Abstract
The vagal afferent system is strategically positioned to mediate rapid changes in motility and satiety in response to systemic glucose levels. In the present study we aimed to identify glucose-excited and glucose-inhibited neurons in nodose ganglia and characterize their glucose-sensing properties. Whole-cell patch-clamp recordings in vagal afferent neurons isolated from rat nodose ganglia demonstrated that 31/118 (26%) neurons were depolarized after increasing extracellular glucose from 5 to 15 mm; 19/118 (16%) were hyperpolarized, and 68/118 were non-responsive. A higher incidence of excitatory response to glucose occurred in gastric- than in portal vein-projecting neurons, the latter having a higher incidence of inhibitory response. In glucose-excited neurons, elevated glucose evoked membrane depolarization (11 mV) and an increase in membrane input resistance (361 to 437 MΩ). Current reversed at −99 mV. In glucose-inhibited neurons, membrane hyperpolarization (−13 mV) was associated with decreased membrane input resistance (383 to 293 MΩ). Current reversed at −97 mV. Superfusion of tolbutamide, a KATP channel sulfonylurea receptor blocker, elicited identical glucose-excitatory but not glucose-inhibitory responses. Kir6.2 shRNA transfection abolished glucose-excited but not glucose-inhibited responses. Phosphatidylinositol bisphosphate (PIP2) depletion using wortmannin increased the fraction of glucose-excited neurons from 26% to 80%. These results show that rat nodose ganglia have glucose-excited and glucose-inhibited neurons, differentially distributed among gastric- and portal vein-projecting nodose neurons. In glucose-excited neurons, glucose metabolism leads to KATP channel closure, triggering membrane depolarization, whereas in glucose-inhibited neurons, the inhibitory effect of elevated glucose is mediated by an ATP-independent K+ channel. The results also show that PIP2 can determine the excitability of glucose-excited neurons.
Introduction
Although the presence of glucose-sensing neurons in the hypothalamus was first reported more than 50 years ago (Mayer, 1953), little is known about the site and mechanism by which alterations in blood glucose level are sensed. In general, glucose-sensing neurons in the brain are involved in the control of neuroendocrine function, nutrient metabolism and energy homeostasis (Levin et al. 2004). The glucose-sensing neurons in the brain are unlikely to play a major role in mediating digestive function in response to changes in circulating glucose levels because changes in cerebrospinal fluid glucose level ranges between only 10 and 30% of blood glucose levels, and therefore may not be rapid or sensitive enough to reflect changes in peripheral glucose levels (Levin et al. 2004).
Research suggests that acute hyperglycaemia affects a subpopulation of neurons in the nucleus tractus solitarii (NTS) and dorsal motor nucleus of the vagus (DMV) (Mizuno & Oomura, 1984; Kobashi & Adachi, 1994; Ferreira et al. 2001; Balfour et al. 2006). Several investigators have shown that glucose injection into the DMV of anaesthetized rats decreases gastric motility (Sakaguchi et al. 1985). Ferreira et al. (2001) demonstrated that glucose administration into the NTS modulates gastric motor function. However, these studies failed to show that NTS and DMV neurons are true primary sensors of peripheral glucose, thus rendering the physiological relevance of these observations unclear.
Recent studies in our laboratory showed that acute hyperglycaemia in rats reduces gastric contractions in a dose-dependent manner, an action abolished by perivagal capsaicin application or vagal rootlet sectioning (Zhou et al. 2008). Further, we showed that hyperglycaemia stimulates vagal afferent pathways, which in turn activate vagal efferent cholinergic pathways synapsing with intragastric nitric oxide-containing neurons to mediate gastric relaxation (Zhou et al. 2008). Hence, vagal afferent pathways are likely to play an important role in glucose sensing.
The vagovagal pathways appear to be involved in the detection of hypoglycaemia and the regulation of eating behaviour. Traditionally attributed to the CNS, glucose sensors in the portohepatic region are now recognized to play an important role in modulating the response to glycaemia (Hevener et al. 1997). Novin et al. (1973) showed that vagotomy abolished the increased eating observed in rabbits after 2-deoxyglucose (2-DG) infusion into the portal vein, but had no effect on the eating behaviour of rabbits receiving 2-DG infusion into the jugular vein. Del Prete & Scharrer (1990) further demonstrated that the eating response to 2-DG in rats was reduced by hepatic branch vagotomy. We have confirmed and extended these findings, showing that glucose sensing in the portal vein is mediated by vagal afferents (Grabauskas et al. 2008a). These observations are consistent with the idea that portohepatic glucose sensors play a critical role in the detection of the transient pre-meal decline in blood glucose and the reliable translation of this decline into meal initiation. In contrast to the liver, which has little or no vagal afferent innervation, the portal vein is richly innervated by afferent fibres sensitive to changes in metabolite concentration, pressure and osmolarity (Lautt, 1983; Shimazu, 1987). In vivo motility (Zhou et al. 2008) and eating behaviour studies in rodents (Novin et al. 1973; Del Prete and Scharrer 1990; Grabauskas et al. 2008a) suggest that sensing of circulatory glucose levels occurs in the nodose ganglia. Hence, in this study we determined the presence of glucose-excited and glucose-inhibited neurons in the nodose ganglia. Patch-clamp recording, immunocytochemistry, and signal transduction studies using pharmacological tools and small inhibitory RNA (siRNA) technologies facilitated further examination of the physiological and molecular characteristics of these glucose-sensing neurons.
Methods
Ethical approval
All procedures were performed in accordance with National Institutes of Health guidelines and with the approval of the University Committee on Use and Care of Animals at the University of Michigan. We have read the article ‘Reporting ethical matters in The Journal of Physiology: standards and advice’ and our experiments comply with the policies and regulations (Drummond, 2009).
Retrograde tracing of nodose ganglia
Thirty-one Sprague–Dawley rats (2–4 weeks of age) of either sex were deeply anaesthetized with a 4% mixture of halothane in air as described previously (Grabauskas & Moises, 2003). Following laparotomy, crystals of the retrograde tracer 1,1′-dioctadecyl-3,3, 3′3-tetramethylindocarbocyanine (DiI; Molecular Probes/Invitrogen, Carlsbad, CA, USA) were applied to either the gastric corpus or the portal vein. To confine the dye to the site of application, crystals of DiI were embedded in a fast hardening epoxy resin that was allowed to harden for approximately 5 min. This was followed by washing of the surgical area with warm sterile saline and the wound was closed with nylon sutures (4–0). The animal was allowed to recover for 10–15 days prior to being killed for nodose ganglia dissection. In 20 experiments DiI was applied to the corpus and in 11 to the portal vein.
Isolation and culture of nodose ganglia neurons
Forty-eight male Sprague–Dawley rats were anaesthetized with ketamine at 250 mg kg−1 and xylazine at 25 mg kg−1 (Peter et al. 2006). The depth of anaesthesia was monitored by testing a paw withdrawal reflex. Following the dissection of the nodose ganglia the animal was killed by injecting an overdose of urethane (3–5 g kg−1) and severing the aorta. The ganglia were placed in a 35 mm culture dish containing Ca2+- and Mg2+-free Hanks’ balanced salt solution with penicillin and streptomycin. Desheathed ganglia were sliced into small fragments and placed in a 1.5 ml centrifuge tube containing digestion buffer (dispase II and collagenase IA, 1 mg ml−1). After incubation at 37°C for 60 min, cells were dispersed by gentle trituration through Pasteur pipettes and washed in Dulbecco's modified Eagle's medium (DMEM). The cells were resuspended in L15 medium (GIBCO BRL/Invitrogen) containing 10% fetal bovine serum, plated onto poly-l-lysine-coated (100 μg ml−1) coverslips for 30 min, and cultured in DMEM with 10% fetal calf serum at 37°C. Neurons were stuck to coverslips and maintained in culture for 24–48 h at 37°C prior to recording.
