Abstract
The cysteine (Cys) residue at position 312 in the third transmembrane domain (M3) is conserved among 5-hydroxytryptamine type 3 (5-HT3) receptor subunits and many other subunits of the nicotinic acetylcholine (nACh) related Cys-loop receptor family, including most of the γ-aminobutyric acid type A (GABAA) and glycine receptor subunits. To elucidate a possible role for the Cys-312 in human 5-HT3A receptors, we replaced it with alanine and expressed the 5-HT3A(C312A) mutant in HEK293 cells. The mutation resulted in an absence of 5-HT-induced whole-cell current without reducing homopentamer formation, surface expression or 5-HT binding. The 5-HT3A(C312A) mutant, when co-expressed with the wild-type 5-HT3A subunit, did not affect functional expression of receptors, suggesting that the mutant is not dominant negative. Interestingly, co-expression of 5-HT3A(C312A) with 5-HT3B led to surface expression of heteropentamers that mediated small 5-HT responses. This suggests that the Cys-312 is essential for homomeric but not heteromeric receptor gating. To further investigate the relationship between residue 312 and gating we replaced it with amino acids located at the equivalent position within other Cys-loop subunits that are either capable or incapable of forming functional homopentamers. Replacement of 5-HT3A Cys-312 by Gly or Leu (equivalent residues in the nACh receptor δ and γ subunits) abolished and severely attenuated function, respectively, whereas replacement by Thr or Ser (equivalent residues in nACh receptor α7 and GABAAρ subunits) supported robust function. Thus, 5-HT3A residue 312 and equivalent polar residues in the M3 of other Cys-loop subunits are essential determinants of homopentameric gating.
Introduction
5-Hydroxytryptamine type 3 (5-HT3) receptors mediate rapid serotonergic excitatory synaptic transmission and modulate neurotransmitter release (Barnes et al. 2009). 5-HT3 receptors are members of the family of cysteine (Cys)-loop ligand-gated ion channels, which also includes nicotinic acetylcholine (nACh), γ-aminobutyric acid type A (GABAA) and glycine receptors. Separate genes encode five human 5-HT3 subunits (5-HT3A–5-HT3E). 5-HT3A subunits are unique among them in their ability to form functional homopentamers (Barnes et al. 2009). Recombinant homomeric 5-HT3A receptors exhibit robust functional expression in heterologous systems and are closely related to the nACh receptor, for which there is a 4 Å resolution structural model (Maricq et al. 1991; Unwin, 2005). These factors make the 5-HT3A receptor an appealing model for studying the relationship between Cys-loop receptor structure and function (Reeves & Lummis, 2002). Indeed several mutagenesis studies have probed the extracellular agonist binding site, residues in the first (M1), second (M2) and fourth (M4) transmembrane domains and the intracellular and extracellular loops of the 5-HT3A receptor (Barnes et al. 2009). However, there are no studies examining the functional role of residues in the third (M3) transmembrane domain.
Unlike the 5-HT3A subunit most of the other 44 human Cys-loop subunits are unable to form functional homomeric receptors. The remaining exceptions include the α7 and α9 nACh receptor subunits, the ρ1–3 GABAA receptor subunits, and the glycine α1–3 subunits (Lester et al. 2004). The large majority of Cys-loop receptor subunits must combine with one or more distinct isoforms in order to form functional heteropentameric receptors. The existence of heteromeric receptors provides considerable heterogeneity of function within the same Cys-loop receptor subfamily. GABAA and nACh receptors are the most heterogeneous of the subfamilies with 19 and 16 different human subunits, respectively. Their subunit composition determines receptor pharmacology, single channel conductance, gating kinetics and ion selectivity (McKernan & Whiting, 1996; Lester et al. 2004; Rudolph & Mohler, 2004; Gotti et al. 2007; Mitchell et al. 2008). Furthermore, an individual subunit within a heteropentamer can potentially dictate the destination of the receptor. For example, the glycine receptor β subunit binds the trafficking protein gephyrin, which selectively targets heteromeric glycine receptors to the postsynaptic density (Fritschy et al. 2008).
There are several mechanisms that can account for the failure of most Cys-loop subunits to form functional homopentamers. Homomeric 5-HT3B receptors fail to express at the surface membrane due to the presence of an amino acid motif which results in 5-HT3B subunit retention within the endoplasmic reticulum (Boyd et al. 2003). The motif is masked by the 5-HT3A subunit allowing surface expression of functional heteromeric 5-HT3AB receptors. Neurotransmitter agonists bind to Cys-loop receptors at the interface between adjacent subunits which form positive and negative binding surfaces. However, not all subunits can serve both as the positive and negative interface and this can lead to a failure of homomeric receptor assembly, as is the case for the GABAA receptor α subunits (Bollan et al. 2003). Alternatively, homomeric receptor assembly occurs, but the receptor cannot support agonist binding. This is the case for the GABAA receptor β3 subunit, which forms surface receptors that can be activated by the general anaesthetic propofol, but cannot bind GABA (Davies et al. 1997). Another mechanism that could prevent functional homomeric receptor formation is the failure of channel gating in a surface receptor that successfully binds agonist. Such a gating deficit would have to be recessive in order for the subunit to successfully combine with other subunits to form functional heteromeric receptors.
In this study we identified a residue in the M3 domain that is polar in 5-HT3A, nACh α7 and α9, GABAAρ1–3 and glycine α1 subunits, all of which form functional homomeric receptors and non-polar in nACh δ, γ and ɛ subunits which require other subunits to combine into functional heteromeric receptors. We therefore investigated the contribution of this residue, at position 312 in the 5-HT3A subunit, to homomeric receptor expression, agonist binding and function. This is the first report of a functional role for an M3 residue in the 5-HT3 receptor.
Methods
DNA constructs and transient transfection of subunit cDNAs
HA epitope (YPYDVPDYA) or Myc epitope (EQKLISEEDL) tagged wild-type (WT) human 5-HT3A and 5-HT3B receptor subunits in the pGW1 vector were provided by Dr C. N. Connolly and Prof. J. J. Lambert (University of Dundee). Point mutations were introduced into cDNA encoding the Myc-tagged 5-HT3A subunit using the QuikChange II site-directed mutagenesis kit (Stratagene, Cedar Creek, TX, USA). All cDNAs were sequenced to confirm their fidelity. Transient transfection of HEK293 cells with subunit cDNAs at equimolar ratios was performed by the calcium phosphate precipitation method as described previously (Koch et al. 2006). Cells were incubated at 37°C in an atmosphere of 5% CO2 (100% relative humidity) overnight during the transfection and were washed after 16 h with fresh medium. Transfected cells were routinely cultured for 24–72 h before use. A stable HEK293 cell line expressing Myc-5-HT3A receptors was generated by positive selection using 500 μg ml−1 geneticin G418 (Invitrogen, Carlsbad, CA, USA). Clones were isolated and screened using fluorescence microscopy to verify consistent labelling with an antibody against the Myc-epitope. Electrophysiological recording from positive clones was used to confirm functional 5-HT3A receptor expression. A stable 5-HT3A receptor-expressing cell line was chosen and used where indicated in the text. All cell culture reagents were purchased from Invitrogen.
Electrophysiology
The whole-cell configuration of the patch clamp technique was used to record 5-HT-activated currents from transiently transfected human embryonic kidney 293 (HEK293) cells. Experiments were performed at room temperature (20–24°C). The recording chamber was continuously perfused (5 ml min−1) with an extracellular solution comprising (in mm): NaCl, 140; KCl, 2.8; MgCl2, 2.0; CaCl2, 1.0; Hepes, 10; and glucose, 10 (pH 7.4 adjusted with NaOH). The electrode solution contained (in mm): CsCl, 140; MgCl2, 2.0; CaCl2, 0.1; EGTA, 1.1; ATP (Mg2+ salt), 0.1; and Hepes-CsOH, 10 (pH 7.4). Cells were voltage-clamped at an electrode potential of –60 mV. Junction potentials were nulled with an open electrode in the recording chamber prior to each experiment. The liquid junction potential was trivial (∼2 mV), and its inappropriate compensation was ignored. 5-HT (30 or 100 μm) was diluted from frozen stocks into the extracellular solution on the day of recording. 5-HT3 receptors were activated by locally applying 5-HT to the cell by pressure (10 psi) ejection (Picospritzer II, General Valve Corp., Fairfield, NJ, USA). Currents were recorded using an Axopatch 200B amplifier, low-pass filtered at 1 kHz, digitized at 10 KHz using a Digidata 1320A interface, and acquired using pCLAMP 8 software (all from Molecular Devices Corp., Sunnyvale, CA, USA) onto the hard drive of a personal computer for subsequent off-line analysis.
