: insights from the 5-HT3 receptor. Trends Pharmacol Sci. 2005;26:587–594. doi: 10.1016/j.tips.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Reeves DC, Jansen M, Bali M, Lemster T, Akabas MH. A role for the β1–β2 loop in the gating of 5-HT3 receptors. J Neurosci. 2005;25:9358–9366. doi: 10.1523/JNEUROSCI.1045-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucktooa P, Smit AB, Sixma TK. Insight in nAChR subtype selectivity from AChBP crystal structures. Biochem Pharmacol. 2009;78:777–787. doi: 10.1016/j.bcp.2009.06.098. [DOI] [PubMed] [Google Scholar]
- Schug KA, Lindner W. Noncovalent binding between guanidinium and anionic groups: focus on biological- and synthetic-based arginine/guanidinium interactions with phosph[on]ate and sulf[on]ate residues. Chem Rev. 2005;105:67–114. doi: 10.1021/cr040603j. [DOI] [PubMed] [Google Scholar]
- Song C, Corry B. Role of acetylcholine receptor domains in ion selectivity. Biochim Biophys Acta. 2009;1788:1466–1473. doi: 10.1016/j.bbamem.2009.04.015. [DOI] [PubMed] [Google Scholar]
- Thompson AJ, Lummis SCR. A single ring of charged amino acids at one end of the pore can control ion selectivity in the 5-HT3 receptor. Br J Pharmacol. 2003;140:359–365. doi: 10.1038/sj.bjp.0705424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin N. Refined structure of the nicotinic acetylcholine receptor at 4Å resolution. J Mol Biol. 2005;346:967–989. doi: 10.1016/j.jmb.2004.12.031. [DOI] [PubMed] [Google Scholar]
- van Hooft JA, Wadman W. Ca2+ ions block and permeate serotonin 5-HT3 receptor channels in rat hippocampal interneurons. J Neurophysiol. 2003;89:1864–1869. doi: 10.1152/jn.00948.2002. [DOI] [PubMed] [Google Scholar]
- Wang HL, Cheng X, Taylor P, McCammon JA, Sine SM. Control of cation permeation through the nicotinic receptor channel. PLoS Comput Biol. 2008;4:e41. doi: 10.1371/journal.pcbi.0040041. [DOI] [PMC free article] [PubMed] [Google Scholar]