Patch-clamp electrophysiology
Prior to electrophysiological recording, retrogradely labelled nodose ganglia neurons were identified using a Nikon E600 FN microscope equipped with TRITC epifluorescence filters. Unless otherwise indicated, recordings were made in neurons that were unequivocally identified as labelled with DiI. All measurements were made in a physiological saline solution composed of (in mm): 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 5 glucose, 10 mannitol and 10 Hepes; pH adjusted to 7.3 with NaOH. Mannitol was used to adjust the osmolarity of the test solution depending on the concentration of glucose used (5 or 10 mm). Whole-cell or perforated patch recordings were performed using borosilicate glass electrodes with resistance between 3 and 6 MΩ (A-M Systems, Carlsborg, WA, USA) backfilled with a saline solution composed of (in mm): 130 potassium gluconate, 10 Hepes, 10 EGTA, 1.0 MgCl2, 2.5 CaCl2, 1.0 ATP and 0.3 GTP. For the perforated patch recording, the internal solution also included 0.5 mg ml−1 amphotericin B (Sigma-Aldrich). Current and voltage recordings were obtained from discrete isolated nodose ganglia neurons using the Axopatch 200B patch-clamp amplifier (Axon Instruments, Sunnyvale, CA, USA) filtered at 2 kHz using a four-pole low-pass Bessel filter. For data analysis, signals were digitized using an analog-to-digital converter Digidata 1322B (Axon Instruments/Molecular Devices), and stored and analysed on a personal computer running pCLAMP 9 software (Axon Instruments/Molecular Devices).
Western blotting
Nodose ganglia were obtained from three rats and pooled for Western blot analyses. The neurons were lysed and centrifuged at 14 000 g for 10 min. Protein samples were then run on Ready Gel 12% Tris-HCl (Bio-Rad, Hercules, CA, USA) for 1.5 h at 80 V. Proteins were then transferred to polyvinylidene difluoride membranes for 1 h at 80 V. The membranes were blocked with StartBlock buffer T20 (Thermo Fisher Scientific, Waltham, MA, USA) for 1 h at room temperature, probed with primary antibodies against Kir6.2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 1: 600 dilution or antibodies against sulfonylurea receptor 1 (SUR1) (Santa Cruz Biotechnology) at 1: 500 dilution at 4°C overnight, and then washed in Tris-buffered saline for 1 h. The membranes were probed with corresponding horseradish peroxidase-conjugated secondary antibodies at 1: 2000 dilution. The resulting bands were scanned with an Epson 2400 and analysed using ImageJ.
Immunocytochemistry
Immunocytochemistry studies to localize the inwardly rectifying K+ channel (Kir6.2) and the sulfonylurea receptor 1 (SUR1) were performed on nodose ganglia neurons. Following anaesthesia with urethane, a transcardial perfusion was performed with ice-cold heparinized phosphate-buffered saline (PBS) and subsequently with fixative containing 4% paraformaldehyde, 0.2% picric acid and 0.35% glutaraldehyde in phosphate buffer (0.1 mol l−1, pH 7.4). The left and right nodose ganglia were removed and placed in the same fixative for 2 h at room temperature and then in 25% sucrose in PBS (0.1 mol l−1) overnight at 4°C. The ganglia were cut in longitudinal sections measuring 5 μm using a precision cryostat (Leica Microsystems, Bannockburn, IL, USA). The sections were collected in serially ordered sets, thaw-mounted on gelatin–chromium coated slides, and stored at −70°C. For permeabilization and background reduction, sections of the nodose ganglia were incubated in 5% normal donkey serum in PBS for 1 h at room temperature. Single or double labelling was performed using the primary antibodies against Kir6.2 (H-55, 1: 250) and SUR1 (H-80, 1: 250). The primary antibodies were diluted in PBS containing 2% normal donkey serum, 0.3% Triton X-100 and 0.1% sodium azide, and tissues were incubated overnight at room temperature. The tissues were washed in PBS and then exposed for 1 h to species-specific Alexa Fluor 488 (Molecular Probes/Invitrogen,) or cyanine 3-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) diluted in PBS containing 0.3% Triton X-100. The sections were coverslipped and then sealed. The specificity of antibodies was demonstrated by leaving out the primary antibodies or secondary antibodies during the staining procedure, which resulted in complete abolition of staining of the nodose ganglia.
All preparations were examined with an epifluorescence microscope or a Zeiss LSM 510 laser scanning confocal microscope. The images were analysed by using filter combinations that enabled separate visualization of multiple fluorophores. The images from the epifluorescence microscope were taken with a Zeiss AxioCam digital camera. Images were stored and analysed with Image-Pro Plus software (Media Cybernetics, Bethesda, MD, USA) or Adobe Photoshop CS2. The digital images taken by the confocal microscope were analysed by Zeiss LSM Image Browser and Adobe Photoshop CS2.
Transfection of nodose ganglia with Kir6.2 short hairpin RNA
Construction of pLentilox 3.7 TRESK shRNA
Kir6.2 short hairpin RNA (shRNA) sequences were designed with the Kir6.2 nucleotides (NM_031358) flanked with blunt T/A bp on the 5′ end and an XhoI sticky end on the 3′ end (sense strand: 5′-Phos-TGTTTAGCATCTCTCCGGATTCAAGAGATCCGGAGAGATGCTAAATTTTTTC-3′; antisense strand: 5′-Phos-TCGAGAAAAAATTTAGCATCTCTCCGGATCTCTTGAATCCGGAGAGATGCTAAACA-3′; Integrated DNA Technologies, Coralville, IA, USA). Both sense and antisense strands were generated by oligonucleotide synthesis with 5′-phosphate and polyacrylamide gel electrophoroesis (PAGE) purification. To introduce RNAi stem-loop, complementary strands of the Kir6.2 sequences were annealed and inserted into the HpaI and XhoI sites of a shRNA-expressing vector pLL3.7, which was contained the mouse U6 promoter upstream of CMV-EGFP expression cassette (Rubinson et al. 2003).
Generation of the lentiviral vector
pLL3.7–Kir6.2 shRNA plasmids and packaging plasmid (pMLDg/pRRE, pRSV-Rev, pCI-VSVG) were transfected into 293T cells by standard calcium phosphate precipitation methods. The supernatant was collected after 72 h and pelleted by centrifugation at 12 300g in a Beckman JA-17 rotor (Beckman Coulter, Fullerton, CA, USA). The viral pellet was then resuspended in DMEM at 10X the original concentration. The resulting shRNA lentiviruses were quality tested in A549 cells. The lenti-Kir6.2 shRNA was shown to transduce greater than 60% of A549 cells, as determined by fluorescence-activated cell sorter (FACS) analysis, which measured the enhanced green fluorescent protein (EGFP) marker in the recombinant lentiviral genome. Scrambled siRNA (siRNA-A; Santa Cruz Biotechnology) was used as control siRNA.
Cell infection
Primary nodose ganglia neuronal cultures were supplemented with (20–100) × 106 TU of lenti-Kir6.2 shRNA or scrambled siRNA viral particles at 37°C. After 3 h, media containing lentiviral particles were aspirated and replaced with DMEM/F-12 media containing 100 mg dl−1 of glucose and 10% fetal calf serum supplemented with gentamycine (100 U ml−1). The neurons were cultured for 72–96 h at 37°C in a 5% CO2 atmosphere.
Statistical analyses
All values are expressed as means ±s.e.m. Significance levels were set at P < 0.05 and were calculated using two-way anova or Fisher's exact test. All statistical analyses were performed using InStat software (GraphPad Software, Inc, La Jolla, CA, USA).