Confocal microscopy
One day after transfection cells were subcultured and seeded onto poly-l-lysine-coated coverslips, and then routinely cultured for ∼40 h before use. For total labelling, the cells were incubated with polyclonal anti-HA (Invitrogen) and/or monoclonal anti-Myc antibodies (Clontech Laboratories, Mountain View, CA, USA) after fixation, permeabilization and blocking. For the observation of surface expression or internalization, HA- or Myc-tagged subunits were surface-labelled as appropriate with anti-HA and/or anti-Myc antibodies at a concentration of 1.5 μg ml−1 at ∼22°C for 10 min. Subsequently, cells were treated as indicated in the text prior to fixation and permeabilization. Bound primary antibody was detected with Alexa Fluor 488 and/or Alexa Fluor 594-conjugated secondary antibodies (Invitrogen) as described previously (Wu et al. 2007; Liang et al. 2008). Cells were permanently mounted in Fluoromount-G (SouthernBiotech, Birmingham, AL, USA) and examined using a confocal microscope (510 Meta, Carl Zeiss MicroImaging, Inc., Thornwood, NY, USA).
Quantitative analysis of surface expression
Quantification was carried out using a modification of a previously described approach (Wu et al. 2008). HEK293 cells were grown on poly-l-lysine-coated coverslips in 24-well plates overnight, and then transfected. Approximately 65 h after transfection, cells were surface labelled as described above. Subsequently, cells were fixed immediately with Zamboni's fixative (4% paraformaldehyde and 0.2% picric acid in phosphate buffer, pH 6.9) for 30 min, and incubated with peroxidase-conjugated secondary antibody (1: 1000, GE Healthcare, Piscataway, NJ, USA) in OptiMEM (Invitrogen) at 4°C overnight. Plates were developed with 250 μl of ABTS solution (Roche Molecular Biomedicals, Mannheim, Germany). Surface expression of WT or mutant subunits was revealed by the level of colour development. After ∼30 min, 200 μl of the substrate solution from each well was transferred to a 96-well plate and analysed at 405 nm using a Microplate Reader (Molecular Devices Corp.).
Western blotting
HEK293 cells were transiently transfected in a 6-well plate with the appropriate cDNAs, as described above. Approximately 65 h after transfection cells were washed twice with phosphate-buffered saline and harvested with ice-cold homogenization buffer (50 mm Tris-HCl, pH 7.4) supplemented with complete protease inhibitors (Pierce Biotechnology Inc., Rockford, IL, USA). Subsequently, the cell suspension was homogenized with 25 strokes in a 1 ml tight-fitting Dounce homogenizer (Fisher Scientific, Pittsburgh, PA, USA) followed by five passages through a syringe fitted with an ultra-fine needle (31 gauge), and unbroken cells and intact nuclei were removed by centrifugation at 1500 g (4°C) for 10 min. This centrifugation procedure was repeated and the supernatant was separated from the pellet. The supernatant was centrifuged for 10 min at 16 000 g at 4°C. The resultant pellet contained the crude membrane fraction. Radio immunoprecipitation assay (RIPA) solubilization buffer (Pierce Biotechnology Inc.) plus complete protease inhibitors was added to the pellets and the resulting suspension was incubated for 30 min at 4°C to extract integral membrane proteins. Protein concentrations were determined using the BCA Protein Assay kit (Pierce Biotechnology Inc.) and then frozen at −80°C. For Western blot, ∼60 μg of total protein was used. The same volume of 2× SDS loading buffer (125 mm Tris-HCl, 4% SDS, 20% glycerol, 0.02% Bromophenol Blue) with and without 200 mm dithiothreitol (DTT) was added. The samples were incubated in ice for ∼20 min immediately prior to SDS-PAGE separation (7.5% Tris-HCl Ready Gel, Bio-Rad, Hercules, CA, USA). Electrophoresis was carried out for 1.5 h using the Mini-PROTEAN 3 Cell (Bio-Rad). Protein bands were subsequently transferred to a nitrocellulose membrane using a mini Trans-Blot electrophoretic transfer cell (Bio-Rad). The membranes were blocked for 2 h with 3.5% non-fat milk in PBS containing 0.05% Tween 20 (Bio-Rad) and processed with anti-Myc monoclonal antibody (0.1 μg ml−1, Invitrogen) and subsequently with anti-mouse horseradish peroxidase-conjugated secondary antibody (1: 10 000, GE Healthcare). The membranes were developed using an Amersham ECL Western Blotting Detection System (GE Healthcare).
Radioligand binding assay
HEK293 cells transiently expressing WT or mutant 5-HT3A receptors were washed and harvested in Hanks’ buffered salt solution (HBSS) (Invitrogen), removing culture medium. Cells were centrifuged at 3000 g for 5 min, supernatant was removed and pellets were either used immediately for [3H]GR65630 binding or stored at −80°C for later use. A crude membrane fraction of thawed cells was obtained by suspension in 10 ml ice-cold 50 mm Hepes buffer (pH 7.5) and centrifugation for 30 min at 40 000 g. Membranes were then resuspended in 50 mm Hepes buffer by ultrasonication and the total protein concentration was measured using a NanoDrop ND-1000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA). For competition binding studies, 100 μl aliquots of cell suspensions were incubated with [3H]GR65630 (∼100 pm, Perkin Elmer, Waltham, MA, USA) for 60 min at 25°C with 5-HT (100 nm to 100 μm) in a final volume of 200 μl. The reaction was halted by rapid vacuum filtration using a Brandel cell harvester (Brandel, Gaithersburg, MD, USA) with Whatman GF/B filter papers presoaked in ice-cold 0.5 m polyethyleneimine in 50 mm Hepes buffer. Filters were allowed to air dry for 15 min and then assayed for radioactivity by liquid scintillation counting. For saturation binding studies, samples were incubated with concentrations of [3H]GR65630 ranging from 100 pm to 30 nm. Non-specific binding for both types of assay was determined in the presence of 1 μm ondansetron and all experiments were performed in triplicate. Values of agonist binding affinity (Ki) were calculated using the Cheng–Prusoff equation (Cheng & Prusoff, 1973):
where, IC50 is the concentration of 5-HT required to displace 50% of the bound [3H]GR65630, L is the concentration of [3H]GR65630 in competition binding experiments, and Kd is the dissociation constant for [3H]GR65630 determined from saturation binding.
Co-immunoprecipitation
HEK293 cells were grown in 60 mm culture dishes overnight and transiently transfected with wild-type and mutant 5-HT3A cDNA constructs at equimolar ratios as described above. Approximately 65 h after transfection, cells were lysed in RIPA solubilization buffer plus complete protease inhibitors and the resulting extract was subjected to either Western blot analysis or immunoprecipitation as described previously (Liang et al. 2007). Briefly, the targeting proteins were immunoprecipitated with 50 μl of Protein A Sepharose 4 Fast Flow (GE Healthcare) preloaded with 5 μg of anti-HA or anti-Myc antibody. After SDS-PAGE and electroblotting, membranes were incubated with anti-Myc or anti-HA antibody followed by detection using horseradish peroxidase-conjugated secondary antibody as described above.
Homology modelling
The human 5-HT3A receptor sequence was successfully rendered as a three dimensional structure using Deepview-Swiss PDB Viewer to generate a homology model based on the Torpedo marmorata nicotinic acetylcholine receptor (PDB ID: 2bg9) (Unwin, 2005) as described previously (Deeb et al. 2007). Briefly, the human 5-HT3A amino acid sequence was aligned with equivalent residues of the Torpedoα, β, γ, δ nACh receptor subunits and, where necessary, modified in length to generate a superposition that enabled successful homology modelling. The model was then submitted to the online SwissModel website (http://www.swissmodel.expasy.org) for energy minimization. The final model was then rendered using Deepview software for analysis and the generation of images.
