Abstract
Cystic fibrosis is caused by mutations in CFTR, the cystic fibrosis transmembrane conductance regulator gene. Disruption of CFTR-mediated anion conductance results in defective fluid and electrolyte movement in the epithelial cells of organs such as the pancreas, airways and sweat glands, but the function of CFTR in salivary glands is unclear. Salivary gland acinar cells produce an isotonic, plasma-like fluid, which is subsequently modified by the ducts to produce a hypotonic, NaCl-depleted final saliva. In the present study we investigated whether submandibular salivary glands (SMGs) in ΔF508 mice (CftrΔF/ΔF) display ion transport defects characteristic of cystic fibrosis in other tissues. Immunolocalization and whole-cell recordings demonstrated that Cftr and the epithelial Na+ (ENaC) channels are co-expressed in the apical membrane of submandibular duct cells, consistent with the significantly higher saliva [NaCl] observed in vivo in CftrΔF/ΔF mice. In contrast, Cftr and ENaC channels were not detected in acinar cells, nor was saliva production affected in CftrΔF/ΔF mice, implying that Cftr contributes little to the fluid secretion process in the mouse SMG. To identify the source of the NaCl absorption defect in CftrΔF/ΔF mice, saliva was collected from ex vivo perfused SMGs. CftrΔF/ΔF glands secreted saliva with significantly increased [NaCl]. Moreover, pharmacological inhibition of either Cftr or ENaC in the ex vivo SMGs mimicked the CftrΔF/ΔF phenotype. In summary, our results demonstrate that NaCl absorption requires and is likely to be mediated by functionally dependent Cftr and ENaC channels localized to the apical membranes of mouse salivary gland duct cells.
Introduction
The first stage of the digestion process takes place in the oral cavity, where saliva is secreted in response to ingested food. Saliva is a combination of the fluid secreted by three major pairs of glands (parotid, submandibular and sublingual) and numerous minor salivary glands. Salivary gland acinar cells are classified according to the type of secretion they produce: parotid glands are composed of serous acini which secrete a watery saliva rich in amylase, an enzyme for digesting starches; the sublingual glands are primarily composed of mucous cells that secrete a viscous mucin-rich fluid that lubricates the ingested food and protects the oral cavity from mechanical, chemical and bacterial insults; and the submandibular glands are composed of a mixture of serous and mucous acinar cells.
The significance of saliva in the upper gastrointestinal tract can be inferred from individuals displaying hyposalivation defects, causing difficulties in tasting, chewing and swallowing, and increased incidence and severity of infections leading to dental caries, periodontal disease, and oral and oesophageal ulcers. Salivary gland dysfunction is most frequently observed as a side-effect of medications and irradiation therapy, as well as diseases such as Sjögren's syndrome and cystic fibrosis.
Cystic fibrosis (CF) is the most common genetic disease in Caucasians, occurring in approximately 1 out of 3200 live births (Quinton, 1999). Identification of the gene associated with this condition (CFTR) has led to the characterization of the functional properties of the CFTR protein (Riordan et al. 1989). Of the more than 1000 mutations associated with CF, deletion of phenylalanine 508 (ΔF508) is the most common, occurring in about 70% of cases (Kerem et al. 1989). CFTR codes for a cAMP-activated anion channel highly expressed in epithelial tissues, including salivary glands.
Studies of the effects of CF on human salivary gland function have produced conflicting results. For example, it has been reported that either CF has no effect on flow (Blomfield et al. 1973, 1976) or it reduces salivation (Davies et al. 1990, 1991). Moreover, little effect of CF has been generally noted on the concentration of Na+, K+, Cl− or protein in submandibular gland (SMG) saliva, but defective Cl− reabsorption and HCO3− secretion have been described in SMG ductal saliva from some CF patients (Blomfield et al. 1973; Davies et al. 1990, 1991). The reason for the large variability in the reported effects of CF on salivary gland function is unknown. Most of these studies were performed prior to the advent of methods for determining the nature of the mutation, which relates to the severity of disease.
To gain further insight into the effects of CF on salivary gland function, we have utilized the mouse CF model system which reproduces many of the defects observed in the human disease (Zeiher et al. 1995). Specifically, we examined whether mice homozygous for the ΔF508 mutation (CftrΔF/ΔF) express defects characteristic of CF related to fluid secretion or NaCl reabsorption. The stimulated saliva flow was normal in CftrΔF/ΔF mice whereas the NaCl content of saliva was significantly elevated in mice lacking Cftr, verifying that this channel is a major regulator of NaCl reabsorption by SMG duct cells. The mouse CF model may therefore provide a valuable system for examining the physiological significance and the regulation of ion absorptive mechanisms.
Methods
General methods and materials
CftrΔF/ΔF mice were generated by introduction of a deletion corresponding to the human ΔF508 allele via gene targeting (Zeiher et al. 1995). Targeted disruptions of the murine Nhe2 and Nhe3 genes were performed previously (Schultheis et al. 1998; Bell et al. 1999). All animals were bred and housed in micro-isolator cages with ad libitum access to laboratory chow and water. Water used for Cftr+/+ and CftrΔF/ΔF mice was supplemented with GoLYTELY (Braintree Laboratories Inc., Braintree, MA, USA), an oral osmotic laxative used to increase the survival of CftrΔF/ΔF mice (Clarke et al. 1996). Experiments were performed at the University of Rochester using protocols approved by the University Committee on Animal Resources and in compliance with the policies and regulations described by Drummond (2009). Sex- and age-matched adult mice between 2 and 6 months old were used. Reagents were purchased from Sigma-Aldrich unless otherwise indicated.
Tissue processing and immunohistochemistry
Mouse submandibular gland fixation and immunostaining procedures were performed as described elsewhere (Romanenko et al. 2008). The rabbit anti-CFTR polyclonal antibody was from Cell Signaling Technology, Inc. (Danvers, MA, USA; cat. no. 2269) and an anti-mouse α-ENaC polyclonal antibody was generated by injecting rabbits with a peptide directed at amino acids 46–68 of the mouse α-ENaC sequence (LGKGDKREEQALGPEPSEPRQPTC). This region was selected based on the rat α-ENaC epitope employed previously (Masilamani et al. 1999) and differs from the rat sequence in two amino acids. Rabbit serum was affinity purified using a kit, following the manufacturer's instructions (Sulfolink Immobilization Kit, Pierce Biotechnology Inc., Rockford, IL, USA). Dilutions were used for both antibodies (1: 100). The specificity of the anti α-ENaC antibody was tested using CHO cells expressing the murine α, β and/or γ subunits of ENaC (online Supplemental Material, Fig. S1B).
Measurement of fluid secretion in vivo
Mice were anaesthetized by intraperitoneal injection of chloral hydrate (400 mg (kg body wt)−1) prior to saliva collection as described (Romanenko et al. 2008). The level of anaesthesia was monitored by assessing the animals responsive to toe pinch. In vivo fluid secretion data were normalized by body weight (μl (kg body wt)−1). Immediately after collection of saliva, animals were killed by carbon dioxide inhalation, and the diaphragm and aorta were severed to ensure that the animals were dead.
Ex vivo submandibular gland perfusion
Ex vivo SMG perfusion was performed as previously reported (Romanenko et al. 2007). Secretion was stimulated by addition of a combination of muscarinic (0.3 μm carbachol, CCh) and β-adrenergic (5 μm isoproterenol, IPR) agonists to the perfusion solution. Where indicated, ENaC (amiloride) and Cftr (CFTR(INH)-172) channel blockers were added to the perfusion solution. Salivary gland acini are leaky epithelia, and consequently relatively large compounds like amiloride and CFTR(INH)-172 have access to the luminal surface (Romanenko et al. 2008).