Results
Demonstration of glucose-excited and glucose-inhibited neurons in the nodose ganglia
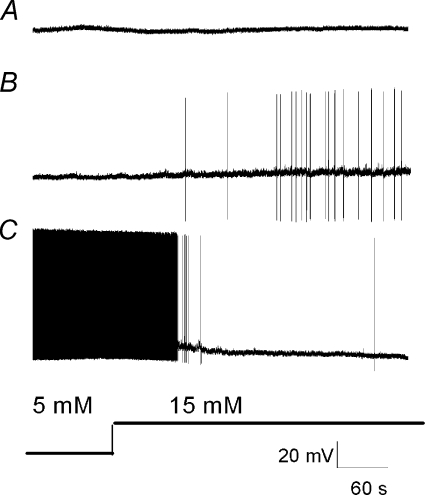
We conducted whole-cell patch-clamp recordings of isolated nodose neurons. Some recordings were performed on retrogradely labelled afferent neurons whose projections to the gastrointestinal tract had been established using the retrograde fluorescent tracer DiI. Whole-cell recordings were obtained from 118 nodose neurons, including 51 innervating the gastric corpus and 35 projecting to the portal vein. Thirty of 118 neurons were spontaneously active. Resting membrane potentials were between −52 and −65 mV (mean −59 ± 3 mV). Overshooting action potential amplitudes ranged from 70 to 123 mV (mean 93 ± 4.1 mV). In response to a 500 ms −100 pA hyperpolarizing current pulse, input resistance ranged between 205 and 490 MΩ (mean 360 ± 23 MΩ). Under these conditions, 31/118 (26%) neurons examined were glucose excited; the neuronal membrane potential was depolarized on increasing the extracellular glucose concentration from 5 to 15 mm (Figs 1 and 2). Nineteen of 118 (16%) neurons were glucose inhibited, exhibiting hyperpolarization in response to the elevated glucose concentration; 68 neurons (58%) did not respond to the glucose concentration transition. Interestingly, the proportion of glucose-excited and glucose-inhibited neurons differed significantly among the gastric- and portal vein-projecting neurons. Gastric-projecting neurons had a significantly higher incidence of glucose-excitatory response (19/51, 37%) when compared with portal vein-projecting neurons (4/36, 11%) (P < 0.05). Conversely, portal vein-projecting neurons had a significantly higher incidence of glucose-inhibitory response (10/36, 28%) when compared with gastric-projecting neurons (4/51, 8%) (P < 0.05).
Figure 1.
Continuous membrane potential recordings depict typical responses of glucose-insensitive (A), glucose-excited (B) and glucose-inhibited (C) nodose ganglia neurons to an increase in extracellular glucose (5 to 15 mm)
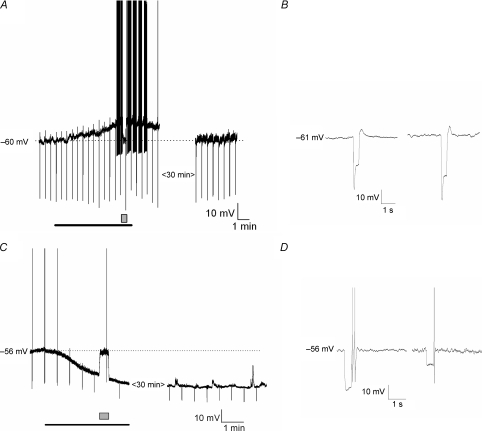
Figure 2. Effects of glucose on the membrane properties of nodose ganglia neurons.
A, continues membrane potential recording shows that increase in extracellular glucose concentration from 5 to 15 mm (bar) depolarized the membrane potential sufficient to elicit action potentials. The depolarization was associated with the increase in neuronal input resistance; the neuronal input resistance was tested every 40 s by injecting 500 ms, 100 pA amplitude current pulses (negative membrane potential deflections). To evaluate changes in the membrane input resistance the membrane potential was current clamped back (box). Note that after glucose concentration was restored to 5 mm, membrane potential and input resistance returned to baseline within 30 min. B, the same experiment illustrated in A is depicted on an expanded time scale illustrating the membrane potential response to injection of 100 pA current pulse at 5 mm (left) and 15 mm (right) glucose concentration (potential was current clamped back). C, continues membrane potential recording shows that increase in extracellular glucose concentration from 5 mm to 15 mm hyperpolarized the membrane potential in a glucose inhibited neuron. The hyperpolarization was associated with a decrease in neuronal input resistance. To evaluate changes in the membrane input resistance the membrane potential was current clamped back (box). Note that the effect of hyperpolarization was sustained after 30 min of recording. D, the same experiment illustrated in C is depicted on an expanded time scale to show the membrane potential response to injection of 100 pA current pulse at 5 mm (left) and 15 mm (right) glucose concentration (potential was current clamped back).
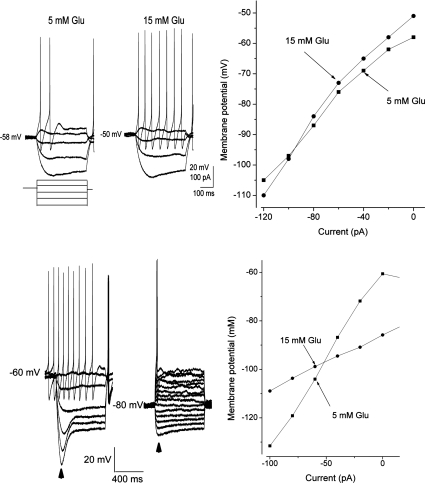
In general, many of the glucose-excited neurons were quiescent under basal conditions, whereas the majority of glucose-inhibited neurons were spontaneously active (Fig. 1). However, this baseline characteristic is by no means uniform. Both types of basal activities were observed in both glucose-excited and glucose-inhibited neurons. The general characteristics of glucose-excited neurons are shown in Fig. 3A and B. In response to an increase of the extracellular glucose concentration, glucose-excited neurons showed an increased membrane input resistance from 361 ± 22 to 437 ± 27 MΩ (n= 31, P < 0.05) and this was associated with membrane potential depolarization of 11 ± 3 mV lasting 30–60 min. The I–V relationship showed that the current reversed at −99 ± 6 mV (n= 31), which is close to the theoretical K+ equilibrium potential of −105 mV in the solution used, suggesting that glucose-evoked excitation was due to closure of K+ channels. In glucose-inhibited neurons, the higher glucose concentration decreased the membrane input resistance from 383 ± 26 to 293 ± 16 MΩ (n= 19, P < 0.05) and this was associated with membrane potential hyperpolarization of 13 ± 4 mV (Fig. 3C and D). The hpyperpolarization persisted throughout the time of recording, ∼1 h. The current reversal potential was −98 ± 4 mV (n= 19), which is also similar to the K+ equilibrium potential of −105 mV. The electrophysiological properties of the nodose ganglia neurons in response to transition of glucose concentrations from 5 to 15 mm did not change after 72–96 h in tissue culture (n= 20) (data not shown).
Figure 3. Glucose levels modulate the membrane properties of nodose ganglia neurons.
A, current–clamp responses show that increase in extracellular glucose (Glu) increased the excitability of glucose-excited neurons, and this was associated with an increase in membrane input resistance; note the increase in amplitude of the negative membrane potential deflection evoked by negative current pulses. B, current–voltage (I–V) relationships from a glucose-excited neuron show that an increase in extracellular glucose concentration increased the slope of the relationship; the effect reversed at −108 mV, close to the estimated K+ equilibrium potential. C, current-clamp responses demonstrate that an increase in extracellular glucose hyperpolarized the recorded neuron from −60 mV to −80 mV. This increase also decreased membrane input resistance and suppressed the action potentials generated by positive current pulses. The arrowheads indicate the time when the data for I–V relationship were acquired. D, current–voltage relationship for a glucose-inhibited type of neuron demonstrates that an increase of glucose concentration reduced the slope of the relationship; the effect reversed at −98 mV, close to the estimated K+ equilibrium potential.
Protein expression of Kir6.2 in the rat nodose ganglia
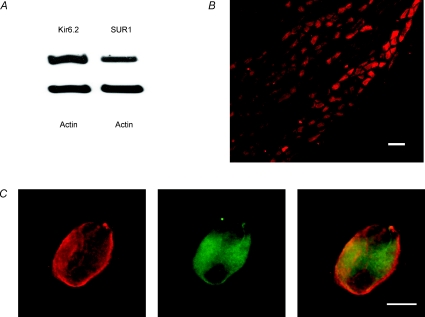
The KATP channel is formed from Kir6.2 channel-forming units and the regulatory sulfonylurea receptor (SUR) (Enkvetchakul & Nichols, 2006). Kir6.2 protein and SUR1 protein were abundant in rat nodose ganglia as demonstrated by Western blot analyses (Fig. 4A). Immunocytochemistry revealed intense staining of Kir6.2 in almost all nodose ganglia neurons (Fig. 4B). This same group of neurons also displayed intense immunostaining of SUR1 (Fig. 4C), confirming that the full KATP channel is present in almost all the neurons in the rat nodose ganglia.