Results
Replacement of the 5-HT3A subunit M3 Cys residue by Ala abolishes function
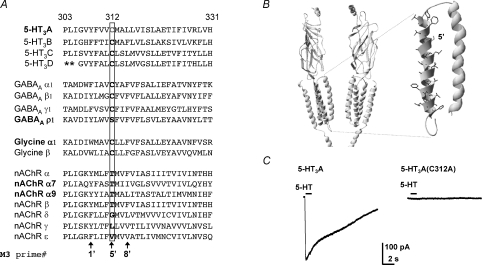
Alignment of the human 5-HT3 subunit M3 amino acid sequences reveals that the Cys residue at position 312 in the 5-HT3A subunit is completely conserved among paralogous subunits (Fig. 1A). While not shown in the alignment the residue is also conserved among orthologous mouse and rat 5-HT3A and 5-HT3B subunits (Hanna et al. 2000). The inclusion of additional representative human Cys-loop receptor subunit sequences in the alignment reveals that the Cys residue is also conserved in most GABAA receptor subunits (the exceptions being ρ1–3 in which the equivalent residue is Ser) and all glycine receptor subunits (Fig. 1A); Thr is the equivalent residue in nACh receptor α1–10 and β subunits. The nACh receptor δ, γ and ɛ subunits have a Gly, Leu and Val, respectively, at the position equivalent to 5-HT3A subunit residue 312. 5-HT3A, GABAAρ1–3, glycine α1–3, nACh α7 and α9 subunits all form functional homomeric receptors and each has a polar residue at the position equivalent to that of residue 312 in the 5-HT3A subunit.
Figure 1. The mutant 5-HT3A(C312A) subunit does not form functional receptors.
A, amino acid sequence alignment of the M3 of human Cys-loop receptor subunits. Note that the human 5-HT3A Cys (C) 312 residue, labelled by shading (grey in print, red online) and boxed, is highly conserved in 5-HT3 subunits, GABAAα, β and γ subunits, and glycine α and β subunits. A prime numbering system is used (De Rosa et al. 2002) to identify homologous M3 residues across all Cys-loop receptor subunits. Asterisks indicate a poorly aligned region of the 5-HT3B subunit sequence, which is omitted for clarity. B, a ribbon rendering of a homology model of the 5-HT3D receptor based on the T. marmorata nACh receptor cryo-EM structure (Unwin, 2005). Two subunits are shown each with four transmembrane domains, a large extracellular N-terminus, the top portion of the cytoplasmic membrane associated helix, and a short C terminal tail. Inset, the M2 and M3 helices expanded with M3 residue side chains rendered and Cys-312 shaded (grey in print, red online). C, representative currents recorded from HEK293 cells transiently expressing WT 5-HT3A or mutant 5-HT3A(C312A) receptors before, during and after 5-HT (100 μm) application for 1 s (where indicated by the bar) at a holding potential of −60 mV. Each trace is a representative of at least eight recordings.
We used the whole-cell configuration of the patch-clamp technique and mutagenesis to examine whether Cys-312 in the 5-HT3A subunit is required for the formation of functional homomeric receptors. 5-HT (100 μm), locally applied to HEK293 cells transiently expressing wild-type human 5-HT3A receptors, evoked robust inward currents recorded at −60 mV (Fig. 1C). By contrast, HEK293 cells expressing mutant 5-HT3A(C312A) subunits, in which Cys-312 was replaced by Ala, failed to respond to 5-HT (Fig. 1C).
Mutant 5-HT3A(C312A) subunits form homopentamers
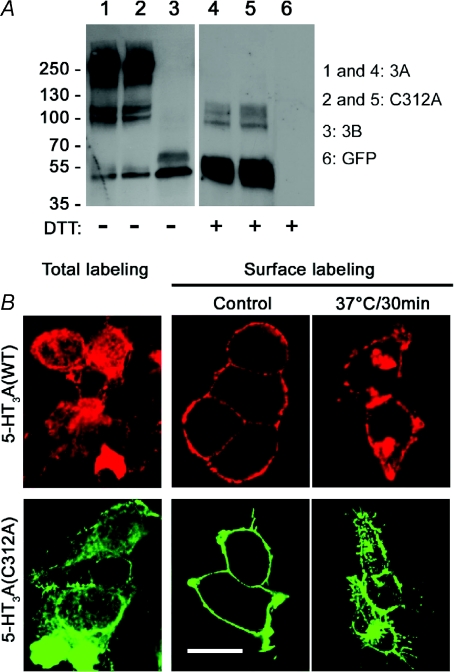
The lack of 5-HT3A(C312A) function could be caused by a failure of assembly of mutant subunits into homopentamers. We used Western blot analysis with antibodies against the Myc epitope to investigate whether 5-HT3A(C312A) subunits combine to form pentameric proteins. Western-blot analysis revealed a dominant protein band at ∼250 kDa representing the homopentameric Myc-tagged WT 5-HT3A receptor extracted from transiently transfected HEK293 cells (Fig. 2A, lane 1). Less prevalent, but clearly visible were bands at ∼50 kDa and ∼100 kDa representing monomeric and dimeric 5-HT3A proteins. Likewise, analysis of protein extracted from cells expressing mutant Myc-tagged 5-HT3A(C312A) subunits revealed a predominant band corresponding to pentameric protein with fainter bands similar to those seen in lane 1 corresponding to monomeric and dimeric 5-HT3A(C312A) proteins (Fig. 2A, lane 2). By contrast, analysis of protein extracted from cells expressing Myc-tagged 5-HT3B subunits alone revealed a single predominant band at ∼50 kDa (Fig. 2A, lane 3), consistent with the known failure of the subunit, when expressed alone, to form homopentamers (Boyd et al. 2002; Ilegems et al. 2004). Inclusion of the reducing agent DTT (100 mm for 20 min) in the loading buffer with WT 5-HT3A (Fig. 2A, lane 4) and mutant 5-HT3A(C312A) protein (Fig. 2A, lane 5) caused the disappearance of ∼250 kDa bands resulting in a predominant band at 50 kDa.
Figure 2. The 5-HT3A(C312A) mutation does not disrupt pentamer formation or receptor internalization.
A, HEK293 cells transiently expressing Myc-tagged 5-HT3A (lane 1 and 4), its mutant C312A (lane 2 and 5), 5-HT3B (lane 3) or GFP (lane 6) were homogenized and membrane proteins were solubilized in RIPA buffer. The extracted membrane protein was subjected to Western blot analysis in the presence or absence of DTT. The positions of molecular mass markers (in kDa) are indicated on the left. Two additional experiments gave similar results. B, for total labelling HEK293 cells, transiently expressing HA-5-HT3A or mutant Myc-5-HT3A(C312A) receptors, were fixed, permeabilized and then exposed to anti-HA or Myc antibodies. To examine receptor trafficking, cells were first surface-labelled at room temperature with anti-HA or anti-Myc antibodies. Subsequently, cells were either fixed immediately (control) or incubated at 37°C for 30 min before fixation. Bound primary antibodies were detected with Alexa Fluor 488 (rendered in green) or 594 (rendered in red) conjugated secondary antibodies, and examined by confocal microscopy. Representative images from two independent experiments performed in duplicate are shown. Scale bar is 16 μm.
Taken together these results indicate that recombinant WT 5-HT3A and mutant 5-HT3A(C312A) subunits, unlike 5-HT3B subunits, exist predominantly as homopentamers.
Mutant 5-HT3A(C312A) pentamers are expressed in the surface membrane
Since pentameric assembly appears normal (Fig. 2A), the lack of 5-HT3A(C312A) homopentamer function could be caused by a failure of incorporation into the surface membrane. We compared the ability of WT 5-HT3A and mutant 5-HT3A(C312A) subunits to access the plasma membrane of HEK293 cells using confocal microscopy with fluorescently labelled antibodies against HA and Myc epitopes. When cells were surface-labelled with antibodies prior to fixation and permeabilization WT 5-HT3A and mutant 5-HT3A(C312A) receptors were readily observed in the surface membrane (Fig. 2B). Total labelling, in which cells were labelled after fixation and permeabilization, revealed additional intracellular receptors. 5-HT3A receptors rapidly internalize through an agonist-independent pathway (Morton et al. 2008; Xiong et al. 2008). We examined whether WT 5-HT3A and mutant 5-HT3A(C312A) receptors exhibited similar constitutive internalization. Live cells were surface labelled with antibodies and then incubated at 37°C for 30 min. Confocal microscopy revealed that a large proportion of surface labelled WT 5-HT3A and mutant 5-HT3A(C312A) receptors became internalized during this time (Fig. 2B).
Taken together these data demonstrate that, like WT 5-HT3A receptors, mutant 5-HT3A(C312A) pentamers are expressed in the surface membrane and undergo constitutive internalization.