Analysis of saliva composition
Saliva pH was measured immediately after collection with a pH-sensitive electrode (Thermo Scientific, Beverly, MA, USA). Saliva samples were then stored at −86°C until further analysis. Na+, K+ and Cl− concentrations were analysed as previously described (Romanenko et al. 2008).
Electrophysiological recordings
Whole-cell recordings were performed on isolated SMG acinar and granular duct cells as described elsewhere (Romanenko et al. 2007, 2008).
Solutions used for Cftr-mediated Cl− currents recordings were as follows: high Cl− external solution (in mm): 150 NaCl, 1 CaCl2, 1 MgCl2, 10 Hepes, 20 sucrose, pH 7.4; low Cl− external solution (in mm): 10 NaCl, 140 sodium gluconate, 1 CaCl2, 1 MgCl2, 10 Hepes, 20 sucrose, pH 7.4; the internal pipette solution (in mm): 120 CsCl, 10 TEACl, 1 MgCl2, 0.5 EGTA, 1 ATP, 10 Hepes, pH 7.4. Cftr-mediated Cl− currents were elicited by addition to the perfusate of a cAMP-increasing cocktail (10 μm forskolin + 100 μm 3-isobutyl-1-methylxanthine (IBMX)). CdCl2 at 300 μm and 10 μm amiloride were added to external solutions to prevent ClC-2-mediated Cl− currents and ENaC-mediated Na+ currents, respectively (Dinudom et al. 1993; Romanenko et al. 2008). Solutions used to record ENaC-mediated Na+ currents were as follows: external solution (in mm): 135 sodium glutamate, 5 potassium glutamate, 2 CaCl2, 2 MgCl2, 10 Hepes, 20 sucrose, pH 7.2; the internal pipette solution (in mm): 145 potassium glutamate, 5 Na4EGTA, 3 CaCl2, 10 Hepes, pH 7.2.
Biotinylation procedure
In brief, dispersed submandibular cells from Cftr+/+ and CftrΔF/ΔF mice were rinsed twice with ice-cold phosphate-buffered saline (PBS; pH 8.0) prior to the addition of 80 μl of 10 mm Sulfo NHS-SS-Biotin (Pierce) stock solution per ml of PBS as previously described (Gonzalez-Begne et al. 2007).
Western blot analysis
Western blot experiments from whole-cell lysates and affinity enriched biotinylated plasma membrane fractions were performed as previously described (Gonzalez-Begne et al. 2007; Romanenko et al. 2008). Antibodies used were anti-β and anti-γ rabbit polyclonal antibodies (Ergonul et al. 2006) or an anti-mouse α-ENaC rabbit polyclonal antibody. Dilutions used for α-, β- and γ-ENaC antibodies were 1: 100, 1: 1000 and 1: 250, respectively.
Statistical analysis
Results are presented as the mean ±s.e.m. Statistical significance was determined using Student's t test or ANOVA, followed by Bonferroni's test for multiple comparisons using Origin 7.0 Software (OriginLab Corp., Northampton, MA, USA). P values of less than 0.05 were considered statistically significant. All experiments were performed using three or more separate preparations.
Results
Cftr is functionally expressed in the apical pole of SMG duct cells
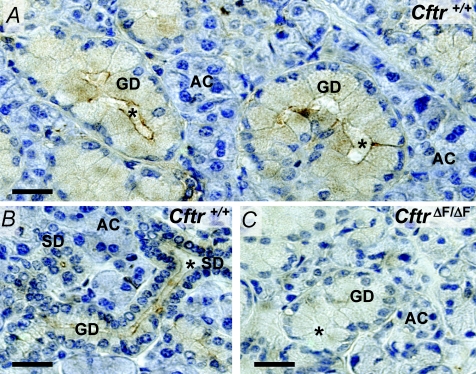
Activation of an apical anion channel is the rate limiting step for fluid secretion by the acinar secretory cells of most exocrine glands, including salivary glands. The Cftr anion channel has been postulated to fulfil this secretory role in the SMG (Zeng et al. 1997b). We performed immunolocalization studies to verify the expression and localization of Cftr channels in the mouse SMG. Figure 1A and B show that Cftr-dependent immunostaining in male and female glands, respectively, was associated with granular and striated duct cells, but little if any staining was detected in acinar cells. The Cftr-associated immunostaining pattern in the SMG was similar to the distribution of Cftr previously described in mouse SMG (Zeiher et al. 1995). At the subcellular level, most of the immunostaining was confined to the apical pole of ducts cells, consistent with the apical expression of Cftr channels in other epithelial tissues (Zeiher et al. 1995; Bothe et al. 2008). Essentially no specific staining was found in SMG tissue from CftrΔF/ΔF mice (Fig. 1C), in agreement with the lack of Cftr protein in the CftrΔF/ΔF SMG (Zeiher et al. 1995).
Figure 1.
Immunohistochemical localization of Cftr channel protein in mouse SMG A and B, immunoperoxidase labelling for Cftr in submandibular glands from male (A) and female (B) Cftr+/+ mice. Sections show staining of striated (SD) and granular ducts (GD) with no apparent immunolabelling in acinar cells (AC). C, immunostaining is not present in SMG from ΔF508 (CftrΔF/ΔF) mice. Asterisks show luminal spaces. Bars = 50 μm.
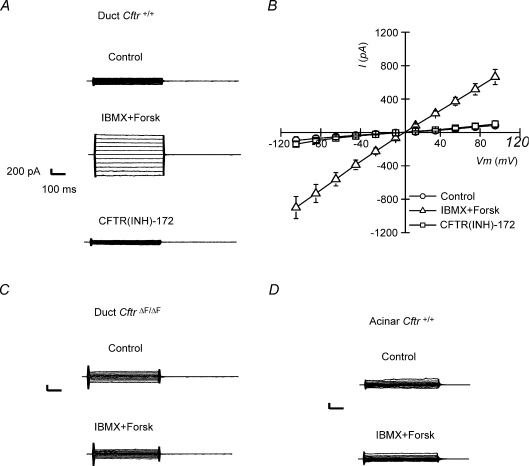
To verify if the staining pattern correlates with functional Cftr channels, single acinar and duct cells were isolated and tested for Cftr-mediated Cl− currents using the whole-cell configuration of the patch clamp technique. Figure 2A and B shows that an ohmic conductance was elicited upon addition of a cAMP-increasing cocktail to the perfusate (10 μm Forskolin + 100 μm IBMX) in SMG duct cells isolated from Cftr+/+ mice. This conductance was carried by Cl−, since its replacement by gluconate in the perfusate shifted the reversal potential to more positive voltages: reversal potential was −3.8 ± 1 mV at [Cl−]o= 154 mm and +54.1 ± 14.7 mV at [Cl−]o= 14 mm (paired experiments, n= 3). The equilibrium potentials for Cl− (ECl) under these conditions at room temperature were −3.9 mV and +56.1 mV, respectively. Moreover, the cAMP-activated Cl− current was completely abolished by 10 μm CFTR(INH)-172, a selective CFTR channel blocker (Ma et al. 2002). cAMP did not activate a Cl− current in granular duct cells from CftrΔF/ΔF mice (Fig. 2C), confirming that Cftr encodes the cAMP-activated anion channel in mouse SMG duct cells. We also failed to detect a cAMP-activated Cl− current in SMG acinar cells (Fig. 2D).