Figure 4. Kir6.2 and SUR1 protein expression in the rat nodose ganglia.
A, representative immunoblot from the nodose ganglia shows Kir6.2 and SUR1 protein expression; actin used as loading control. B, immunohistochemistry studies to identify neurons containing the inwardly rectifying K+ channels in nodose ganglion cross-section. Calibration bar, 100 μm. C, representative immunofluorescence staining for SUR1 (left), the inwardly rectifying K+ channel Kir6.2 (middle), and overlay (right) in a nodose ganglion neuron. Calibration bar, 20 μm.
Electrophysiological evidence that glucose-evoked current is mediated by the KATP channel
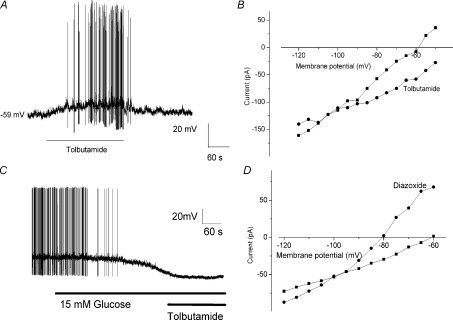
Our data suggest that K+ ions mediate the effects of glucose in nodose ganglia neurons. KATP channels have been implicated as the underlying mechanism responsible for the glucose-sensing mechanism in pancreatic β-cells and ventromedial hypothalamic neurons (Levin et al. 2004). Opening of the KATP channel produces a K+ current that hyperpolarizes the cell and reduces electrical excitability. Conversely, KATP channel closure increases membrane excitability (Song & Ashcroft, 2001). We used pharmacological activation and inhibition of KATP channels to determine the presence of these channels in nodose ganglia neurons. Tolbutamide and diazoxide were used as a KATP channel inhibitor and opener, respectively. Bath application of tolbutamide (200 μm) depolarized recorded neurons (Fig. 5A). I–V relationships revealed that tolbutamide application inhibited the membrane current with a reversal potential of −92 ± 4 mV in 6/20 neurons recorded (Fig. 5B). In contrast, bath application of diazoxide (200 μm) activated a membrane current with a reversal potential of −93 ± 5 mV (n= 5, Fig. 5D). In addition, no further increase in neuronal excitability in response to a glucose concentration transition from 5 to 15 mm was observed following tolbutamide (200 μm) treatment. (n= 6; data not shown). In glucose inhibited neurons, tolbutamide (200 μm) did not attenuate the hyperpolarization current generated by ‘high’ glucose concentration (15 mm) (Fig. 5C), indicating that KATP channels do not modulate the excitability of glucose inhibited neurons. These electrophysiological data support the notion that the KATP channel plays a role in the mediation of glucose-excitatory responses.
Figure 5. Effects of KATP channel modulators on membrane properties of nodose ganglia neurons.
A, continuous membrane potential recordings depict the typical response to extracellular superfusion of tolbutamide (200 μm) in an isolated nodose ganglion neuron. B, tolbutamide (200 μm) shifted the I–V relationship in a depolarized direction, and changed the slope of the relationship, indicating increased input resistance. I–V relationships crossed at −90 mV. The I–V relationship was recorded in the presence of 2 mm extracellular glucose. C, continuous membrane potential recording demonstrating that tolbutamide (200 μm) did not attenuate the duration or the amplitude of the hyperpolarization current generated by ‘high’ glucose concentration, indicating that KATP channels do not modulate the excitability of glucose-inhibited neurons. D, extracellular application of diazoxide (100 μm), a KATP channel activator, generated a shift of the I–V relationship, hyperpolarizing duration and decreasing membrane input resistance. The I–V relationship was recorded in the presence of 15 mm extracellular glucose.
Kir6.2 subunit silencing with a lentiviral vector
Viral vectors are efficient gene delivery tools in mammalian cells (Rubinson et al. 2003). We developed a lentiviral vector that expresses RNA, including Kir6.2 subunit shRNAs. This vector was engendered to co-express EGFP as a reporter gene, permitting identification of infected cells. Our Western blot data demonstrated a 55% reduction in Kir6.2 protein expression in primary neuronal cultures of nodose ganglia neurons infected with the lentiviral vector (Fig. 6A) (n= 3). About 65–75% of the cultured neurons were EGFP positive and showed weak or no immunoprecipitation for anti-Kir6.2 (Fig. 6B and C). On the other hand, nodose ganglia neurons that were transfected with scrambled siRNA were positive for both EGFP and anti-Kir6.2 (Fig. 6D and E). Thus, the lentiviral vector is capable of stably expressing transgenes that silence Kir6.2 mRNA in nodose ganglia neurons.
Figure 6. Lentivirus-based transfection of shRNA for Kir6.2 suppresses endogenous protein and mRNA expression.
A, Western blot demonstrating a significant reduction of Kir6.2 protein 96 h after the primary nodose ganglion neuron cultures were transfected with lenti-Kir6.2 shRNA. Actin was used as a loading control. B, photomicrograph of nodose ganglion neuron transfected with lenti-shKir6.2 and cultured for 96 h displaying intense EGFP signal (arrow). C, faint immunostain signal for anti-Kir6.2 antibody in the same neuron shown in B (arrow). D, photomicrograph of nodose ganglion neuron transfected with scrambled siRNA and cultured for 96 h displaying intense EGFP signal (arrow). E, immunostain signal for anti-Kir6.2 antibody in the same neuron shown in D (arrow).
To demonstrate the role of the KATP channel in the mediation of glucose-evoked depolarization, we used primary nodose ganglia cultures transfected with the scrambled siRNA or lentiviral vector that targeted Kir6.2 mRNA expression. After incubation for 72–96 h, we examined nodose ganglia neuronal responsiveness to extracellular glucose concentration transition. Only lentiviral vector transfected EGFP-positive cells were used for electrophysiogical recordings. None of the recorded transfected neurons with siRNA for Kir6.2 responded to the glucose concentration transition from 5 to 15 mm (n= 20) or the extracellular application of tolbutamide (n= 5, data not shown). However, 2/20 (10%) of the transfected neurons recorded were inhibited by the increase in extracellular glucose concentration. On the other hand, 3 and 1 of 10 recorded neurons transfected with scrambled siRNA were excited and inhibited, respectively, in response to glucose concentrating transition from 5 to 15 mm. Thus neurons transfected with siRNA for Kir6.2 showed no excitatory response to elevated glucose but the incidence of glucose-inhibited neurons among these transfected neurons was not different from that of the non-transfected nodose ganglia neurons. In contrast, nodose ganglia neurons transfected with scrambled siRNA showed a frequency of glucose excitation and inhibition similar to non-infected neurons. The demonstration that the KATP channel modulators diazoxide and tolbutamide mimic the effects of low and high extracellular glucose concentration on neuron excitability, and that the depolarizing effects of hyperglycaemia were blocked by silencing Kir6.2 subunit expression in the nodose ganglia neurons indicate that KATP channels play a critical role in the glucose-excitatory response. On the other hand, the hyperpolarizing effects of hyperglycaemia observed in glucose-inhibited neurons are not mediated by KATP channels.
