Abstract
Background
Although many neuroimaging studies have examined changes in brain function in adults with substance use disorders, far fewer have examined adolescents. This study investigated patterns of brain activation in adolescents with severe substance and conduct problems (SCP) compared to controls.
Methods
Functional magnetic resonance imaging (fMRI) at 1.5 Tesla assessed brain activation in 12 adolescent males with SCP, ranging in age from 14 to 18, and 12 controls similar in age, gender, and neighborhood while performing the attentionally-demanding Stroop task.
Results
Even though the adolescents with SCP performed as well as the controls, they activated a more extensive set of brain structures for incongruent (e.g., “red” in blue ink) versus congruent (e.g. “red” in red ink) trials. These regions included parahippocampal regions bilaterally, posterior regions involved in language-related processing, right-sided medial prefrontal areas, and subcortical regions including the the thalamus and caudate.
Conclusion
These preliminary results suggest that the neural mechanisms of attentional control in youth with SCP differ from youth without such problems. This difficulty may prevent SCP youth from ignoring salient but distracting information in the environment, such as drug-related information.
1. Introduction
This paper is a preliminary investigation of whether adolescents with severe substance and conduct problems (SCP) exhibit atypical patterns of neural activation when they must exert internal control over their behavior in the face of salient distracting information. Among adolescents, substance dependence (SD) and conduct disorder (CD) are often comorbid with vulnerability thought to arise from shared or similar genetic mechanisms (Hicks et al. 2004). Both are associated with “disinhibitory psychopathology” (McGue et al., 2001; van den Bree et al., 1998), including a disregulation of adaptive self-control in the face of strong environmental cues or previous experience (Filmore and Rush, 2002). Experimentally, such disregulation in adults is associated with poor performance on the Stroop task (Mintzer and Stitzer, 2002; Simon et al., 2000), a classic measure of attentional control (MacLeod, 1992). In this task, individuals identify a word’s ink color, while ignoring the word’s meaning. Strong internal control over behavior is required to attend to the ink color and override the more automatic process of reading the word, especially when the word and ink color conflict (e.g., the word “red” in blue ink).
The far less extensive research on attentional control in adolescents with substance problems also suggests that attentional control is compromised (Giancola, et al., 1998), while the data regarding conduct disorder alone are mixed (Bauer and Hesselbrock, 1999 Herba, Tranah, Rubia & Yule, 2006; Oosterlaan et al., 2005). More specifically, poorer attentional abilities, low constructive thinking and high antisocial behavior predict substance use disorders in 14–18 year old adolescent females (Aytaclar et al. 1999; Giancola, et al., 2001, Tapert & Brown, 1999, 2000; Tapert et al. 2002).
The neural bases of these attentional deficits have been relatively unexplored. The few studies examining patterns of brain activation in adolescents with substance use disorder have focused more on memory than attention. In two pilot studies, one with 7 adolescent cannabis users (Jacobsen et al., 2004a) and another with 6 adolescent users of ecstasy (Jacobsen et al., 2004b), greater hippocampal activation relative to a fixation baseline was observed in the substance users than controls. Separately, teens with alcohol use disorders performing a working memory task exhibit greater activation in bilateral posterior parietal cortex and less activation in precuneus and posterior cingulate regions than controls (Tapert et al., 2004). Additional differences in prefrontal regions are observed when alcohol use disorder is comorbid with marijuana use disorder (Schweinsburg et al., 2005) and effects are more pronounced in females than males (Caldwell et al., 2005). Hence, although atypical brain activation is observed in adolescents with substance use disorders, no clear consistent pattern has yet emerged. Moreover, to our knowledge, no imaging studies have been performed on attentional abilities in adolescents with or comorbid for conduct disorder.
In the current study we examine the neural bases of deficits in attentional control in adolescents with severe SCP using functional magnetic resonance imaging (fMRI) and the color-word Stroop task. We use this task because our extensive prior fMRI research with this task (Banich et al., 2000a, 2000b, Milham et al. 2001, 2002, 2003a, 2003b; Milham and Banich, 2005; Liu et al., 2004, 2006) has shown that our procedures are sensitive to detecting the neural substrates of attentional control as well as to group differences in such substrates (Milham et al., 2002). Moreover, this task has yielded differences between adults with and without substance use disorder (Gruber and Yurgelun-Todd, 2005). In that study, two effects were observed in regions typically linked to attentional control. First, the 9 adult, heavy users of cannabis activated mid-cingulate regions for the incongruent Stroop condition (the word “red” displayed in blue ink) relative to a fixation baseline, whereas the 10 non-smoking controls activated more rostral regions of the cingulate. Second, while the dorsolateral prefrontal activity of marijuana users was diffuse and bilateral, that of the controls was more focal and right-sided.