Figure 2.
Cftr-mediated Cl− currents in granular duct cells Whole-cell Cl− currents were recorded as described in Methods. A, cAMP-activated Cl− currents elicited in response to square voltage steps in Cftr+/+ granular duct cells (from −100 to +100 mV, 20 mV steps). CFTR(INH)-172 at 10 μm fully blocked Cftr-mediated currents. Scale: current 200 pA and time 100 ms. B, current–voltage relations from data like those shown in panel A (n= 5). C, c-AMP-activated Cl− currents were not present in granular duct cells isolated from ΔF508 (CftrΔF/ΔF) mice (n= 4). D, submandibular acinar cells isolated from Cftr+/+ mice also failed to display cAMP-activated Cl− currents (n= 4).
Defective NaCl absorption in CftrΔF/ΔF SMG
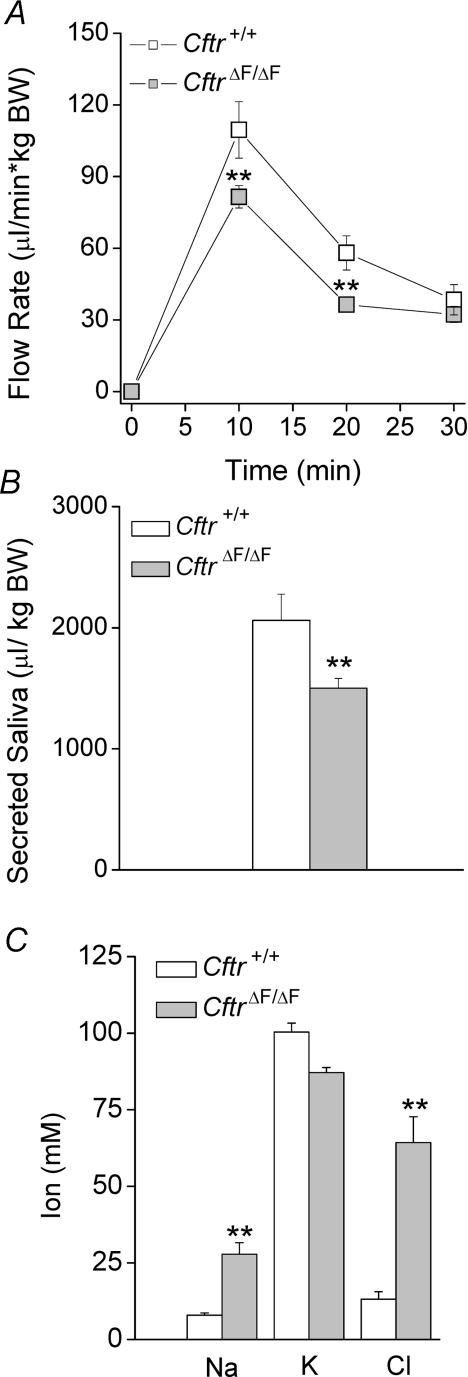
Most of whole saliva (∼90%) is secreted by two of the three major salivary glands, the submandibular and parotid glands. To directly evaluate whether Cftr is involved in acinar fluid secretion and/or ductal function in the SMG, the in vivo saliva flow rates and ionic compositions were determined in littermate Cftr+/+ and CftrΔF/ΔF mice (Snouwaert et al. 1992; Zeiher et al. 1995). Figure 3A and B shows that the flow rate and total volume of saliva generated during stimulation with the broad-range cholinergic secretagogue pilocarpine were modestly decreased in CftrΔF/ΔF mice, suggesting that Cftr contributes little to fluid secretion in this organ under these conditions. To investigate the role of Cftr in ductal modification of saliva, we determined the ion composition of pilocarpine-stimulated SMG saliva (Fig. 3C). The [Cl−] and [Na+] were significantly increased, whereas the [K+] was not affected, in the CftrΔF/ΔF mouse CF model.
Figure 3.
In vivo effects of the CftrΔF508 mutation on fluid secretion and ion composition of SMG saliva Secretion was stimulated by intraperitoneal injection of pilocarpine HCl (10 mg (kg body weight)−1). In vivo fluid secretion data were normalized by body weight (μl (kg body weight)−1). A, saliva flow in response to stimulation was slightly affected in ΔF508 (CftrΔF/ΔF) mice. B, total submandibular saliva secreted. C, Na+ and Cl− saliva concentrations were significantly increased in the ΔF508 CF mouse model. Data are given as the mean ±s.e.m.**P < 0.05, t test; n= 16 for each genotype.
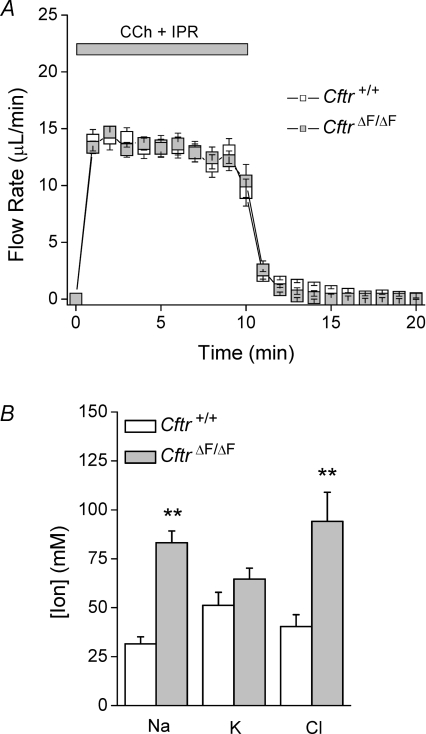
We previously found that in vivo saliva production in response to systemic administration of pilocarpine may not faithfully reflect perturbations of the saliva production machinery (Romanenko et al. 2007). Consequently, we also used an ex vivo, perfused SMG preparation to eliminate the circulating factors and neural inputs that complicate the interpretation of in vivo experiments. A combination of muscarinic (0.3 μm CCh) and β-adrenergic (5 μm IPR) stimulation was used to enhance ductal function (Romanenko et al. 2008). Supplemental Table 1 shows that Na+ and Cl− reabsorption was strongly stimulated in the SMG upon β-adrenergic stimulation, while K+ secretion was not significantly different, mimicking the in vivo effects of pilocarpine on salivary gland function (compare to Fig. 3C). The salivary flow rates and secretion kinetics were essentially identical in SMG from wild-type and CftrΔF/ΔF mice (Fig. 4A). In contrast, both the [Cl−] and [Na+] were significantly increased more than 2-fold in CftrΔF/ΔF saliva, whereas the [K+] was unchanged (Fig. 4B). It should be noted that the [Cl−] in saliva is less than the sum of the [K+] and [Na+], suggesting that other anions must be secreted to maintain electroneutrality. HCO3−, which permeates the Cftr anion channel and is a major regulator of saliva pH, is the second most abundant physiological anion after Cl− in biological fluids, including saliva (Sommer et al. 1975). Consequently, we measured the saliva pH in littermate wild-type (pH 8.18 ± 0.04, n= 13) and CftrΔF/ΔF (pH 7.63 ± 0.06, n= 8; P < 0.01, t test) mice. This decrease in saliva pH suggests that the [HCO3−] is likely to have decreased in parallel with the [Cl−] increase in CftrΔF/ΔF mice.
Figure 4.