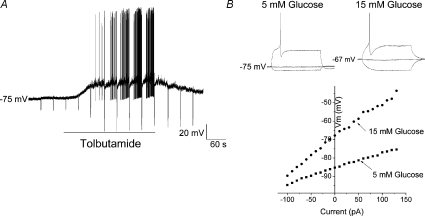
Regulation of KATP channels by phosphatidylinositol bisphosphate
Our Western blot analysis and immunocytochemical data indicated that all nodose ganglia neurons expressed the KATP channel. However, only a fraction (∼26%) of nodose ganglia neurons were depolarized by the KATP channel modulator tolbutamide. This suggests that intracellular messengers other than ATP may be involved in the glucose-induced modulation of KATP channels. Hilgeman & Ball (1996) demonstrated that the application of phosphatidylinositol bisphosphate (PIP2) to inside-out patches of cardiomyocytes modulated KATP channel sensitivity to ATP. It has been shown that PIP kinases are required for PIP2 synthesis (Doughman et al. 2003). Our studies showed that intracellular treatment of nodose neurons with the PIP kinase inhibitor wortmannin (50 nm, Vanhaesebroeck et al. 2001) hyperpolarized 7/17 (41%) recorded neurons by 17 mV (mean 17 ± 4 mV) and the hyperpolarization was associated with a decrease in membrane input resistance from 353 (mean 353 ± 34 MΩ) to 120 MΩ (mean 120 ± 31 MΩ). The effect of wortmannin reversed at −91 ± 4 mV. Of the remaining 10 recorded neurons, two were depolarized and eight were unaffected by wortmannin treatment. In response to extracellular superfusion of tolbutamide, 5/7 (72%) wortmannin-treated neurons were depolarized and showed an increased membrane input resistance (n= 7, Fig. 7A), indicating that this effect is mediated by the closure of K+ channels. Moreover, intracellular dialysis with wortmannin, which depleted intracellular PIP2, increased the excitability of neurons to glucose. Four of five wortmannin-treated neurons (80%) responded to elevation of extracellular glucose from 5 to 15 mm (Fig. 7B). These data indicate that PIP2 can determine the excitability of the nodose ganglia neurons to extracellular glucose by modulating the activity of KATP channels.
Figure 7. Effects of wortmannin on the membrane properties of nodose ganglia neurons.
A, continuous membrane potential recording from wortmannin (50 nm)-treated neuron in response to extracellular superfusion of tolbutamide (200 μm). Tolbutamide depolarized the recoded neuron from −75 to −46 mV, an effect associated with the increase in membrane input resistance from 110 to 596 MΩ. The effect of tolbutamide was reversible. B, wortmannin treatment enhanced the neuronal sensitivity to glucose. Current-clamp recordings demonstrate that an increase in extracellular glucose concentration depolarized the recorded neuron from −85 to −67 mV and increased the membrane input resistance from 98 to 250 MΩ. In addition, the glucose concentration transition decreased the threshold for action potential triggering from −560 to 150 pA. The I–V relationship demonstrates that the glucose effect reversed at ∼−110 mV, which is close to the theoretical K+ ion reversal potential in the conditions recorded.
Effects of glucose metabolism inhibitor 2-DG on nodose ganglia neuron membrane properties
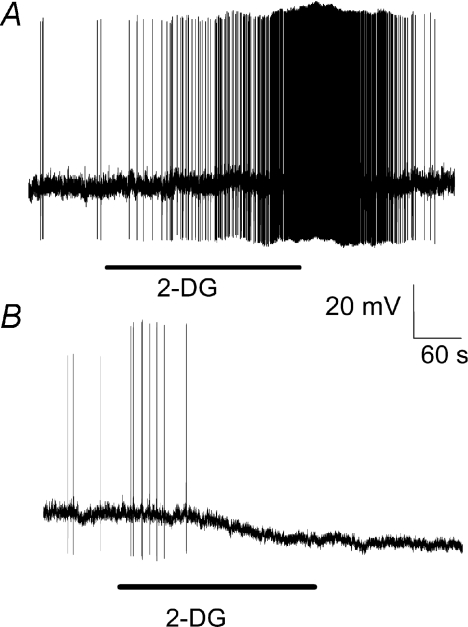
In 3/15 recorded nodose ganglia neurons, extracellular application of 15 mm 2-DG, a non-metabolizable glucose analogue, depolarized the membrane potential to a level sufficient to evoke action potentials (Fig. 8A). In 4/15 neurons, 2-DG generated membrane hyperpolarization (Fig. 8B). These observations indicate that inhibition of glucose metabolism with 2-DG can either excite or inhibit nodose ganglia neurons, suggesting that glucose metabolism is involved in both types of glucose-evoked response. Alternatively, glucose uptake may also be involved in these actions of glucose.
Figure 8.
Continuous membrane potential recording demonstrates that extracellular superfusion of 2-deoxyglucose (2-DG), a glucose metabolism inhibitor, generated either depolarization or hyperpolarization of neuronal membrane potential
Discussion
Our studies demonstrate the existence of a subpopulation of glucose-excited and glucose-inhibited neurons in the rat nodose ganglia. Differential distribution of these two responses occurred among gastric- and portal vein-projecting nodose ganglia neurons. In glucose-excited neurons, glucose metabolism leads to closure of KATP channels, triggering membrane depolarization, whereas in glucose-inhibited neurons, the inhibitory effects of an elevated glucose concentration is mediated by an ATP-independent K+ channel. Our studies also showed that PIP2 is capable of regulating the sensitivity of KATP channels to ATP, thus determining the excitability of glucose-excited neurons to extracellular glucose. This provides a possible mechanism for control of neuronal excitability through signal transduction pathways.
Glucose sensing to date has been considered the domain of the CNS. First identified in the hypothalamus (Mayer, 1953), glucose-sensing neurons are likely to be involved in the control of neuroendocrine function, nutrient metabolism and energy metabolism (Levin et al. 2004). The physiological significance of reports that acute hyperglycaemia affects a subpopulation of neurons in the NTS and DMV (Mizuno & Oomura, 1984; Kobashi & Adachi, 1994; Ferreira et al. 2001; Balfour et al. 2006) has remained unclear because the studies did not show that NTS or DMV neurons are the true primary sensors of peripheral glucose.
Animal studies clearly indicate that vagovagal pathways play an important role in the regulation of gastric motility and eating behaviour in response to acute changes in peripheral blood glucose levels (Schmitt 1973; Vanderweele et al. 1974; Grabauskas et al. 2008a; Zhou et al. 2008). The site of glucose sensing appears to be vagal afferent fibres projecting to the stomach (Zhou et al. 2008) and the portal vein (Schmitt 1973; Vanderweele et al. 1974; Grabauskas et al. 2008a). Wan & Browning (2008) reported that changes in blood glucose levels caused a postsynaptic response in 79% of NTS neurons, implying that glucose modulates synaptic transmission from the central terminals of vagal afferent fibres. Our demonstration of the existence of a subpopulation of glucose-excited and glucose-inhibited neurons in the nodose ganglia supports this implication.
Niijima (1982, 1983, 1984, 1988) reported that there were glucose sensitive afferent nerve fibres in the hepatic branch of the vagus nerve in the guinea pig whose firing was inhibited by elevated blood glucose levels. His methods differed from ours in both experimental design and the species of animal used. He observed decreased frequency in electrical firing of the distal cut end of the vagus nerve in response to administration of glucose into the portal vein. His recording was made from bundles of fibres rather than single fibres, and hence the net result represents a summation of responses to different fibres, some of which may be excitatory while others may be inhibitory. Our data show that the majority of the glucose sensing nodose ganglia neurons projecting to the portal vein were inhibited by elevated ambient glucose.
Mei et al. (1978) reported that perfusion of the upper small intestine stimulated some vagal neurons in the cat. The stimulating effect of intestinal glucose may be mediated by direct action of glucose on vagal afferent fibres or by serotonin released in response to glucose in the lumen (Zhu et al. 2001). These data differ from ours in species and the methods of recording. Mei et al. used sharp electrodes to record vagal discharges and did not address the effects of glucose on membrane potential and input resistance. These are important properties for the characterization of glucose inhibitory actions.
Chambert et al. (1993) and Adachi et al. (1984) measured the activities of NTS neurons in response to infusion of glucose into the rat portal vein. Both excitatory and inhibitory responses were reported (Chambert et al. (1993), Table 2). In both the abstract and the discussion the authors stated that only glucose inhibitory responses were observed. It is important to note that in contrast to our studies, the glucose concentration used was 300 mm, significantly higher than our 5–15 mm. Furthermore, NTS neurons often serve as relay neurons which integrate signals coming from other sensing neurons, including the nodose ganglia. This group also reported that NTS neurons which decreased firing during portal infusion of glucose were also glucose sensitive to topical application of glucose (Adachi et al. 1984). Hence it is plausible that the observed effects were due to systemic glucose acting directly on NTS neurons or were merely due to relayed signals from the nodose ganglia. Explanation of these results is complex and requires further studies for clarification.