Mutant 5-HT3A(C312A) receptors bind 5-HT
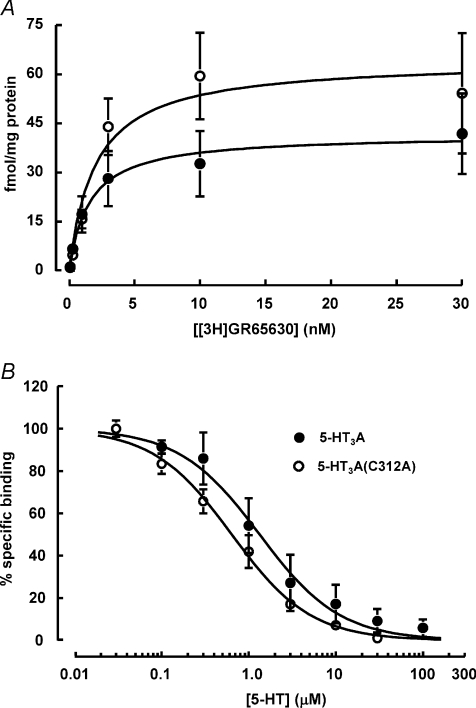
Since both pentameric assembly and surface expression appear normal, the lack of 5-HT3A(C312A) function could instead be caused by a failure of homopentamers in the cell membrane to bind 5-HT. We investigated whether mutant 5-HT3A(C312A) receptors bind 5-HT using the potent and selective 5-HT3 receptor antagonist [3H]GR65630 (Kilpatrick et al. 1987). [3H]GR65630 exhibited higher levels of saturable binding to HEK293 cell membranes containing mutant 5-HT3A(C312A) receptors (Bmax= 64.3 ± 6.3 fmol (mg protein)−1) compared to membranes containing WT 5-HT3A receptors (Bmax= 41.6 ± 2.1 fmol (mg protein)−1; Fig. 3A). 5-HT displaced [3H]GR65630 in a concentration-dependent manner (Fig. 3B). The IC50 values for 5-HT were 1.3 ± 0.2 and 0.64 ± 0.05 μm for WT 5-HT3A and mutant 5-HT3A(C312A) receptors, respectively. The Ki values, determined for 5-HT using the Cheng–Prusoff equation (see Methods), were 1.3 μm for WT 5-HT3A receptors and 0.61 μm for mutant 5-HT3A(C312A) receptors. These data suggest that 5-HT binds with a higher affinity to 5-HT3A(C312A) receptors compared to WT 5-HT3A receptors. Therefore, the lack of 5-HT3A(C312A) receptor function is not a result of an inability to bind 5-HT; instead it results from a failure of channel gating.
Figure 3. The 5-HT3A(C312A) mutation increases Bmax and the affinity of 5-HT.
Binding of the antagonist [3H]GR65630 to HEK293 membranes containing WT 5-HT3A (○) or mutant 5-HT3A(C312A) (○) receptors. A, compared to 5-HT3A, 5-HT3A(C312A) receptors exhibited higher saturable binding of [3H]GR65630. The Bmax and Kd values for 5-HT3A were 41.6 ± 2.1 fmol (mg protein)−1 and 1.6 ± 0.3 nm, respectively, whereas the Bmax and Kd values for 5-HT3A(C312A) were 64.3 ± 6.3 fmol (mg protein)−1 and 1.99 ± 0.74 nm, respectively. B, 5-HT inhibited [3H]GR65630 binding with a higher potency for 5-HT3A(C312A) receptors compared to WT 5-HT3A receptors. The IC50 values for 5-HT were 1.33 ± 0.2 μm for 5-HT3A receptors and 0.64 ± 0.05 μm for 5-HT3A(C312A) receptors. The Ki values for 5-HT, determined using the Cheng–Prusoff equation (see Methods) were 1.3 μm and 0.61 μm for 5-HT3A and 5-HT3A(C312A) receptors, respectively. Data are presented as means ±s.e.m. of at least three experiments done in triplicate. Specific [3H]GR65630 binding was determined by displacement with ondansetron (1 μm).
The 5-HT3A(C312A) mutation is not dominant negative
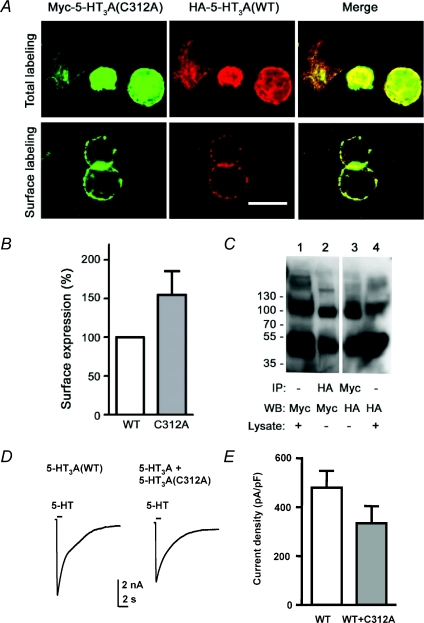
The 5-HT3A(C312A) construct forms receptors in the surface membrane, which bind 5-HT but fail to gate. The mutant may act in a dominant negative fashion, which would preclude its participation in functional heteromeric receptors. We transiently expressed 5-HT3A(C312A) with the WT 5-HT3A subunit to investigate this possibility. Confocal fluorescence microscopy revealed that WT 5-HT3A and mutant 5-HT3A(C312A) subunits co-localized at the plasma membrane (Fig. 4A). In addition, quantitative measurement of surface expression by ELISA indicated that, when expressed alone, the 5-HT3A(C312A) mutant receptors tended to be more stable at the plasma membrane compared to WT 5-HT3A receptors (Fig. 4B). Moreover, epitope-tagged WT 5-HT3A and 5-HT3A(C312A) subunits expressed together could be co-immunoprecipitated using anti-Myc or anti-HA antibodies (Fig. 4C). These data demonstrate that 5-HT3A(C312A) and WT 5-HT3A subunits combine in receptors at the plasma membrane.
Figure 4. The 5-HT3A(C312A) mutant is not dominant negative.
A, colocalization of HA-5-HT3A subunits with mutant Myc-5-HT3A(C312A) at the plasma membrane. After either total or surface labelling (see Methods), bound primary antibodies were detected with Alexa Fluor 488- and 594-conjugated secondary antibodies. Representative images from one of three independent experiments are shown. Co-localization of 5-HT3A (red) and 5-HT3A(C312A) (green) is evident as yellow in the merged image. Scale bar is 16 μm. B, ELISA was used to quantify the levels of surface expression of Myc-5-HT3A and Myc-5-HT3A(C312A) subunits in parallel experiments using HEK293 cells transiently expressing either construct. Surface expression was normalized to that of the WT 5-HT3A subunit. Data are presented as means ±s.e.m. of 3–5 independent experiments performed in triplicate. C, co-immunoprecipitation of 5-HT3A and 5-HT3A(C312A) subunits. Lysates from HEK293 cells transiently co-expressing HA-5-HT3A and Myc-5-HT3A(C312A) subunits were extracted and either immunoblotted directly (lane 1 and 4) or immunoprecipitated using anti-HA antibody (lane 2, IP HA) or anti-Myc antibody (lane 3, IP Myc). Note that samples were treated with DTT (100 mm). Molecular mass markers are indicated on the left (in kDa). Two additional experiments gave similar results. IP, immunoprecipitation; WB, Western blot. D, traces show representative currents activated by 5-HT (100 μm) recorded from cells stably expressing WT 5-HT3A receptors either transiently transfected with GFP alone or in combination with mutant 5-HT3A(C312A) receptors. E, bar graph showing the maximal 5-HT-activated current densities, recorded from cell expressing WT 5-HT3A subunits alone (n= 13) or WT plus 5-HT3A(C312A) subunits (n= 12), which were determined by normalizing current amplitude (pA) to cell membrane capacitance (pF). Transient expression of non-functional 5-HT3A(C312A) mutant subunits with stably expressed WT 5-HT3A subunits did not significantly reduce receptor function (Student's t test).