Ex vivo effects of the CftrΔF508 mutation on fluid secretion and ion composition of SMG saliva Ex vivo submandibular glands were used to determine the flow rate and ion composition of saliva in Cftr+/+ and ΔF508 (CftrΔF/ΔF) mice. Carbachol (CCh, 0.3 μm) and isoproterenol (IPR, 5 μm) were used to stimulate secretion. Flow rate is expressed as μl min−1. A, saliva flow in response to stimulation (Cftr+/+, open squares, n= 13; CftrΔF/ΔF, grey squares, n= 8). B, ion composition of saliva collected in panel A. Data are given as the mean ± the s.e.m.**P < 0.05, t test.
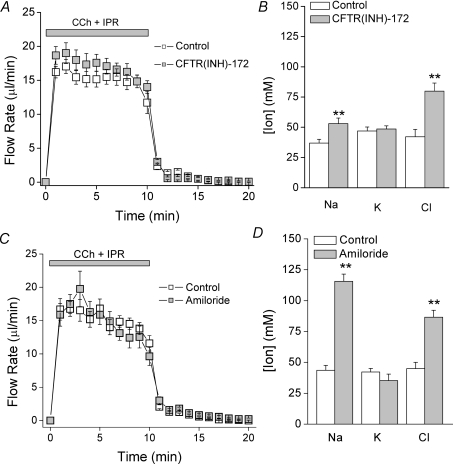
The above functional data demonstrate that Cl− reabsorption is dependent on Cftr expression, but the molecular mechanism involved in this process remains unclear. The simplest interpretation of the above results is that the apical Cftr channel acts as the Cl− influx pathway in SMG duct cells. However, it has been postulated that CFTR modulates the activity and expression of several ion channels and ion transporters (Kunzelmann, 2001). To test whether Cftr channels are essential for Cl− reabsorption, ex vivo pharmacological experiments were performed. Treatment with the CFTR-specific blocker CFTR(INH)-172 mimicked the CftrΔF/ΔF phenotype, i.e. fluid secretion was not affected (Fig. 5A) but the saliva [NaCl] was increased (Fig. 5B), suggesting that functional Cftr protein is required to mediate Cl− reabsorption in the mouse SMG.
Figure 5.
Pharmacological blockade of Cftr and ENaC ion channels mimics the CftrΔF/ΔF phenotype Wild-type ex vivo submandibular glands (Control, Cftr+/+) were perfused with 3 μm CFTR(INH)-172 or 10 μm amiloride for 30 min prior to and during stimulation. Carbachol (CCh, 0.3 μm) and isoproterenol (IPR, 5 μm) were used to stimulate secretion. A, saliva flow in response to stimulation (Control, open squares, n= 8; CFTR(INH)-172 perfused glands, grey squares, n= 8). B, ion composition of saliva collected in panel A. C, saliva flow in response to stimulation (Control, open squares, n= 15; amiloride-perfused glands, grey squares, n= 12). D, ion composition of saliva collected in panel C. Data are given as the mean ± the s.e.m.**P < 0.05, t test.
The role of ENaC in SMG NaCl reabsorption
Both genetic (Fig. 4B) and pharmacological (Fig. 5B) disruption of Cftr activity increased the saliva Na+ concentration. One explanation of these results is that Na+ influx is dependent upon Cl− reabsorption in the SMG. Several coupled NaCl absorption mechanisms have been described in absorptive epithelia (Kunzelmann & Mall, 2002; Baum, 2008), all of which require a Na+-influx pathway in the apical membrane. Consistent with this model, Na+-transporting proteins have been detected in SMG, including Nhe2 and Nhe3 Na+/H+ exchangers and the ENaC Na+ channel (Dinudom et al. 1993; Lee et al. 1998). Table 1 shows a summary of the ex vivo experiments performed with Nhe2−/− and Nhe3−/− glands to test whether these Na+ ion transporters mediate Na+ reabsorption. NaCl reabsorption was not impaired by null mutations in either Na+/H+ exchanger (nor was K+ secretion), similar to previous in vivo results in the mouse parotid gland (Park et al. 2001).
Table 1.
Nhe2 and Nhe3 Na+/H+ exchangers are not involved in NaCl reabsorption
| (mm) | Wild-type | Nhe2−/− | Nhe3−/− |
|---|---|---|---|
| Na+ | 31.5 ± 3.6 (13) | 31.1 ± 9.5 (5) | 25.9 ± 6.4 (8) |
| K+ | 51.2 ± 6.7 (13) | 52.2 ± 5.0 (5) | 64.3 ± 7.5 (8) |
| Cl− | 40.41 ± 6.0 (13) | 51.28 ± 4.9 (5) | 42.9 ± 7.0 (8) |
Ion composition analysis of ex vivo submandibular saliva from wild-type, Nhe2−/− and Nhe3−/− mice stimulated with carbachol (CCh, 0.3 μm) and isoproterenol (IPR, 5 μm). Wild-type mice values were taken from Supplemental Table 1 (0.3 μm CCh + 5 μm IPR). Data are given as the mean ±s.e.m. and the number of experiments is given in parentheses. Note that there were no significant differences between groups, ANOVA.
Considering that NaCl reabsorption was normal in Nhe2−/− and Nhe3−/− mice, we hypothesized that the epithelial Na+ channel ENaC is likely to be involved in this process. Taking advantage of ENaC's sensitivity to low concentrations of amiloride (Kellenberger et al. 2003), ex vivo experiments revealed that neither the kinetics nor the total volume of secreted saliva was affected by amiloride treatment (Fig. 5C). This result rules out potential side-effects on other amiloride-sensitive proteins involved in fluid secretion, in particular the Na+/H+ exchanger Nhe1 (Park et al. 2001). Conversely, ion composition analysis demonstrated that amiloride treatment impaired Na+ reabsorption nearly 3-fold in the SMG (Fig. 5D). Qualitatively similar to Cftr inhibition (Figs 3, 4 and 5), this manoeuvre also impaired Cl− reabsorption, providing additional evidence that Na+ and Cl− transport are functionally linked in this NaCl absorptive tissue.
Functional ENaC channels are expressed in the apical membrane of SMG duct cells
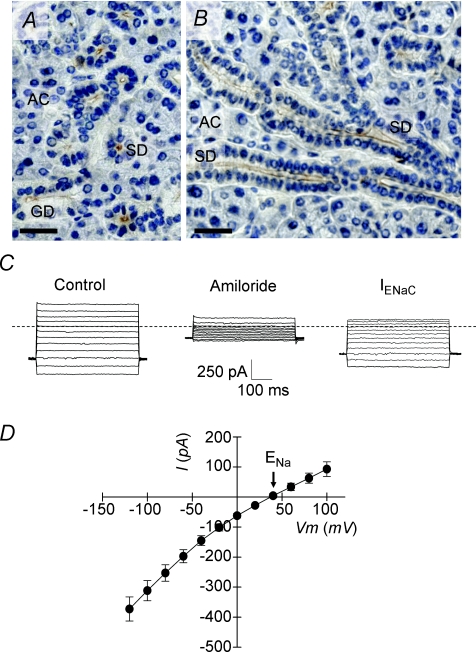
Based on the amiloride sensitivity of NaCl reabsorption, ENaC appears to be directly linked to Na+ absorption, the molecular counterpart to the Cftr-mediated Cl− influx in the SMG. This model requires that ENaC be localized to the apical membrane of SMG duct cells. Consequently, immunolocalization studies were performed on mouse SMG. The specificity of a rabbit polyclonal antibody generated against the N-terminal segment of α-ENaC was tested on CHO cells expressing the murine α, β and/or γ subunits of ENaC (Supplementary Fig. S1A). Similar to the staining pattern for Cftr (Fig. 1), Fig. 6A and B shows strong α-ENaC-associated immunostaining at the apical poles of granular and striated SMG duct cells, but no staining was detected in acinar cells. To verify that the α-ENaC staining pattern correlates with functional ENaC channels, SMG granular duct cells were isolated and assessed for an amiloride-sensitive, ENaC-mediated Na+ current using the whole-cell configuration of the patch clamp technique. A robust, time-independent Na+ current was elicited in response to different voltage steps in isolated granular duct cells (Fig. 6C and D). This Na+ conductance relied on ENaC activity because it was essentially abolished by 10 μm amiloride treatment (80.2 ± 4.4% inhibition at −60 mV, n= 8). The current–voltage relation of the amiloride-sensitive current revealed that the reversal potential was more than +40 mV, consistent with a Na+ conductance (ENa=+47.7 mV at room temperature). Together, these results provide further evidence for the involvement of ENaC channels in the functional coupling of NaCl absorption in submandibular duct epithelium.