Gastric-projecting neurons from the nodose ganglia have a significantly higher incidence of an excitatory response to glucose compared with portal vein-projecting neurons. When stimulated by hyperglycaemia, this group of glucose-excited neurons may activate vagal efferent cholinergic pathways synapsing with intragastric nitric oxide-containing neurons to mediate gastric relaxation (Zhou et al. 2008). The findings of Zhou et al (2008) who showed that hyperglycaemia stimulates vagal afferents, which inhibits gastric motility, should be contrasted with that reported by Shi et al (2003) who showed that hyperglycaemia failed to inhibit spontaneous or bethanechol induced contractions in rats, as measured by a strain gauge force transducer sutured to the antrum. On the other hand, intravenous infusion of glucose inhibited antral contractions stimulated by insulin-induced hypoglycaemia. This inhibitory action of glucose was not affected by sectioning the hepatic branch of the vagus nerve, nor by capsaicin treatment. This led to the suggestion that afferent pathways are not involved in glucose sensing. These differences in findings may be related to the methods used to stimulate and record gastric pressure. Shi et al. (2003) measured antral motility using a strain gauge transducer sutured to the antrum, whereas Zhou et al. (2008) recorded intragastric presence with the use of a water-filled balloon and hence the stomach was partially distended and more sensitive for detecting gastric relaxation. Furthermore, it is not surprising that the inhibitory effect of glucose on antral contraction induced by hypoglycaemia was not sensitive to capsaicin treatment. Since insulin-induced hypoglycaemia acts centrally to stimulate gastric contractions, we would not expect this action to be affected by glucose acting on the vagal afferent fibres.
Animal studies indicate that changes in glucose concentrations in the portal vein also significantly affect eating behaviour (Schmitt 1973; Vanderweele et al. 1974; Grabauskas et al. 2008a). Our demonstration that portal vein-projecting neurons have a significantly higher incidence of inhibitory response to glucose may explain the observations of Russek (1963) and others (Tordoff & Friedman, 1986; Tordoff et al. 1989) who showed that intraportal infusion of glucose suppressed food intake in experimental animals. In general it is believed that mediators that increase afferent firings in the vagus nerve result in a reduction of food intake, while a decrease in vagal activity results in increased food intake. This concept, however, is based on older studies where recordings were made from bundles of afferent fibres (Niijima, 1982, 1983, 1984, 1988), which may contain fibres that may be excited or inhibited by glucose. Furthermore, whether stimulation of vagal afferents results in increased or decreased feeding may depend on their distal projections in the hypothalamus where some of the hypothalamic neurons may contain anorexigenic peptides while others process orexigenic peptides.
Regulation of the KATP channel by ATP derived from glucose metabolism has been proposed to be responsible for glucose-excitatory action in CNS glucose-sensing neurons (Trapp et al. 1997). Miki et al. (2001) demonstrated that mice with the Kir6.2 gene deleted lack glucose-excited neurons in the hypothalamus. In the present study, we showed that nodose ganglia neurons express KATP channel-forming subunits Kir6.2 and SUR1. Further, we showed that transfection with Kir6.2 shRNA abolished the response of nodose ganglia neurons to elevated glucose concentration or extracellular application of tolbutamide. These observations support the notion that KATP channels play a critical role in the glucose excitatory response in the rat nodose ganglia.
Although most neurons in the rat nodose ganglia contain KATP channels, only 26% exhibit glucose excitatory properties. This finding is similar to observations in the hypothalamus (Karschin et al. 1997; Dunn-Meynell et al. 1998) which suggest that other elements may contribute to regulating the sensitivity of the KATP channel to glucose stimulation. As shown in our 2-DG experiments, inhibition of glucose metabolism can either inhibit or excite nodose ganglia neurons. In pancreatic β cells, glucokinase regulates glycolytic flux and intracellular ATP production (Heimberg et al. 1996) and is a primary regulator of ATP production, KATP channel activity, and insulin secretion (Routh et al. 1997). A similar mechanism may be applicable to glucose-excited neurons in the nodose ganglia. Glycolysis of glucose mediated by glucokinase raises the ATP: ADP ratio and inactivates the KATP channel. The increased ATP: ADP ratio leads to membrane depolarization, calcium influx, and increased firing rates in glucose-excited neurons. Hence, as demonstrated in the ventromedial hypothalamic nucleus, ATP production may be a key regulatory step in the glucose excitatory response (Routh et al. 1997).
A number of regulatory molecules may modulate the gating of KATP channels. Among these, PIP2 has been shown to reduce the sensitivity of KATP channels to ATP (Hilgemann & Ball, 1996; Baukrowitz et al. 1998). Our data indicate that depletion of intracellular PIP2 using wortmannin increases the fraction of glucose-excited neurons from 26% to 80%, suggesting that intracellular PIP2 levels affect the glucose-sensing ability of nodose ganglia neurons. The specificity of wortmannin may depend on the concentration used. We employed a concentration of 50 nm, which has been shown to be rather specific in inhibiting PIP kinase (Vanhaesebroeck et al. 2001). Although wortmannin may also inhibit myosin-light kinase, phosphatidylinositol 4-kinase, PKA, PKC and PKG, these inhibitory actions are not observed in concentrations up to 1 μm (Nakanishi et al. 1995; Ui et al. 1995).
In contrast to the glucose-excited neurons in the nodose ganglia, KATP channels are not involved in mediating the hyperpolarization response to high glucose levels in glucose-inhibited neurons. The mechanism of glucose-induced neuronal inhibition is less understood. Several mechanisms have been proposed, including activation of an electronic Na+ pump (Oomura et al. 1974), activation of ATP sensitive Cl− channels (Song et al. 2001; Routh, 2002) or inhibition of Na+/K+-ATPase (Routh, 2002). Oomura et al. (1974) found that extracellular applications of the Na+/K+-ATPase blocker ouabain reversed the inhibitory effects of high glucose on the firing of the lateral hypothalamus neurons. These findings, together with the observation that glucose inhibition was abolished by the metabolic poison azid (Oomura et al. 1974), led these investigators to propose that glucose stimulates the Na+/K+-ATPase pump resulting in hyperpolarization and inhibition of the hypothalamic neurons. However, there are inconsistencies in this interpretation as ouabain inhibition of the pump is irreversible but in Oomura's studies, the inhibitory action of ouabain on glucose action is reversible. The contribution of Na+/K+-ATPase to glucose-induced inhibition remains unresolved to this day.
Glucose-induced activation of post-synaptic Cl− channels has also been proposed to explain the inhibitory action of glucose on the hypothalamic neurons. Song et al. (2001) reported that in rats ventromedial hypothalamic (VMH) neurons, an increase in intracellular glucose concentration induced membrane hyperpolarization and activated a current with a reversal potential of −50 mV, a value close to that of Cl− (−57 mV) in the solution. Similar observations were made by others (Routh, 2002; Fioramonti et al. 2007). However the Cl− theory has been rejected by recent studies using the VMH neurons (Burdakov et al. 2006; Williams & Buradakov, 2009). These studies showed that biophysical elimination of all inhibitory Cl− currents did not affect glucose induced inhibition of the VMH neurons. The membrane current–voltage relationship demonstrated that glucose activates a current selective for K+ ions. Therefore it appears that at least in the hypothalamic neurons, the final effector mediating glucose induced inhibition is K+ currents (Burdakov & Lesage, 2009). In our study, the hyperpolarization response of the nodose ganglia neurons to high glucose levels appears to be mediated by the opening of a K+ channel that is insensitive to ATP. Although the exact nature of this current is unclear, our preliminary studies indicate that it is abolished by the Ca2+ chelator BAPTA but not attenuated by the specific Ca2+-activated K+ channel antagonists ibertoxin and apamin (Grabauskas et al. 2008b). Identification of this current requires further investigation.
In summary, we have demonstrated the existence of subpopulations of glucose-excited and glucose-inhibited neurons in the rat nodose ganglia. In glucose-excited neurons, metabolism of glucose leads to increased ATP production and closure of the KATP channel, triggering a membrane depolarization. The inhibitory effect of elevated glucose in glucose-inhibited neurons is mediated by an ATP-independent K+ channel. Understanding peripheral glucose sensing as it relates to digestive function and feeding behaviour will provide information critical to the medical management of obesity and the treatment of diabetic patients with gastrointestinal complications.