The group of adolescents we examined is characterized by poly-substance abuse as well as co-morbidity with other psychiatric disorders, most notably conduct disorder. This sample clearly cannot be considered a “pure” homogenous group for the purposes of certain research questions (e.g., “Do different drugs of abuse have differential effects on the neural substrates of attentional control in the adolescent brain?”). Nonetheless, the sample recruited very well characterizes the type of adolescent patient we typically treat in our clinical program. As such, the results may provide insight into alterations of neural functioning in such a severely affected group.
2. Method
2.1 Participants
2.1.1 Patient selection
All patients were enrolled in a treatment program for adolescents with serious SCP. Admission into the treatment program required (1) significant conduct and substance problems, (2) judgment by clinical staff of no current psychosis, mental retardation, or risk of homicide, suicide, or arson, (3) no physical illness that would prevent participation in group treatment. All individuals were between the ages of 14 and 18, with English as the primary language. Because severe SCP is much less common in females, our sample was all male.
2.1.2. Control selection
Male controls were recruited by a market research firm and through word of mouth from zip codes well-represented by patients in the treatment program. They were selected to achieve racial/ethnic and age distributions similar to the patients. Potential control participants were excluded if they had any history of court convictions (except minor traffic violations), school expulsions, substance-related arrests, or substance treatment.
2.1.3 Exclusion criteria
Potential participants (both patients and controls) were excluded if they (1) had a history of head injury with loss of consciousness for more than 15 minutes, neurological illness, or history of neurosurgical procedures, (2) were extremely claustrophobic, (3) wore orthodontic braces, (4) had other contraindications to MR scanning, such as intracranial, intraorbital, or intraspinal metal or non-MR-compatible devices, (5) were color-blind, or (6) had body piercings or other jewelry that could not be removed. Procedures were approved by the Colorado Multiple Institutional Review Board.
These criteria led 16 patients and 17 controls to be recruited to participate. Of these, we could not use data from 4 patients (three due to excessive motion and one due to a brain abnormality) and 5 controls (one had undetected agenesis of the corpus callosum, one due to an fMRI scan that was corrupted by reconstruction artifact, two due to excessive motion, and one due to previous brain surgery not revealed during initial evaluation). Hence, we obtained useful data from 12 patients and 12 controls.
2.2 Clinical Assessments
2.2.1 Patients and Controls
All participants answered yes or no to questions regarding symptoms or behaviors of 10 items related to substance abuse/dependence and 15 questions related to CD. This questionnaire allowed us to check, at least according to self-report, that none of the controls had high level of conduct disorder and/or substance abuse/dependence.
2.2.2 Patients Only
The NIMH Diagnostic Interview Schedule for Children-Version IV (DISC-IV) (Shaffer et al., 2000) was given to assess symptoms for various psychiatric disorders based on youth report. Patients were administered the modules for Generalized Anxiety Disorder, Major Depression/Dysthymia, Attention Deficit Hyperactivity Disorder, Oppositional Defiant Disorder, and Conduct Disorder. The Composite International Diagnostic Interview-Substance Abuse Module (CIDI-SAM) (Cottler et al., 1989) was used to provide a measure of DSM-IV diagnoses of SUD for tobacco, alcohol, and 10 different substances of abuse via self-report of symptoms. Only patients completed the DISC-IV and CIDI-SAM as these lengthy assessments of pathology yield a very low prevalence of diagnoses in a sample that has been previously screened for past drug and conduct problems (Crowley et al., 2001). Both instruments were administrated by professional research assistants trained by supervisors who had received special training from the developers of the DISC-IV and CIDI-SAM respectively.
2.3 Procedures
Consent/assent from the adolescent and consent from the parent/guardian (if younger than 18 years) were obtained prior to entering the study. As part of the initial assessment upon entry into the treatment program, patients were administered the DISC-IV and CIDI-SAM and consented to the use of these interviews in the study. Both patients and controls completed the CD/SUD questionnaire. All participants also provided urine and saliva samples for toxicology screening of amphetamine, THC, cocaine, methamphetamine, and alcohol on the same day as the MRI scan. They also completed a brief MRI screen to assure MR compatibility and also to check for previous neurological insult.
2.4. Imaging Protocol
Each participant was involved in one imaging session in which both functional and anatomical images were obtained.
2.4.1. Stimuli
Stimuli were words printed in 36-point font against a black background in different “ink” colors. The ink color and words used were blue, red, yellow, and green. The neutral words were not color-related but like the color words were semantically related (i.e., bond, money, option, invest; mirror, sofa, closet, door).
2.4.2. Equipment
Stimuli were back-projected on a high-resolution screen at the participant’s feet, which was viewed through a mirror mounted on the head coil. To avoid head motion associated with vocal responses that interferes with image registration, as in our prior studies, participants identified the item’s ink color via a button press on a 4-button response device that was interfaced via fiber optic cables to a workstation. E-Prime was used to control stimuli presentation and record responses. Presentation of stimuli was synchronized with the magnet via a trigger pulse.