Figure 6.
Immunohistochemical and functional evidence for α-ENaC expression in mouse SMG Immunoperoxidase labelling of α-ENaC in wild-type submandibular glands (Control, Cftr+/+). Transverse (A) and longitudinal (B) sections showing the staining of striated (SD) and granular ducts (GD) with no apparent immunolabelling in acinar cells (AC). Bars = 50 μm. C, whole-cell Na+ currents were recorded as described in Methods. Na+ currents elicited in response square voltage steps in wild-type (Control, Cftr+/+) granular duct cells (from −120 to +100 mV, 20 mV steps). Amiloride at 10 μm blocked ENaC-mediated currents. Scale: current 250 pA and time 100 ms, respectively. Dashed line indicates zero current level. D, ENaC-mediated current–voltage relation was obtained by subtracting whole-cell currents after amiloride treatment from those recorded before amiloride addition (n= 8). Data are given as the mean ±s.e.m.
α-ENaC protein level and activity are reduced in CftrΔF/ΔF SMG
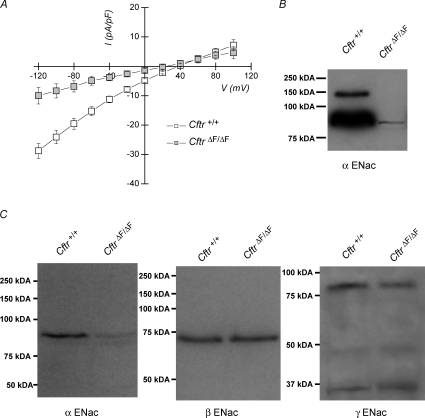
The above results demonstrate that absorption of Na+ and Cl− is dependent on both ENaC and Cftr channels in the duct epithelium of the mouse SMG. Because Na+ reabsorption was impaired in the CftrΔF/ΔF SMG, we predicted that functional disruption of Cftr might also affect ENaC activity in this epithelium. To test this hypothesis, whole-cell recordings were performed on isolated SMG granular duct cells from Cftr+/+ and CftrΔF/ΔF mice and the ENaC-mediated Na+ current magnitudes compared. Indeed, the results from these experiments (Fig. 7A) show that the ENaC-mediated Na+ current was severely decreased in CftrΔF/ΔF SMG duct cells (CftrΔF/ΔF=−4.9 ± 1.5 pA pF−1vs. Cftr+/+=−15.3 ± 1.5 pA pF−1 at −60 mV; P < 0.001, t test).
Figure 7.
α-ENaC currents and protein level are reduced in CftrΔF/ΔF SMG A, whole-cell Na+ currents were recorded as described in Methods. ENaC-mediated current densities versus voltage plots in Cftr+/+ (open squares) and ΔF508 (CftrΔF/ΔF; grey squares) SMG duct cells. Current densities were obtained by dividing the current magnitude by the cell capacitance and expressing as pA pF−1 (n= 8 for Cftr+/+ and n= 9 for CftrΔF/ΔF). Data are given as the mean ±s.e.m.**P < 0.05, t test. Data for Cftr+/+ mice are the same as those shown in Fig. 6C. B, α-ENaC immunoreactivity in submandibular plasma membrane-enriched proteins from Cftr+/+ and CftrΔF/ΔF mice. C, α-, β- and γ-ENaC immunoreactivities in submandibular whole-cell lysates from Cftr+/+ and CftrΔF/ΔF mice. α-, β- and γ-ENaC immunoreactive bands ran at molecular weights of approximately 90, 73 and 80 kDa, respectively, similar to the expected sizes of these proteins in mice.
The decrease in ENaC-mediated Na+ currents in CftrΔF/ΔF SMG duct cells could be due to either changes in channel properties or a decrease in the expression of ENaC at the plasma membrane. To monitor the plasma membrane expression of α-ENaC, western blots were performed on biotinylated proteins isolated by streptavidin affinity chromatography. Figure 7B shows that expression of the α-ENaC subunit in the plasma membrane was dramatically reduced in CftrΔF/ΔF SMG. Western blots of whole-cell lysates also found that the total cellular level of α-ENaC protein was considerably decreased in CftrΔF/ΔF SMG (left panel, Fig. 7C), demonstrating that the decrease in α-ENaC expression was not restricted to the plasma membrane. This effect was limited to the α-ENaC subunit because no apparent changes were observed in the total cellular expression of β- or γ-ENaC (middle and right panels of Fig. 7C, respectively) using previously characterized antibodies (Ergonul et al. 2006).
Discussion
The fluid secreted by salivary gland acinar cells is an isotonic NaCl-rich fluid. This NaCl-rich fluid is modified during its passage through the ductal epithelium, where much of the NaCl is reabsorbed, and because the ducts are relatively impermeable to water, NaCl reabsorption results in the production of a hypotonic saliva. Apical Cl− channels are key components of both the fluid secretion and NaCl reabsorption mechanisms in exocrine salivary glands, while fluid secretion by acinar cells also requires the apical water channel Aqp5 (Ma et al. 1999; Krane et al. 2001). Although results are conflicting, some clinical studies suggest that CFTR may be important for fluid secretion and NaCl reabsorption in salivary glands (Blomfield et al. 1973; Davies et al. 1990, 1991), but the role of CFTR in these processes has not been experimentally demonstrated. The present study provides evidence that NaCl absorption requires and is likely to be mediated by functionally interdependent Cftr and ENaC channels localized to the apical membranes of mouse SMG duct cells.
We found that mouse SMG tissue displayed strong Cftr immunostaining in the apical membrane of duct cells, with no apparent Cftr-specific staining in acinar cells, as previously noted (Zeiher et al. 1995). Consistent with our immunolocalization studies, we also failed to detect Cftr-mediated Cl− currents in whole-cell recordings of SMG acinar cells, and the total volume of ex vivo-stimulated SMG fluid was not affected by disruption of Cftr. These ex vivo results are in agreement with the lack of cAMP-activated Cl− current and Cftr protein in acinar cells. Conversely, it has previously been reported that the secretion of in vivo whole saliva stimulated with a β-adrenergic agonist was reduced in Cftr−/− mice (Best & Quinton, 2005) and that Cftr channels are expressed in the apical membranes of both rat and mouse submandibular acinar and duct cells (Zeng et al. 1997b; Ishibashi et al. 2008). We do not know the basis for these apparent differences, but it could be related to several factors including differences in stimulation protocols, whole saliva vs. gland-specific saliva collection methods, type of Cftr mutation, and/or mouse background. Alternatively, we detected a modest decrease in the secretion of in vivo whole saliva (Fig. 3) that was not detected in the ex vivo-stimulated SMG fluid in CftrΔF/ΔF mice. We believe that the differences between the in vivo and ex vivo model systems are likely to be due to absorptive defects in the intestinal tract of CF mice (Clarke et al. 1996), or circulating factors and/or neural inputs that complicate the interpretation of in vivo experiments, rather than an effect on the ion transport machinery itself (Romanenko et al. 2007).