Acknowledgments
We would like to acknowledge Thomas Lanigan with the Vector Core at the University of Michigan for his assistance in designing the viral vector for transfection. This study was supported by the Michigan Peptide Research Center (G.G.) and the National Institute of Diabetes and Digestive and Kidney Diseases Grants P30 DK34933 and R01 DK58913 (C.O.). Gene sequence number for Kir6.2 (GenBank Accession No. NM_031358). The authors disclose no conflicts.
Glossary
Abbreviations
- KATP
ATP-sensitive K+
- 2-DG
2-deoxyglucose
- DMV
dorsal motor nucleus of the vagus
- EGFP
enhanced green fluorescent protein
- NTS
nucleus tractus solitarii
- PIP2
phosphatidylinositol bisphosphate
- shRNA
short hairpin RNA
- siRNA
small inhibitory RNA
Author contributions
All of the authors have contributed to conception and design of the experiments, collection, analysis and interpretation of data, and drafting the article or revising it critically for important intellectual content. The studies were carried out at the Division of Gastroenterology, University of Michigan. All the authors approved the final version to be published.
References
- Adachi A, Shimizu N, Oomura Y, Kobashi M. Convergence of hepatoportal glucose-sensitive afferent signals to glucose-sensitive units within the nucleus of the solitary tract. Neurosci Lett. 1984;46:215–218. doi: 10.1016/0304-3940(84)90444-0. [DOI] [PubMed] [Google Scholar]
- Balfour RH, Hansen AM, Trapp S. Neuronal responses to transient hypoglycaemia in the dorsal vagal complex of the rat brainstem. J Physiol. 2006;570:469–484. doi: 10.1113/jphysiol.2005.098822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baukrowitz T, Schulte U, Oliver D, Herlitze S, Krauter T, Tucker SJ, Ruppersberg JP, Fakler B. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science. 1998;282:1141–1144. doi: 10.1126/science.282.5391.1141. [DOI] [PubMed] [Google Scholar]
- Burdakov D, Jensen LT, Alexopoulos H, Williams RH, Fearon IM, O’Kelly I, Gerasimenko O, Fugger L, Verkhratsky A. Tandem-pore K+ channels mediate inhibition of orexin neurons by glucose. Neuron. 2006;50:711–722. doi: 10.1016/j.neuron.2006.04.032. [DOI] [PubMed] [Google Scholar]
- Burdakov D, Lesage F. Glucose-induced inhibition: how many ionic mechanisms? Acta Physiol (Oxf) 2009 doi: 10.1111/j.1748-1716.2009.02005.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambert G, Kobashi M, Adachi A. Convergence of gastric and hepatic function information in brain stem neurons of the rat. Brain Res Bull. 1993;32:525–529. doi: 10.1016/0361-9230(93)90302-r. [DOI] [PubMed] [Google Scholar]
- Del Prete E, Scharrer E. Hepatic branch vagotomy attenuates the feeding response to 2-deoxy-d glucose in rats. Exp Physiol. 1990;75:259–261. doi: 10.1113/expphysiol.1990.sp003400. [DOI] [PubMed] [Google Scholar]
- Doughman RL, Firestone AJ, Anderson RA. Phosphatidylinositol phosphate kinases put PI4,5P2 in its place. J Membr Biol. 2003;194:77–89. doi: 10.1007/s00232-003-2027-7. [DOI] [PubMed] [Google Scholar]
- Dunn-Meynell AA, Rawson NE, Levin BE. Distribution and phenotype of neurons containing the ATP sensitive K+ channel in rat brain. Brain Res. 1998;814:41–54. doi: 10.1016/s0006-8993(98)00956-1. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enkvetchakul D, Nichols CG. Gating mechanism of KATP channels: function fits form. J Gen Physiol. 2006;122:471–480. doi: 10.1085/jgp.200308878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira M, Browning KN, Sahibzada N, Verbalis JG, Gillis RA, Travagli RA. Glucose effects on gastric motility and tone evoked from the rat dorsal vagal complex. J Physiol. 2001;536:141–152. doi: 10.1111/j.1469-7793.2001.t01-1-00141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fioramonti X, Contie S, Song Z, Routh VH, Lorsignol A, Penicaud L. Characterization of glucosensing neuron subpopulations in the arcuate nucleus: integration in neuropeptide Y and pro-opio melanocortin networks? Diabetes. 2007;56:1219–1227. doi: 10.2337/db06-0567. [DOI] [PubMed] [Google Scholar]
- Grabauskas G, Li Y, Owyang C. Glucose-inhibited neurons in the nodose ganglia modulate feeding behaviour in response to changing glucose concentration in the portal vein. Neurogastroenterol Motil. 2008a;20:128. Ab SAT 147. [Google Scholar]
- Grabauskas G, Moises HC. Gastrointestinal-projecting neurones in the dorsal motor nucleus of the vagus exhibit direct and viscerotopically organized sensitivity to orexin. J Physiol. 2003;549:37–56. doi: 10.1113/jphysiol.2002.029546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabauskas G, Song I, Lanigan T, Owyang C. Hyperpolarization of nodose ganglia in chronic hyperglycemia rats is mediated by activation of the tandem-pore K+ channel TRESK. Gastroenterology. 2008b;134:A–94. Ab 651. [Google Scholar]
- Heimberg H, De Vos A, Moens K, Quartier E, Bouwens L, Pipeleers D, Van Schaftingen E, Madsen O, Schuit F. The glucose sensor protein glucokinase is expressed in glucagon-producing α-cells. Proc Natl Acad Sci U S A. 1996;93:7036–7041. doi: 10.1073/pnas.93.14.7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevener AL, Bergman RN, Donovan CM. Novel glucosensor for hypoglycemic detection localized to the portal vein. Diabetes. 1997;46:1521–1525. doi: 10.2337/diab.46.9.1521. [DOI] [PubMed] [Google Scholar]
- Hilgemann DW, Ball R. Regulation of cardiac Na+, Ca2+ exchange and KATP potassium channels by PIP2. Science. 1996;73:956–959. doi: 10.1126/science.273.5277.956. [DOI] [PubMed] [Google Scholar]
- Karschin C, Ecke C, Ashcroft FM, Karschin A. Overlapping distribution of KATP channel-forming Kir6.2 subunit and the sulfonylurea receptor SUR1 in rodent brain. FEBS Lett. 1997;401:59–64. doi: 10.1016/s0014-5793(96)01438-x. [DOI] [PubMed] [Google Scholar]
- Kobashi M, Adachi A. Effect of topical administration of glucose on neurons innervating abdominal viscera in dorsal motor nucleus of vagus in rats. Jpn J Physiol. 1994;44:729–734. doi: 10.2170/jjphysiol.44.729. [DOI] [PubMed] [Google Scholar]
- Lautt WW. Afferent and efferent neural roles in liver function. Prog Neurobiol. 1983;21:323–348. doi: 10.1016/0301-0082(83)90016-3. [DOI] [PubMed] [Google Scholar]
- Levin BE, Routh VH, Kang L, Sanders NM, Dunn-Meynell AA. Neuronal glucosensing: what do we know after 50 years? Diabetes. 2004;53:2521–2528. doi: 10.2337/diabetes.53.10.2521. [DOI] [PubMed] [Google Scholar]
- Mayer J. Glucostatic mechanism of regulation of food intake. N Engl J Med. 1953;249:13–16. doi: 10.1056/NEJM195307022490104. [DOI] [PubMed] [Google Scholar]
- Mei N. Vagal glucoreceptors in the small intestine of the cat. J Physiol. 1978;282:485–506. doi: 10.1113/jphysiol.1978.sp012477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T, Liss B, Minami K, Shiuchi T, Saraya A, Kashima Y, Horiuchi M, Ashcroft F, Minokoshi Y, Roeper J, Seino S. ATP-sensitive K+ channels in the hypothalamus are essential for the maintenance of glucose homeostasis. Nat Neurosci. 2001;4:507–512. doi: 10.1038/87455. [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Oomura O. Glucose responding neurons in the nucleus tractus solitarius of the rat: in vitro study. Brain Res. 1984;307:109–116. doi: 10.1016/0006-8993(84)90466-9. [DOI] [PubMed] [Google Scholar]