5-HT3A(C312A) expression did not appear to affect maximal current density in cells simultaneously transiently transfected with WT 5-HT3A receptor cDNA; however, 5-HT-evoked current amplitudes varied considerably between cells, potentially obscuring an effect of the mutation on functional expression (data not shown). More consistent current amplitudes can be achieved by stable receptor expression and we have used this approach successfully to describe the effect of subsequent transient introduction of an additional subunit (Davies et al. 2001). Positive selection was used to generate a stable 5-HT3A receptor expressing HEK293 line (described in Methods). 5-HT (100 μm) application caused the activation of robust currents (Fig. 4D). Stable cells were transiently transfected with green fluorescent protein (GFP) cDNA either alone or in the presence of cDNA encoding the mutant 5-HT3A(C312A) subunit. Recordings from these cells 48 h after transfection revealed no significant change in current density caused by introduction of the 5-HT3A(C312A) subunit (Fig. 4E). These data suggest that the 5-HT3A(C312A) mutation is recessive. The failure of 5-HT3A(C312A) to significantly affect the density of current in cells expressing both the mutant and WT constructs suggests that the latter can at least partially compensate for the functional deficit of the former.
Mutant 5-HT3A(C312A) subunits combine with 5-HT3B subunits into cell surface receptors
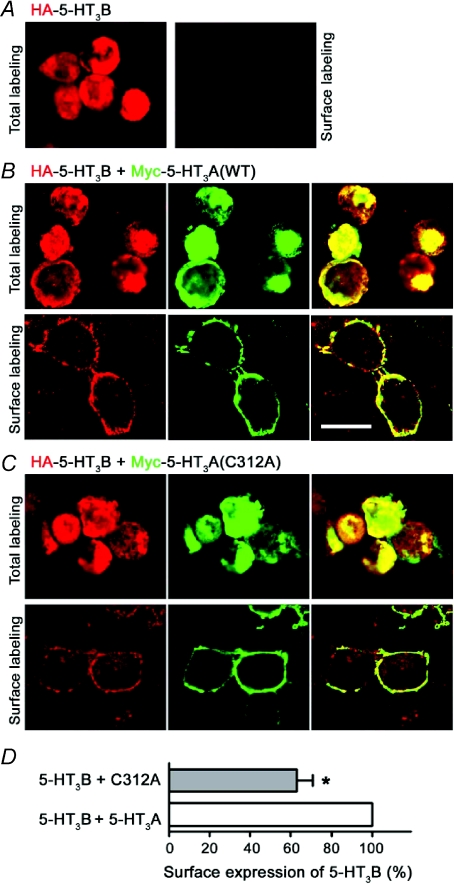
5-HT3B subunits fail to form functional homomers, but combine with 5-HT3A subunits to form functional heteromeric 5-HT3AB receptors with distinctive biophysical characteristics (Davies et al. 2001). Since 5-HT3A(C312A) subunits form homopentamers, which express in the surface membrane and bind 5-HT, the mutant subunit may combine with the 5-HT3B subunit into heteropentamers. Consistent with a previous study (Boyd et al. 2002), when expressed alone 5-HT3B subunits failed to access the cell surface (Fig. 5A); however when co-expressed with WT 5-HT3A subunits, 5-HT3B subunits were robustly expressed at the plasma membrane (Fig. 5B). Co-expression with the mutant subunit also led to the surface expression of the 5-HT3B subunit, indicating the formation of heteromeric 5-HT3A(C312A)/5-HT3B receptors (Fig. 5C). Quantification of 5-HT3B receptor subunit expression using ELISA revealed that the 5-HT3A(C312A) mutant is less effective than the WT 5-HT3A subunit at inducing the surface expression of 5-HT3B subunits (Fig. 5D).
Figure 5. WT 5-HT3A and mutant 5-HT3A(C312A) subunits enable surface localization of the 5-HT3B subunit.
Total and surface labelling of HEK293 cells transiently expressing HA-5-HT3B alone (A) or in combination with either Myc-5-HT3A (B) or Myc-5-HT3A(C312A) (C). Labelling was performed as described for Fig. 4. The distribution of receptor subunits was examined by confocal microscopy. Representative images from two independent experiments performed in duplicate are shown. Scale bar is 16 μm. D, surface expression level of 5-HT3B with co-expression of WT 5-HT3A or mutant 5-HT3A(C312A) quantified using ELISA. Expression level of 5-HT3B with co-expression of 5-HT3A was normalized as 100%. Data are presented as means ±s.e.m. of 3–5 independent experiments performed in triplicate. *P < 0.05 (t test).
Mutant 5-HT3A(C312A) and 5-HT3B subunits form weakly functional heteromeric receptors
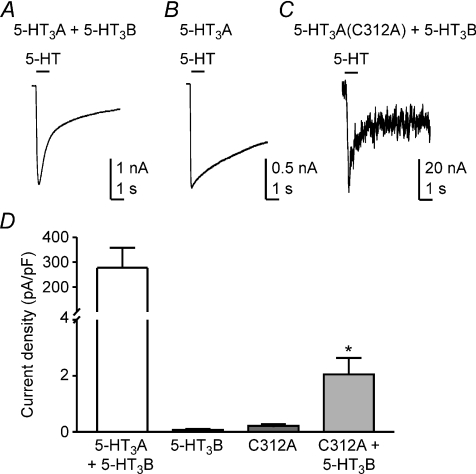
We used the whole-cell patch-clamp technique to determine whether heteromeric 5-HT3A(C312A)/5-HT3B receptors are functional. Co-expression of 5-HT3B subunits with WT 5-HT3A subunits produces functional heteromeric receptors with modified biophysical characteristics (Davies et al. 2001; Stewart et al. 2003). As previously reported, human WT 5-HT3AB receptor mediated currents exhibited rapid desensitization during the application of 5-HT (Fig. 6A), while homomeric WT 5-HT3A receptor mediated currents desensitized more slowly (Fig. 6B). Heteromeric 5-HT3A(C312A)/5-HT3B receptors mediated small currents that resembled those of WT 5-HT3AB receptors (Fig. 6C). Cells expressing 5-HT3A(C312A)/5-HT3B receptors had markedly reduced current densities compared to those expressing WT 5-HT3AB receptors (Fig. 6D). However, currents mediated by 5-HT3A(C312A)/5-HT3B receptors were significantly larger than those observed when either the 5-HT3A(C312A) or 5-HT3B subunits were expressed alone. The lower current density in cells expressing 5-HT3A(C312A)/5-HT3B receptors compared to WT 5-HT3AB receptors is likely to be due to a combination of reduced cell surface expression (Fig. 5D) and impaired function. These data demonstrate that mutant 5-HT3A(C312A) subunits can combine with 5-HT3B subunits to form functional heteromeric receptors.
Figure 6. Heteromeric 5-HT3A(C312A)/5-HT3B receptors exhibit weak function.
A, 5-HT (100 μm) activated large whole-cell currents mediated by heteromeric 5-HT3AB receptors. Representative currents recorded from a cell transiently transfected with cDNAs encoding WT 5-HT3A and WT 5-HT3B subunits. Note that the current desensitized rapidly, a hallmark of heteromeric receptor function. B, a representative 5-HT-evoked current recorded from a cell expressing homomeric 5-HT3A receptors. The current decayed slowly. C, a representative 5-HT-evoked current recorded from a cell transfected with cDNAs encoding 5-HT3A(C312A) and 5-HT3B subunits desensitized rapidly. D, bar graph depicting the maximal 5-HT-activated current densities expressed as current amplitude (pA) normalized to cell membrane capacitance (pF), recorded from cells expressing WT 5-HT3AB receptors (n= 8), 5-HT3B subunits (n= 3), 5-HT3A(C312A) receptors (n= 5) and 5-HT3A(C312A)/5-HT3B receptors (n= 4). Error bars represent ±s.e.m. Statistical analysis (ANOVA, post hoc Bonferroni's test) reveals that the 5-HT-evoked current density recorded from cells expressing 5-HT3A(C312A) and 5-HT3B subunits together was greater than current densities recorded from cells expressing either subunit alone (*P < 0.05).
A polar residue at position 312 in the 5-HT3A subunit is important for function
Our data demonstrate that the 5-HT3A(C312A) mutation blocks homomeric receptor function without preventing the formation of surface 5-HT3A(C312A) homopentamers that bind 5-HT. The function of the 5-HT3A(C312A) mutant can be partially restored by co-expression of 5-HT3B subunits. Examination of the alignment of Cys-loop receptor subunit M3 domains (Fig. 1A) reveals that GABAAρ1–3, glycine α1–3, nACh α7 and α9 subunits, which form functional homomeric receptors, all have polar residues at positions equivalent to that of residue 312 in the 5-HT3A subunit. Since replacement of the polar Cys residue by non-polar Ala led to a lack of homomeric 5-HT3A(C312A) receptor function, we examined the role of residue polarity in this effect. We made a series of 5-HT3A subunit mutants in which Cys-312 was replaced by polar residues Thr and Ser (found at the equivalent position in ACh α and GABAAρ subunits, respectively), or non-polar residues Gly and Leu (found at the equivalent position in ACh δ and γ, respectively) and tested their function in transiently transfected HEK293 cells.