In contrast to acinar cells, granular duct cells displayed large cAMP-activated Cl− currents. The currents in granular duct cells were sensitive to the CFTR-specific blocker CFTR(INH)-172 and were absent in duct cells isolated from CftrΔF/ΔF mice, confirming that Cftr encodes the cAMP-activated anion channel in this cell type. The Cftr-mediated Cl− current in SMG duct cells was similar to the cAMP-activated Cl− current previously described in mouse granular duct cells (Dinudom et al. 1995). Furthermore, both in vivo and ex vivo experiments revealed that the [Cl−] of SMG saliva was dramatically increased in CftrΔF/ΔF mice, suggesting that Cftr anion channels are involved in Cl− reabsorption. Because the ex vivo volume of fluid was essentially unchanged in CftrΔF/ΔF mice, the increase in [Cl−] was not due to changes in saliva flow rate. The most straight forward interpretation of the increase in the [Cl−] in CftrΔF/ΔF mice is that the Cftr anion channel is involved in Cl− reabsorption. However, Cftr modulates the activity and expression of other ion channels and transporters involved in NaCl reabsorption (Kunzelmann, 2001). Consequently, disruption of Cftr expression may not directly test whether Cftr mediates the Cl− influx in mouse submandibular duct cells. To examine this possibility, we used a pharmacological approach to avoid possible effects of Cftr gene disruption on the expression of other ion-transporting proteins. Pharmacological blockade of Cftr with CFTR(INH)-172 in the SMG of wild-type mice mimicked the CftrΔF/ΔF phenotype, i.e. fluid secretion was not affected whereas Cl− reabsorption was impaired. Taken together, the results of these pharmacological and genetic experiments suggest that Cftr is directly involved in Cl− reabsorption. It should be noted that in both in vivo and ex vivo experiments, the saliva [Cl−] in CftrΔF/ΔF mice was less than predicted if Cftr is the only Cl− reabsorption pathway. This suggests that either residual ΔF508 channels are present or that SMG duct cells express other Cl− absorption pathways.
The Na+ absorption mechanism in mouse SMG is robust, reabsorbing much of the Na+ secreted by acinar cells (typically from 145 mm to less than 30 mm). Nhe2 and Nhe3 Na+/H+ exchangers, which are expressed in the apical membrane of salivary gland duct cells (Park et al. 2001), may contribute to Na+ absorption in other epithelial tissues (Schultheis et al. 1998; Guan et al. 2006). Consequently, we hypothesized that Nhe2 and/or Nhe3 might be involved in SMG Na+ reabsorption, either directly as Na+ influx pathways or by regulating intracellular pH, which may regulate Cftr and/or ENaC activity (Reddy et al. 2008). However, ex vivo submandibular gland experiments performed on Nhe2−/− and Nhe3−/− mice revealed that neither Na+/H+ exchanger was required for Na+ reabsorption, consistent with previous in vivo results in the mouse parotid gland (Park et al. 2001). On the other hand, Na+ reabsorption was severely impaired by 10 μm amiloride. Amiloride is a relatively specific inhibitor of ENaC at this low concentration, suggesting that ENaC is involved in submandibular Na+ reabsorption. In agreement with this observation, immunolocalization and patch clamp experiments showed that ENaC channels are expressed in the apical membrane of submandibular duct cells. The ENaC-mediated Na+ current in duct cells was similar to the amiloride-sensitive Na+ current previously described in mouse granular duct cells (Dinudom et al. 1993).
SMG Na+ reabsorption was also dependent on Cftr expression and/or activity, i.e. like the [Cl−], the Na+ concentration increased in the saliva from CftrΔF/ΔF mice as well as in wild-type glands treated with the CFTR channel blocker CFTR(INH)-172. Similarly, reabsorption of both Na+ and Cl− was dramatically reduced upon amiloride inhibition of ENaC. These results suggest that Na+ and Cl− influx across the apical membrane of SMG duct cells is mediated by ENaC and Cftr channels, respectively, and their coordinated activity drives the NaCl reabsorption process in this gland. In agreement with this NaCl reabsorption model, our immunolocalization and electrophysiology studies provide direct evidence that ENaC and Cftr channels are co-expressed in the same SMG duct cells. Such a functional interaction between ENaC and Cftr, and the resulting coupled NaCl uptake in SMG duct cells, closely resembles the functional interplay found between these two channels in sweat gland duct epithelium (Reddy et al. 1999; Reddy & Quinton, 2003).
The simplest interpretation of our data is that ENaC and Cftr coupling is mediated by electrochemical driving forces. The low intracellular Cl− concentration in SMG ductal cells provides a favourable gradient for Cl− entry upon channel opening (Zeng et al. 1997a). Furthermore, Na+ entry through ENaC would promote apical membrane depolarization, thus increasing the driving force for Cl− influx via Cftr channels. Conversely, it can be postulated that ENaC Na+ channel activity is dependent on Cftr-induced hyperpolarization. Although electrochemical forces are likely to drive much of the functional interaction between ENaC and Cftr in submandibular duct cells, we cannot rule out a direct interaction as previously suggested (Berdiev et al. 2007). It is important to note that Cftr channels are gated by an increase in cAMP levels and salivary ducts are under sympathetic, β-adrenergic control (Denniss et al. 1978). Thus, it is plausible to speculate that the functional interplay between ENaC and Cftr channel activity may require β-adrenergic stimulation, that is through Cftr activation, as previously shown in sweat gland ducts (Reddy et al. 1999; Reddy & Quinton, 2003). Consistent with this hypothesis, we found that β-adrenergic stimulation enhanced not only Cl− reabsorption, but Na+ reabsorption as well (Supplement Table 1).
The duct cells from CftrΔF/ΔF mice also displayed a decreased ENaC-mediated Na+ current. In contrast to the pharmacological experiments where ENaC and Cftr protein expression was unchanged, the level of α-ENaC protein was severely reduced in the CftrΔF/ΔF SMG, but not expression of the β- or γ-ENaC subunits. It can be concluded that the reduced Na+ reabsorption in the CftrΔF/ΔF mice is at least partially due to reduced expression of α-ENaC rather than a reduced driving force or a direct effect on the gating and/or sorting of ENaC channels upon Cftr disruption. Similarly, ENaC activity is dramatically reduced in human CF duct cells or in non-CF duct cells where CFTR activity had been blocked (Reddy et al. 1999; Reddy & Quinton, 2003). The decrease in ENaC activity in duct cells from both salivary and sweat gland cells in CF contrasts with the mechanism observed in CF lungs, where an increased ENaC-dependent Na+ conductance is responsible for the Na+ hyper-absorption, which is postulated to cause the airway pathology in CF disease (Stutts et al. 1995; Kunzelmann, 2001; Riordan, 2008). Consistent with this CF model of airway disease, over-expression of α-ENaC mimicked the CF Na+ hyper-absorption phenotype (Mall et al. 2004). The different mechanisms through which ENaC activity is regulated by Cftr expression in exocrine salivary and sweat glands versus airway epithelium have important implications for our understanding of the pathophysiology of CF. One possibility is that Cftr expression somehow modulates the targeting to the plasma membrane or the functional activity of ENaC in airways (Stutts et al. 1995; Kunzelmann, 2001; Riordan, 2008). In contrast, Cftr is apparently necessary for α-ENaC gene expression in submandibular gland ducts.