- Nakanishi S, Yano H, Matsuda Y. Novel functions of phosphatidylinositol 3-kinase in terminally differentiated cells. Cell Signal. 1995;7:545–557. doi: 10.1016/0898-6568(95)00033-l. [DOI] [PubMed] [Google Scholar]
- Niijima A. Glucose-sensitive afferent nerve fibres in the hepatic branch of the vagus nerve in the guinea-pig. J Physiol. 1982;332:315–323. doi: 10.1113/jphysiol.1982.sp014415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niijima A. Electrophysiological study on nervous pathway from splanchnic nerve to vagus nerve in rat. Am J Physiol Regul Integr Comp Physiol. 1983;244:R888–R890. doi: 10.1152/ajpregu.1983.244.6.R888. [DOI] [PubMed] [Google Scholar]
- Niijima A. Reflex control of the autonomic nervous system activity from the glucose sensors in the liver in normal and midpontine-transected animals. J Auton Nerv Syst. 1984;10:279–285. doi: 10.1016/0165-1838(84)90025-0. [DOI] [PubMed] [Google Scholar]
- Niijima A. The effect of endogenous sugar acids on the afferent discharge rate of the hepatic branch of the vagus nerve in the rat. Physiol Behav. 1988;44:661–664. doi: 10.1016/0031-9384(88)90332-0. [DOI] [PubMed] [Google Scholar]
- Novin D, VanderWeele DA, Rezek M. Infusion of 2-deoxy-D-glucose into the hepatic-portal system causes eating: evidence for peripheral glucoreceptors. Science. 1973;181:858–860. doi: 10.1126/science.181.4102.858. [DOI] [PubMed] [Google Scholar]
- Oomura Y, Ooyama H, Sugimori M, Nakamura T, Yamada Y. Glucose inhibition of the glucose-sensitive neurone in the rat lateral hypothalamus. Nature. 1974;247:284–286. doi: 10.1038/247284a0. [DOI] [PubMed] [Google Scholar]
- Peter JH, Ritter RC, Simasko SM. Leptin and CCK selectively activate vagal afferent neurons innervating the stomach and duodenum. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1544–R1549. doi: 10.1152/ajpregu.00811.2005. [DOI] [PubMed] [Google Scholar]
- Routh VH, McArdle JJ, Levin BE. Phosphorylation modulates the activity of the ATP-sensitive K+ channel in the ventromedial hypothalamic nucleus. Brain Res. 1997;778:107–119. doi: 10.1016/s0006-8993(97)01043-3. [DOI] [PubMed] [Google Scholar]
- Routh VH. Glucose-sensing neurons: are they physiologically relevant? Physiol Behav. 2002;76:403–413. doi: 10.1016/s0031-9384(02)00761-8. [DOI] [PubMed] [Google Scholar]
- Rubinson DA, Dillon CP, Kwiatkowsky AV, Sievers C, Yang L, Kopinja J, Rooney DL, Zhang M, Ihrig MM, McManus MT, Gertler FB, Scott ML, Van Parijs L. A lentivirus-based system to functionally silence genes in primary mammalian cell, stem cells and transgenic mice by RNA interference. Nat Genet. 2003;33:401–406. doi: 10.1038/ng1117. [DOI] [PubMed] [Google Scholar]
- Russek M. Participation of hepatic glucoreceptors in the control of intake of food. Nature. 1963;197:79–80. doi: 10.1038/197079b0. [DOI] [PubMed] [Google Scholar]
- Sakaguchi T, Ohtake M, Yamazaki M. D-Glucose anomers in the nucleus of the vagus nerve can depress gastric motility of rats. Brain Res. 1985;332:390–393. doi: 10.1016/0006-8993(85)90611-0. [DOI] [PubMed] [Google Scholar]
- Schmitt M. Influences of hepatic portal receptors on hypothalamic feeding and satiety centres. Am J Physiol. 1973;225:1089–1095. doi: 10.1152/ajplegacy.1973.225.5.1089. [DOI] [PubMed] [Google Scholar]
- Shi M, Jones AR, Niedringhaus MS, Pearson RJ, Biehl AM, Ferreira M, Jr, Sahibzada N, Verbalis JG, Gillis RA. Glucose acts in the CNS to regulate gastric motility during hypoglycemia. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1192–1202. doi: 10.1152/ajpregu.00179.2003. [DOI] [PubMed] [Google Scholar]
- Shimazu T. Neuronal regulation of hepatic glucose metabolism in mammals. Diabetes Metab Rev. 1987;3:185–206. doi: 10.1002/dmr.5610030109. [DOI] [PubMed] [Google Scholar]
- Song D, Ashcroft FM. ATP modulation of ATP-sensitive potassium channel ATP sensitivity varies with the type of SURs unit. J Biol Chem. 2001;276:7143–7149. doi: 10.1074/jbc.M009959200. [DOI] [PubMed] [Google Scholar]
- Song Z, Levin BE, McArdle JJ, Bakhos N, Routh VH. Convergence of pre- and postsynaptic influences on glucosensing neurons in the ventromedial hypothalamic nucleus. Diabetes. 2001;50:2673–2681. doi: 10.2337/diabetes.50.12.2673. [DOI] [PubMed] [Google Scholar]
- Tordoff MG, Friedman ML. Hepatic portal glucose infusions decrease food intake and increase food preference. Am J Physiol Regul Integr Comp Physiol. 1986;251:R192–R196. doi: 10.1152/ajpregu.1986.251.1.R192. [DOI] [PubMed] [Google Scholar]
- Tordoff MG, Tluczek JP, Friedman ML. Effect of hepatic portal glucose concentration on food intake and metabolism. Am J Physiol Regul Integr Comp Physiol. 1989;257:R1474–R1480. doi: 10.1152/ajpregu.1989.257.6.R1474. [DOI] [PubMed] [Google Scholar]
- Trapp S, Tucker SJ, Ashcroft FM. Activation and inhibition of K-ATP currents by guanine nucleotides is mediated by different channel subunits. Proc Natl Acad Sci U S A. 1997;94:8872–9977. doi: 10.1073/pnas.94.16.8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ui M, Okada T, Hazeki K, Hazeki O. Wortmannin as a unique probe for an intracellular signalling protein, phosphoinositide 3-kinase. Trends Biochem Sci. 1995;20:303–307. doi: 10.1016/s0968-0004(00)89056-8. [DOI] [PubMed] [Google Scholar]
- Vanderweele DA, Novin D, Rezek M, Sanderson JD. Duodenal or hepatic-portal glucose perfusion: evidence for duodenally-based satiety. Physiol Behav. 1974;12:467–473. doi: 10.1016/0031-9384(74)90124-3. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Leevers SJ, Ahmadi K, Timms J, Katso R, Driscoll PC, Woscholski R, Parker PJ, Waterfield MD. Synthesis and function of 3-phosphorylated inositol lipids. Annu Rev Biochem. 2001;70:535–602. doi: 10.1146/annurev.biochem.70.1.535. [DOI] [PubMed] [Google Scholar]
- Wan S, Browning KN. d-Glucose modulates synaptic transmission from the central terminals of vagal afferent fibres. Am J Physiol Gastrointest Liver Physiol. 2008;294:G757–G763. doi: 10.1152/ajpgi.00576.2007. [DOI] [PubMed] [Google Scholar]
- Williams RH, Burdakov D. Silencing of ventromedial hypothalamic neurons by glucose-inhibited K+ currents. Pflugers Arch. 2009;458:777–783. doi: 10.1007/s00424-009-0650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou SY, Lu YX, Owyang C. Gastric relaxation induced by hyperglycemia is mediated by vagal afferent pathways in the rat. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1158–G1164. doi: 10.1152/ajpgi.00067.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JX, Wu XY, Owyang C, Li Y. Intestinal serotonin acts as a paracrine substance to mediate vagal signal transmission evoked by luminal factors in the rat. J Physiol. 2001;530:431–442. doi: 10.1111/j.1469-7793.2001.0431k.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]