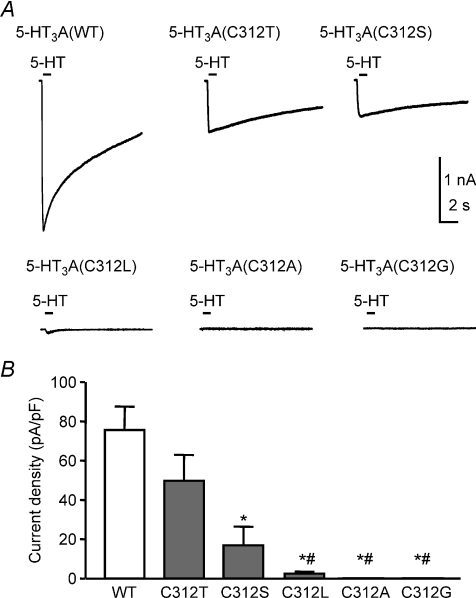
5-HT (30 μm) activated robust currents recorded from cells expressing 5-HT3A, 5-HT3A(C312T) or 5-HT3A(C312S) receptors (Fig. 7A). By contrast, little or no current was activated when 5-HT was applied to cells expressing 5-HT3A(C312A), 5-HT3A(C312G) or 5-HT3A(C312L) receptors (Fig. 7A). 5-HT-evoked current densities were significantly higher in 5-HT3A receptors containing the polar residues Cys or Thr at position 312 compared to those containing non-polar residues at this position (Fig. 7B).
Figure 7. A polar residue at position 312 is essential for robust 5-HT3A receptor function.
A, representative whole-cell currents recorded before, during and after 5-HT (30 μm) application for 1 s at a −60 mV holding potential were recorded from HEK293 cells transiently expressing WT 5-HT3A (n= 5) or mutant 5-HT3A(C312T) (n= 8), 5-HT3A(C312S) (n= 9), 5-HT3A(C312L) (n= 5), 5-HT3A(C312A) (n= 5) or 5-HT3A(C312G) receptors (n= 7). B, bar graph illustrating maximal 5-HT-activated current densities expressed as current amplitude (pA) normalized to cell membrane capacitance (pF). Replacement of Cys-312 by Ser caused a reduction in function compared to WT receptors (*P < 0.01). Replacement of Cys-312 by non-polar residues (Leu, Ala or Gly) significantly reduced receptor function compared to both WT (*P < 0.01) and mutant 5-HT3A(C312T) receptors (#P < 0.05). Statistical analysis using ANOVA with post hoc Bonferroni's test.
Discussion
The Cys residue at position 312 (Cys-312) plays a critical role in homomeric 5-HT3A receptor gating. Replacement of this M3 Cys residue in the human 5-HT3A subunit by Ala abolished function without reducing surface expression of homopentameric receptors that bind 5-HT. Interestingly, 5-HT3A(C312A) subunits combine with WT 5-HT3A receptors without significantly reducing functional expression. Therefore the functional deficit caused by the mutation can be rescued by incorporation of WT 5-HT3A subunits. In addition, mutant 5-HT3A(C312A) subunits combined with 5-HT3B subunits enabling low density expression of functional heteromeric receptors. These data demonstrate that, while replacement of Cys-312 by Ala abolishes function of homomeric 5-HT3A(C312A) receptors, the mutant subunit can combine with other subunits to form functional heteromeric receptors.
Most Cys-loop subunits are unable to form functional homomeric receptors when expressed alone and consequently the majority of subunits combine with other isoforms into heteromeric receptors; this ensures a high level of Cys-loop receptor heterogeneity. The minority of subunits that can form functional homomeric receptors include 5-HT3A, GABAAρ1–3, glycine α1–3, nACh α7 and α9 subunits. All of these subunits have a polar residue at the M3 5′ position (the prime numbering system used by De Rosa and colleagues (De Rosa et al. 2002), illustrated in Fig. 1A) equivalent to 312 in the 5-HT3A subunit. This observation, together with our demonstration that replacement of Cys-312 by the non-polar residue Ala abolishes the function of 5-HT3A receptors, is in keeping with the hypothesis that a polar residue is required at this location in the M3 for normal gating of homomeric receptors. Mutant 5-HT3A subunits in which Cys-312 was replaced by the equivalent non-polar residues found in ACh δ and γ subunits (Gly and Leu, respectively) were unable to form fully functional homomeric receptors.
While we are not aware of previous studies examining M3 residues in 5-HT3 receptors, several studies demonstrate an important role for the M3 domain in heteromeric nACh receptor gating (Campos-Caro et al. 1997; Wang et al. 1999; Cruz-Martin et al. 2001; De Rosa et al. 2002; Cadugan & Auerbach, 2007; De Rosa et al. 2008). The M3 9′, α(V285I) mutation associated with congenital myasthenic syndrome slows channel activation and speeds up closing, resulting in a severe impairment of the end-plate current at the neuromuscular junction (Wang et al. 1999). Simultaneous replacement of the M3 9′ Val residues in α, β, ɛ and δ subunits caused near abolition of channel gating. The adjacent M3 8′ residue is also a critical determinant of gating in αβɛδ nACh receptors (De Rosa et al. 2002). The presence of the aromatic residue Phe at the M3 8′ position reduces mean open time in a stoichiometry-dependent manner. On the basis of this observation it was suggested that a Phe at the M3 8′ position is not compatible with the formation of a functional heteromeric receptor. However, a broader comparison across all Cys-loop subunits reveals that the Phe residue is found in functional homomer-forming GABAAρ subunits and glycine α subunits (Fig. 1A).
The 4 Å resolution structural model of the T. marmorata nACh receptor (Unwin, 2005) reveals that the M3 domain is α-helical (Fig. 1B). While the resolution of this cryo-electron microscopy-derived model is relatively low, the periodicity of the α subunit's M3 helix agrees well with a nuclear magnetic resonance spectroscopy model of a synthetic peptide corresponding to the M3 domain (Lugovskoy et al. 1998) and predictions made by Fourier transform analyses of functional data obtained with M3 Trp scanning mutagenesis (Otero-Cruz et al. 2008). According to these predictions the M3 5′ Thr residue in the nACh α subunit, corresponding to Cys-312 in the 5-HT3A subunit, faces towards the other membrane spanning domains within the same subunit. Likewise the high resolution X-ray crystallography-derived bacterial GLIC (ligand-gated ion channel from Gloeobacter violaceus) and ELIC (ligand gated ion channel from Erwinia chrysanthemi) subunit structural models, in presumed open and closed configurations, respectively, reveal a similar position of the equivalent M3 residue relative to the other membrane-spanning domains (Bocquet et al. 2007, 2009; Hilf & Dutzler, 2008, 2009). However, we chose to model the 5-HT3A receptor using the structure of the nACh receptor due to the higher degree of sequence identity shared by these eukaryotic pentameric ligand-gated ion channels.
Consistent with our findings in the 5-HT3A receptor, replacement of the M3 5′ Thr by the non-polar residue Trp in α(T281W) subunit within αβγδ receptors had no effect on the level of α-bungarotoxin binding (reflecting nACh receptor surface expression) but caused a >10 fold reduction in ACh-evoked current (Guzman et al. 2003). Interestingly, the reduction in current amplitude was associated with a sevenfold reduction in EC50 consistent with the mutation causing an increased affinity of the receptor for ACh. In keeping with this observation we observed that introduction of the non-polar Ala residue at the M3 5′ residue increased the affinity of 5-HT binding to 5-HT3A(C312A) receptors. The observed increase in agonist binding affinity, combined with an absence of function, is consistent with the mutant receptor rapidly transitioning into a non-conducting desensitized state.