Acknowledgments
The authors thank Jennifer Scantlin, Laurie Koek, Margarit Sievert and Yasna Jaramillo for excellent technical assistance. We thank Drs Ted Begenisich and Larry Palmer for critical reading of this manuscript, Dr Palmer for providing anti-β- and anti-γ-ENaC rabbit polyclonal antibodies, and Dr Gary Shull for the Nhe2 and Nhe3 knockout mice. This work was supported in part by NIH grants DE09692, DE08921 (JEM) and DK48816 (LLC).
Glossary
Abbreviations
- CCh
carbachol
- CF
cystic fibrosis
- CFTR (Cftr)
human (murine) cystic fibrosis transmembrane conductance regulator
- ΔF
phenylalanine 508 deletion mutation
- IPR
isoproterenol
- IBMX
3-isobutyl-1-methylxanthine
- SMG
submandibular gland
Author contributions
M.A.C., T.N. and J.E.M. designed the research; M.A.C., T.N., M.G.B. and J.M.C. performed the experiments; M.A.C. and T.N. analysed the data; S.M.W. generated and contributed the anti-α-ENaC rabbit polyclonal antibody. L.L.C. supplied mice for the breeder colonies. All authors wrote and approved the paper. The experiments were performed at the University of Rochester, NY, USA.
Author's present address
T. Nakamoto: Department of Oral Reconstruction and Rehabilitation, Kyushu Dental College, 2-6-1 Manazuru, Kokurakita-ku, Kitakyushu City 803-8580, Japan.
Supplemental material
Supplemental Figure 1. Anti α-ENaC specificity. Expression plasmids were generated by inserting mouse cDNA encoding α, β and γ-ENaC into pCDNA 3.1 (+) plasmid (Invitrogen). (A) α-ENaC immunofluorescence performed on CHO cells 48 hrs after transfection using lipofectamine 2000 (Invitrogen). The secondary antibody used was Alexa Fluor® 488 goat anti-rabbit IgG (Invitrogen). Immunofluorescent labelling was present only when the α-ENaC-containing plasmid was present during the transfection. (B) α-ENaC antibody specificity was assessed by western blot using whole lysates from CHO cells 48 hrs after transfection. Immunoreactivity was present only when the α-ENaC-containing plasmid was present during the transfection. 5 μg per plasmid were used for either immunofluorescence and western blot CHO cells transfections. Bars, 50 μm.
Supplemental Table 1. Submandibular NaCl reabsorption is enhanced by β-adrenergic stimulation.
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors
References
- Baum M. Developmental changes in proximal tubule NaCl transport. Pediatr Nephrol. 2008;23:185–194. doi: 10.1007/s00467-007-0569-0. [DOI] [PubMed] [Google Scholar]
- Bell SM, Schreiner CM, Schultheis PJ, Miller ML, Evans RL, Vorhees CV, Shull GE, Scott WJ. Targeted disruption of the murine Nhe1 locus induces ataxia, growth retardation, and seizures. Am J Physiol Cell Physiol. 1999;276:C788–795. doi: 10.1152/ajpcell.1999.276.4.C788. [DOI] [PubMed] [Google Scholar]
- Berdiev BK, Cormet-Boyaka E, Tousson A, Qadri YJ, Oosterveld-Hut HM, Hong JS, Gonzales PA, Fuller CM, Sorscher EJ, Lukacs GL, Benos DJ. Molecular proximity of cystic fibrosis transmembrane conductance regulator and epithelial sodium channel assessed by fluorescence resonance energy transfer. J Biol Chem. 2007;282:36481–36488. doi: 10.1074/jbc.M708089200. [DOI] [PubMed] [Google Scholar]
- Best JA, Quinton PM. Salivary secretion assay for drug efficacy for cystic fibrosis in mice. Exp Physiol. 2005;90:189–193. doi: 10.1113/expphysiol.2004.028720. [DOI] [PubMed] [Google Scholar]
- Blomfield J, Rush AR, Allars HM, Brown JM. Parotid gland function in children with cystic fibrosis and child control subjects. Pediatr Res. 1976;10:574–578. doi: 10.1203/00006450-197606000-00004. [DOI] [PubMed] [Google Scholar]
- Blomfield J, Warton KL, Brown JM. Flow rate and inorganic components of submandibular saliva in cystic fibrosis. Arch Dis Child. 1973;48:267–274. doi: 10.1136/adc.48.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothe MK, Braun J, Mundhenk L, Gruber AD. Murine mCLCA6 is an integral apical membrane protein of non-goblet cell enterocytes and co-localizes with the cystic fibrosis transmembrane conductance regulator. J Histochem Cytochem. 2008;56:495–509. doi: 10.1369/jhc.2008.950592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke LL, Gawenis LR, Franklin CL, Harline MC. Increased survival of CFTR knockout mice with an oral osmotic laxative. Lab Anim Sci. 1996;46:612–618. [PubMed] [Google Scholar]
- Davies H, Bagg J, Goodchild MC, McPherson MA. Defective regulation of electrolyte and protein secretion in submandibular saliva of cystic fibrosis patients. Acta Paediatr Scand. 1991;80:1094–1095. doi: 10.1111/j.1651-2227.1991.tb11789.x. [DOI] [PubMed] [Google Scholar]
- Davies H, Bagg J, Muxworthy S, Goodchild MC, McPherson MA. Electrolyte concentrations in control and cystic fibrosis submandibular saliva. Biochem Soc Trans. 1990;18:447–448. doi: 10.1042/bst0180447a. [DOI] [PubMed] [Google Scholar]
- Denniss AR, Schneyer LH, Sucanthapree C, Young JA. Actions of adrenergic agonists on isolated excretory ducts of submandibular glands. Am J Physiol Renal Physiol. 1978;235:F548–556. doi: 10.1152/ajprenal.1978.235.6.F548. [DOI] [PubMed] [Google Scholar]
- Dinudom A, Komwatana P, Young JA, Cook DI. A forskolin-activated Cl− current in mouse mandibular duct cells. Am J Physiol Gastrointest Liver Physiol. 1995;268:G806–812. doi: 10.1152/ajpgi.1995.268.5.G806. [DOI] [PubMed] [Google Scholar]
- Dinudom A, Young JA, Cook DI. Amiloride-sensitive Na+ current in the granular duct cells of mouse mandibular glands. Pflugers Arch. 1993;423:164–166. doi: 10.1007/BF00374977. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergonul Z, Frindt G, Palmer LG. Regulation of maturation and processing of ENaC subunits in the rat kidney. Am J Physiol Renal Physiol. 2006;291:F683–693. doi: 10.1152/ajprenal.00422.2005. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Begne M, Nakamoto T, Nguyen HV, Stewart AK, Alper SL, Melvin JE. Enhanced formation of a HCO3− transport metabolon in exocrine cells of Nhe1−/− mice. J Biol Chem. 2007;282:35125–35132. doi: 10.1074/jbc.M707266200. [DOI] [PubMed] [Google Scholar]
- Guan Y, Dong J, Tackett L, Meyer JW, Shull GE, Montrose MH. NHE2 is the main apical NHE in mouse colonic crypts but an alternative Na+-dependent acid extrusion mechanism is upregulated in NHE2-null mice. Am J Physiol Gastrointest Liver Physiol. 2006;291:G689–699. doi: 10.1152/ajpgi.00342.2005. [DOI] [PubMed] [Google Scholar]
- Ishibashi K, Okamura K, Yamazaki J. Involvement of apical P2Y2 receptor-regulated CFTR activity in muscarinic stimulation of Cl− reabsorption in rat submandibular gland. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1729–1736. doi: 10.1152/ajpregu.00758.2007. [DOI] [PubMed] [Google Scholar]
- Kellenberger S, Gautschi I, Schild L. Mutations in the epithelial Na+ channel ENaC outer pore disrupt amiloride block by increasing its dissociation rate. Mol Pharmacol. 2003;64:848–856. doi: 10.1124/mol.64.4.848. [DOI] [PubMed] [Google Scholar]
- Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, Buchwald M, Tsui LC. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989;245:1073–1080. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- Krane CM, Melvin JE, Nguyen HV, Richardson L, Towne JE, Doetschman T, Menon AG. Salivary acinar cells from aquaporin 5-deficient mice have decreased membrane water permeability and altered cell volume regulation. J Biol Chem. 2001;276:23413–23420. doi: 10.1074/jbc.M008760200. [DOI] [PubMed] [Google Scholar]
- Kunzelmann K. CFTR: interacting with everything? News Physiol Sci. 2001;16:167–170. doi: 10.1152/physiologyonline.2001.16.4.167. [DOI] [PubMed] [Google Scholar]
- Kunzelmann K, Mall M. Electrolyte transport in the mammalian colon: mechanisms and implications for disease. Physiol Rev. 2002;82:245–289. doi: 10.1152/physrev.00026.2001. [DOI] [PubMed] [Google Scholar]
- Lee MG, Schultheis PJ, Yan M, Shull GE, Bookstein C, Chang E, Tse M, Donowitz M, Park K, Muallem S. Membrane-limited expression and regulation of Na+–H+ exchanger isoforms by P2 receptors in the rat submandibular gland duct. J Physiol. 1998;513:341–357. doi: 10.1111/j.1469-7793.1998.341bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Song Y, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels. J Biol Chem. 1999;274:20071–20074. doi: 10.1074/jbc.274.29.20071. [DOI] [PubMed] [Google Scholar]
- Ma T, Thiagarajah JR, Yang H, Sonawane ND, Folli C, Galietta LJ, Verkman AS. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J Clin Invest. 2002;110:1651–1658. doi: 10.1172/JCI16112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mall M, Grubb BR, Harkema JR, O’Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med. 2004;10:487–493. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC α, β, and γ subunit proteins in rat kidney. J Clin Invest. 1999;104:R19–23. doi: 10.1172/JCI7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K, Evans RL, Watson GE, Nehrke K, Richardson L, Bell SM, Schultheis PJ, Hand AR, Shull GE, Melvin JE. Defective fluid secretion and NaCl absorption in the parotid glands of Na+/H+ exchanger-deficient mice. J Biol Chem. 2001;276:27042–27050. doi: 10.1074/jbc.M102901200. [DOI] [PubMed] [Google Scholar]
- Quinton PM. Physiological basis of cystic fibrosis: a historical perspective. Physiol Rev. 1999;79:S3–S22. doi: 10.1152/physrev.1999.79.1.S3. [DOI] [PubMed] [Google Scholar]
- Reddy MM, Light MJ, Quinton PM. Activation of the epithelial Na+ channel (ENaC) requires CFTR Cl− channel function. Nature. 1999;402:301–304. doi: 10.1038/46297. [DOI] [PubMed] [Google Scholar]
- Reddy MM, Quinton PM. Functional interaction of CFTR and ENaC in sweat glands. Pflugers Arch. 2003;445:499–503. doi: 10.1007/s00424-002-0959-x. [DOI] [PubMed] [Google Scholar]
- Reddy MM, Wang XF, Quinton PM. Effect of cytosolic pH on epithelial Na+ channel in normal and cystic fibrosis sweat ducts. J Membr Biol. 2008;225:1–11. doi: 10.1007/s00232-008-9126-4. [DOI] [PubMed] [Google Scholar]
- Riordan JR. CFTR function and prospects for therapy. Annu Rev Biochem. 2008;77:701–726. doi: 10.1146/annurev.biochem.75.103004.142532. [DOI] [PubMed] [Google Scholar]
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Romanenko VG, Nakamoto T, Catalan MA, Gonzalez-Begne M, Schwartz GJ, Jaramillo Y, Sepulveda FV, Figueroa CD, Melvin JE. Clcn2 encodes the hyperpolarization-activated chloride channel in the ducts of mouse salivary glands. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1058–G1067. doi: 10.1152/ajpgi.90384.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanenko VG, Nakamoto T, Srivastava A, Begenisich T, Melvin JE. Regulation of membrane potential and fluid secretion by Ca2+-activated K+ channels in mouse submandibular glands. J Physiol. 2007;581:801–817. doi: 10.1113/jphysiol.2006.127498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, Giebisch G, Aronson PS, Lorenz JN, Shull GE. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet. 1998;19:282–285. doi: 10.1038/969. [DOI] [PubMed] [Google Scholar]
- Snouwaert JN, Brigman KK, Latour AM, Malouf NN, Boucher RC, Smithies O, Koller BH. An animal model for cystic fibrosis made by gene targeting. Science. 1992;257:1083–1088. doi: 10.1126/science.257.5073.1083. [DOI] [PubMed] [Google Scholar]
- Sommer HM, Kaiser D, Drack E. pH and bicarbonate excretion in the rat parotid gland as a function of salivary rate. Pflugers Arch. 1975;355:353–360. doi: 10.1007/BF00579856. [DOI] [PubMed] [Google Scholar]
- Stutts MJ, Canessa CM, Olsen JC, Hamrick M, Cohn JA, Rossier BC, Boucher RC. CFTR as a cAMP-dependent regulator of sodium channels. Science. 1995;269:847–850. doi: 10.1126/science.7543698. [DOI] [PubMed] [Google Scholar]
- Zeiher BG, Eichwald E, Zabner J, Smith JJ, Puga AP, McCray PB, Jr, Capecchi MR, Welsh MJ, Thomas KR. A mouse model for the delta F508 allele of cystic fibrosis. J Clin Invest. 1995;96:2051–2064. doi: 10.1172/JCI118253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W, Lee MG, Muallem S. Membrane-specific regulation of Cl− channels by purinergic receptors in rat submandibular gland acinar and duct cells. J Biol Chem. 1997a;272:32956–32965. doi: 10.1074/jbc.272.52.32956. [DOI] [PubMed] [Google Scholar]
- Zeng W, Lee MG, Yan M, Diaz J, Benjamin I, Marino CR, Kopito R, Freedman S, Cotton C, Muallem S, Thomas P. Immuno and functional characterization of CFTR in submandibular and pancreatic acinar and duct cells. Am J Physiol Cell Physiol. 1997b;273:C442–455. doi: 10.1152/ajpcell.1997.273.2.C442. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.