Trp scanning mutagenesis has also been used to infer movement of the M3 domain during gating of the nACh receptor (Cadugan & Auerbach, 2007; Otero-Cruz et al. 2007). A tilted-spring model was proposed to explain how several mutations in the top half of the M3 helix have larger impacts on nACh receptor gating and ACh EC50 values than do mutations of residues closer to the bottom of M3. After analysing the extent to which these mutations altered the opening versus closing rate constants, Cadugan & Auerbach (2007) proposed that the movement of residues in the upper half of the helix is temporally correlated and occurs late during gating, after the movement of M2 and M4 residues. It is possible that movement of the M3 domain is associated with desensitization, a phenomenon which may be exacerbated by the presence of a non-polar residue at the M3 9′ position.
The Zn2+ activated channel (ZAC), another homomeric Cys-loop receptor with a relatively low level of homology to nACh, GABAA and glycine receptors, has considerable spontaneous opening suggesting that its gating may differ considerably compared to most other members of the Cys-loop receptor family (Davies et al. 2003). ZAC contains a Leu residue at the M3 5′ position. The mutant 5-HT3A(C312L) receptor mediated low levels of receptor function compared to WT 5-HT3A receptors. Thus the M3 5′ Leu may contribute to the relatively low level of functional expression seen in cells expressing recombinant ZAC (Davies et al. 2003). Since ZAC is activated by the ion Zn2+ it is possible that the channel gates more like GLIC, which is activated by low pH (Bocquet et al. 2007). GLIC and ELIC homopentameric bacterial channels, which lack the characteristic Cys-loop found in eukaryotic pentameric receptors, but are nevertheless homologues of eukaryotic Cys-loop receptors, have transmembrane domain amino acid sequences that align reasonably well with subunits of this family. In these receptors the gate appears to be located near to the extracellular end of M2. By comparison the structural model of the T. marmorata nACh receptor is consistent with a gate located towards the intracellular end of M2. Alignment of the M3 of GLIC and ELIC with the Cys-loop subunits reveals that the 5′ residues are Ile and Gly, respectively. According to our analysis using the 5-HT3A subunit as a model, non-polar residues are unlikely to support robust functional expression of homomeric Cys-loop receptors. However, this rule may not apply to ZAC, GLIC and ELIC, which may have different gating mechanisms from that of nACh, GABAA and glycine receptors. Future structure–function studies of ZAC and the bacterial channels are required to test the role of M3 residues in their gating.
There are several amino acid determinants that restrict the variety of mammalian Cys-loop receptor subunits that can form neurotransmitter gated homomeric receptors and therefore serve to maximize the capacity for receptor heterogeneity. These include amino acids that (1) prevent the formation of pentamers, (2) retain subunits within the ER, and (3) do not support agonist binding. We have identified a fourth mechanism that retards the formation of functional homomeric neurotransmitter receptors. The presence of a non-polar residue at the 5′ position in M3 impedes the function of cell surface homopentameric receptors that bind neurotransmitter.
Acknowledgments
This work was supported by grants from the National Science Foundation (0818741) and the National Institutes of Health (GM058037). We thank Dr David Perry (George Washington University) for advice regarding radioligand binding assays.
Glossary
Abbreviations
- HA
haemagglutinin-epitope (YPYDVPDYA)
- HA-5-HT3A
HA-tagged 5-HT3A
- Myc
myelocytomatosis-epitope tag (MASMQKLISEEDL)
- ABTS
2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid)
- ELISA
enzyme-linked immunosorbent assay
- M1–M4
transmembrane domains 1–4
- 5-HT
5-hydroxytryptamine
- 5-HT3
5-hydroxytryptamine type 3
- GFP
green fluorescent protein
- nACh
nicotinic acetylcholine
- GR65630
3-(5-methyl-1H-imidazol-4-yl)-1-(1-methyl-1H-indol-3-yl)-1-propanone
- RIPA
radio immunoprecipitation assay
Author contributions
D.-F.W.: conception, design and performance of experiments; collection, analysis and interpretation of data; drafting the manuscript. N.A.O. performed radioligand binding assays and analysed data. D.S. performed electrophysiological experiments. A.M. participated in cDNA preparation and mutagenesis; prepared cells; and performed transfections. T.Z.D.: sequence alignments and homology modelling; participated in drafting the manuscript. T.G.H.: conception, design, analysis and interpretation of experiments; performed electrophysiological experiments; drafted the manuscript. All authors have approved the final version of the manuscript. Experiments were performed in the Department of Pharmacology and Physiology of The George Washington University.
References
- Barnes NM, Hales TG, Lummis SC, Peters JA. The 5-HT3 receptor – the relationship between structure and function. Neuropharmacology. 2009;56:273–284. doi: 10.1016/j.neuropharm.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocquet N, Nury H, Baaden M, Le Poupon C, Changeux JP, Delarue M, Corringer PJ. X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature. 2009;457:111–114. doi: 10.1038/nature07462. [DOI] [PubMed] [Google Scholar]
- Bocquet N, Prado de Carvalho L, Cartaud J, Neyton J, Le Poupon C, Taly A, Grutter T, Changeux JP, Corringer PJ. A prokaryotic proton-gated ion channel from the nicotinic acetylcholine receptor family. Nature. 2007;445:116–119. doi: 10.1038/nature05371. [DOI] [PubMed] [Google Scholar]
- Bollan K, Robertson LA, Tang H, Connolly CN. Multiple assembly signals in gamma-aminobutyric acid (type A) receptor subunits combine to drive receptor construction and composition. Biochem Soc Trans. 2003;31:875–879. doi: 10.1042/bst0310875. [DOI] [PubMed] [Google Scholar]
- Boyd GW, Doward AI, Kirkness EF, Millar NS, Connolly CN. Cell surface expression of 5-hydroxytryptamine type 3 receptors is controlled by an endoplasmic reticulum retention signal. J Biol Chem. 2003;278:27681–27687. doi: 10.1074/jbc.M304938200. [DOI] [PubMed] [Google Scholar]
- Boyd GW, Low P, Dunlop JI, Robertson LA, Vardy A, Lambert JJ, Peters JA, Connolly CN. Assembly and cell surface expression of homomeric and heteromeric 5-HT3 receptors: The role of oligomerization and chaperone proteins. Mol Cell Neurosci. 2002;21:38–50. doi: 10.1006/mcne.2002.1160. [DOI] [PubMed] [Google Scholar]
- Cadugan DJ, Auerbach A. Conformational dynamics of the αM3 transmembrane helix during acetylcholine receptor channel gating. Biophys J. 2007;93:859–865. doi: 10.1529/biophysj.107.105171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Caro A, Rovira JC, Vicente-Agullo F, Ballesta JJ, Sala S, Criado M, Sala F. Role of the putative transmembrane segment M3 in gating of neuronal nicotinic receptors. Biochemistry. 1997;36:2709–2715. doi: 10.1021/bi9623486. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50% inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Cruz-Martin A, Mercado JL, Rojas LV, McNamee MG, Lasalde-Dominicci JA. Tryptophan substitutions at lipid-exposed positions of the gamma M3 transmembrane domain increase the macroscopic ionic current response of the Torpedo californica nicotinic acetylcholine receptor. J Membr Biol. 2001;183:61–70. doi: 10.1007/s00232-001-0051-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PA, Kirkness EF, Hales TG. Modulation by general anaesthetics of rat GABAA receptors comprised of α1β3 and β3 subunits expressed in human embryonic kidney 293 cells. Br J Pharmacol. 1997;120:899–909. doi: 10.1038/sj.bjp.0700987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PA, Kirkness EF, Hales TG. Evidence for the formation of functionally distinct αβγɛ GABAA receptors. J Physiol. 2001;537:101–113. doi: 10.1111/j.1469-7793.2001.0101k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PA, Pistis M, Hanna MC, Peters JA, Lambert JJ, Hales TG, Kirkness EF. The 5-HT3B subunit is a major determinant of serotonin-receptor function. Nature. 1999;397:359–363. doi: 10.1038/16941. [DOI] [PubMed] [Google Scholar]
- Davies PA, Wang W, Hales TG, Kirkness EF. A novel class of ligand-gated ion channel is activated by Zn2+ J Biol Chem. 2003;278:712–717. doi: 10.1074/jbc.M208814200. [DOI] [PubMed] [Google Scholar]
- De Rosa MJ, Corradi J, Bouzat C. Subunit-selective role of the M3 transmembrane domain of the nicotinic acetylcholine receptor in channel gating. Biochim Biophys Acta. 2008;1778:521–529. doi: 10.1016/j.bbamem.2007.10.026. [DOI] [PubMed] [Google Scholar]
- De Rosa MJ, Rayes D, Spitzmaul G, Bouzat C. Nicotinic receptor M3 transmembrane domain: Position 8¢ contributes to channel gating. Mol Pharmacol. 2002;62:406–414. doi: 10.1124/mol.62.2.406. [DOI] [PubMed] [Google Scholar]
- Deeb TZ, Carland JE, Cooper MA, Livesey MR, Lambert JJ, Peters JA, Hales TG. Dynamic modification of a mutant cytoplasmic cysteine residue modulates the conductance of the human 5-HT3A receptor. J Biol Chem. 2007;282:6172–6182. doi: 10.1074/jbc.M607698200. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Harvey RJ, Schwarz G. Gephyrin: Where do we stand, where do we go? Trends Neurosci. 2008;31:257–264. doi: 10.1016/j.tins.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Gotti C, Moretti M, Gaimarri A, Zanardi A, Clementi F, Zoli M. Heterogeneity and complexity of native brain nicotinic receptors. Biochem Pharmacol. 2007;74:1102–1111. doi: 10.1016/j.bcp.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Guzmán GR, Santiago J, Ricardo A, Martí-Arbona R, Rojas LV, Lasalde-Dominicci JA. Tryptophan scanning mutagenesis in the αM3 transmembrane domain of the Torpedo californica acetylcholine receptor: functional and structural implications. Biochemistry. 2003;42:12243–12250. doi: 10.1021/bi034764d. [DOI] [PubMed] [Google Scholar]
- Hanna MC, Davies PA, Hales TG, Kirkness EF. Evidence for expression of heteromeric serotonin 5-HT3 receptors in rodents. J Neurochem. 2000;75:240–247. doi: 10.1046/j.1471-4159.2000.0750240.x. [DOI] [PubMed] [Google Scholar]
- Hilf RJ, Dutzler R. X-ray structure of a prokaryotic pentameric ligand-gated ion channel. Nature. 2008;452:375–379. doi: 10.1038/nature06717. [DOI] [PubMed] [Google Scholar]
- Hilf RJ, Dutzler R. Structure of a potentially open state of a proton-activated pentameric ligand-gated ion channel. Nature. 2009;457:115–118. doi: 10.1038/nature07461. [DOI] [PubMed] [Google Scholar]
- Ilegems E, Pick HM, Deluz C, Kellenberger S, Vogel H. Noninvasive imaging of 5-HT3 receptor trafficking in live cells: From biosynthesis to endocytosis. J Biol Chem. 2004;279:53346–53352. doi: 10.1074/jbc.M407467200. [DOI] [PubMed] [Google Scholar]
- Kilpatrick GJ, Jones BJ, Tyers MB. Identification and distribution of 5-HT3 receptors in rat brain using radioligand binding. Nature. 1987;330:746–748. doi: 10.1038/330746a0. [DOI] [PubMed] [Google Scholar]
- Koch T, Wu DF, Yang LQ, Brandenburg LO, Hollt V. Role of phospholipase D2 in the agonist-induced and constitutive endocytosis of G-protein coupled receptors. J Neurochem. 2006;97:365–372. doi: 10.1111/j.1471-4159.2006.03736.x. [DOI] [PubMed] [Google Scholar]
- Lester HA, Dibas MI, Dahan DS, Leite JF, Dougherty DA. Cys-loop receptors: New twists and turns. Trends Neurosci. 2004;27:329–336. doi: 10.1016/j.tins.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Liang YJ, Wu DF, Stumm R, Hollt V, Koch T. Membrane glycoprotein M6A promotes mu-opioid receptor endocytosis and facilitates receptor sorting into the recycling pathway. Cell Res. 2008;18:768–779. doi: 10.1038/cr.2008.71. [DOI] [PubMed] [Google Scholar]
- Liang YJ, Wu DF, Yang LQ, Hollt V, Koch T. Interaction of the μ-opioid receptor with synaptophysin influences receptor trafficking and signalling. Mol Pharmacol. 2007;71:123–131. doi: 10.1124/mol.106.026062. [DOI] [PubMed] [Google Scholar]
- Lugovskoy AA, Maslennikov IV, Utkin YN, Tsetlin VI, Cohen JB, Arseniev AS. Spatial structure of the M3 transmembrane segment of the nicotinic acetylcholine receptor α subunit. Eur J Biochem. 1998;255:455–461. doi: 10.1046/j.1432-1327.1998.2550455.x. [DOI] [PubMed] [Google Scholar]
- Maricq AV, Peterson AS, Brake AJ, Myers RM, Julius D. Primary structure and functional expression of the 5HT3 receptor, a serotonin-gated ion channel. Science. 1991;254:432–437. doi: 10.1126/science.1718042. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- Mitchell EA, Herd MB, Gunn BG, Lambert JJ, Belelli D. Neurosteroid modulation of GABAA receptors: Molecular determinants and significance in health and disease. Neurochem Int. 2008;52:588–595. doi: 10.1016/j.neuint.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Morton RA, Luo G, Davis MI, Hales TG, Lovinger DM. 2008 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2008. Fluorophore-assisted light inactivation of recombinant 5-HT3A receptors. Programme No. 330.6/D13. [Google Scholar]
- Otero-Cruz JD, Baez-Pagan CA, Caraballo-Gonzalez IM, Lasalde-Dominicci JA. Tryptophan-scanning mutagenesis in the αM3 transmembrane domain of the muscle-type acetylcholine receptor. A spring model revealed. J Biol Chem. 2007;282:9162–9171. doi: 10.1074/jbc.M607492200. [DOI] [PubMed] [Google Scholar]
- Otero-Cruz JD, Torres-Nunez DA, Baez-Pagan CA, Lasalde-Dominicci JA. Fourier transform coupled to tryptophan-scanning mutagenesis: Lessons from its application to the prediction of secondary structure in the acetylcholine receptor lipid-exposed transmembrane domains. Biochim Biophys Acta. 2008;1784:1200–1207. doi: 10.1016/j.bbapap.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves DC, Lummis SC. The molecular basis of the structure and function of the 5-HT3 receptor: A model ligand-gated ion channel (review) Mol Membr Biol. 2002;19:11–26. doi: 10.1080/09687680110110048. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Mohler H. Analysis of GABAA receptor function and dissection of the pharmacology of benzodiazepines and general anaesthetics through mouse genetics. Annu Rev Pharmacol Toxicol. 2004;44:475–498. doi: 10.1146/annurev.pharmtox.44.101802.121429. [DOI] [PubMed] [Google Scholar]
- Stewart A, Davies PA, Kirkness EF, Safa P, Hales TG. Introduction of the 5-HT3B subunit alters the functional properties of 5-HT3 receptors native to neuroblastoma cells. Neuropharmacology. 2003;44:214–223. doi: 10.1016/s0028-3908(02)00376-3. [DOI] [PubMed] [Google Scholar]
- Unwin N. Refined structure of the nicotinic acetylcholine receptor at 4Å resolution. J Mol Biol. 2005;346:967–989. doi: 10.1016/j.jmb.2004.12.031. [DOI] [PubMed] [Google Scholar]
- Wang HL, Milone M, Ohno K, Shen XM, Tsujino A, Batocchi AP, Tonali P, Brengman J, Engel AG, Sine SM. Acetylcholine receptor M3 domain: Stereochemical and volume contributions to channel gating. Nat Neurosci. 1999;2:226–233. doi: 10.1038/6326. [DOI] [PubMed] [Google Scholar]
- Wu DF, Koch T, Liang YJ, Stumm R, Schulz S, Schroder H, Hollt V. Membrane glycoprotein M6a interacts with the μ-opioid receptor and facilitates receptor endocytosis and recycling. J Biol Chem. 2007;282:22239–22247. doi: 10.1074/jbc.M700941200. [DOI] [PubMed] [Google Scholar]
- Wu DF, Yang LQ, Goschke A, Stumm R, Brandenburg LO, Liang YJ, Hollt V, Koch T. Role of receptor internalization in the agonist-induced desensitization of cannabinoid type 1 receptors. J Neurochem. 2008;104:1132–1143. doi: 10.1111/j.1471-4159.2007.05063.x. [DOI] [PubMed] [Google Scholar]
- Xiong W, Hosoi M, Koo BN, Zhang L. Anandamide inhibition of 5-HT3A receptors varies with receptor density and desensitization. Mol Pharmacol. 2008;73:314–322. doi: 10.1124/mol.107.039149. [DOI] [PubMed] [Google Scholar]