2.4.3. Procedure & Design
Participants pressed the button corresponding to an item’s ink color. Participants learned the stimulus-response mappings during the anatomical scans (which preceded the functional scans) by viewing trials in which uniformly colored crosses appeared on the screen. They were given enough practice to ensure that they had memorized the stimulus-response mapping. They were given as much practice as was necessary.
The actual experiment required individuals to identify the ink color in which an item was presented. Participants saw three types of trials: neutral words (e.g., “mirror” printed in blue), congruent words (e.g., “red” printed in red), and incongruent words (e.g., “red” printed in blue). These words were viewed in three types of blocks: congruent (C), incongruent (I) and neutral (N). Within each block, half of the trials were the trial type of interest (i.e., congruent, incongruent, neutral), and half were neutral trials that were similar across all blocks (e.g., bond, option, money, invest). These neutral trials were different from the other half of the neutral trials (e.g., mirror, sofa, closet, door) within the neutral block. This 50/50 combination discourages the use of different strategies across the blocks (e.g., just reading the word and ignoring the ink color during congruent blocks).
A blocked design was implemented with each run consisting of two incongruent blocks, two congruent blocks and two neutral blocks with a baseline block (B) at the beginning and the end of each functional run (e.g., BCNICNIB). The baseline condition consisted of lines of x’s and y’s varying from 3 to 6 items in length (e.g. xxy, yyxyxx) shown in one of the four colors. Each block consisted of 16 trials, with each trial lasting 2500 ms (300 ms of a white cross, 1500 ms of stimulus presentation, and 700 ms of inter-stimulus interval) for a total of 135 trials per run (the first block of baseline contained 23 trials). Each participant completed three runs. The order of blocks (NCI) was counterbalanced across the three runs, and the order used for each of the three runs was counterbalanced across participants.
2.4.4. Data Acquisition
A 1.5 T Siemens Vision magnetic resonance imaging system equipped for echo-planar imaging (EPI) was used for data acquisition. EPI images were acquired using the BOLD technique (TR = 2500 ms, TE = 40 ms, flip angle = 90°) each consisting of 20 contiguous axial slices (matrix = 64 × 64, in-plane resolution = 3.75 × 3.75 mm2, thickness = 6 mm, gap = 0.9 mm), parallel to the AC-PC line. Prior to the EPI images, a high-resolution T1-weighted MP-RAGE anatomical set (168 coronal slices of full head, matrix = 256 × 256, field-of-view = 250 × 250 mm2, slice thickness = 1.5 mm, no gap) was collected for each participant. At the end of the experiment, a T1-weighted spin echo data set (20 axial slices, matrix = 512 × 512, field-of-view = 230 × 230 mm2, slice thickness = 6mm, gap = 0.9 mm) was acquired using the same slice angles as the EPI images.
2.4.5. Image Pre-processing
Prior to statistical analysis, the first seven volumes of each run were discarded to allow the MR signal to reach steady state. The remaining images in each participant’s time series were motion corrected using MCFLIRT module of FSL (FMRIB’s Software Library, v3.1) package (http://www.fmrib.ox.ac.uk/fsl). Images in the data series were then spatially smoothed with a 3D Gaussian kernel (FWHM = 8×8×8 mm) and temporally filtered using a high-pass filter (1.5 repetition cycle). The FEAT (FMRIB’s Expert Analysis Tool) module of FSL was used for these steps and later statistical analysis.
2.4.6. Analysis on individual participant
First, customized square waveforms for each participant were generated for the individual’s specific counterbalanced order of experimental conditions. These waveforms were convolved with a double gamma hemodynamic response function. For each participant, we used FILM (FMRIB’s Improved Linear Model) to estimate the hemodynamic parameters for the different explanatory variables (EVs). Each EV represented a different condition (i.e., incongruent, congruent, neutral). Then statistical contrast maps were generated for the two contrasts of interest. One contrast identifies brain regions that are more activated when the stimuli contain two sources of color information, one in the ink color and one in the word’s meaning (incongruent and congruent blocks) as compared to just one source of color information, that contained in the word (neutral blocks). The other contrast identifies brain regions that are more activated when the two competing sources of color information conflict (incongruent blocks) as compared to when they do not (congruent blocks). Our prior research has shown independent effects for these two contrasts (Milham et al. 2002; Milham and Banich, 2005). After statistical analysis for each subject’s time series, contrast maps were warped into common stereotaxic space before mixed-effects group analyses were performed. This involved registering average EPI image to the T1 spin echo image, then to the MP-RAGE image, and finally to the ICBM152 T1 template using FLIRT (FMRIB’s Linear Image Registration Tool) module of FSL package.
2.4.7. Group Comparisons
Whole-brain group analyses were performed with FLAME (FMRIB’s Local Analysis of Mixed Effects). Afterwards we used the cluster tool in FSL to identify clusters in which the peak activation value differed significantly from zero (Z>2.81, p<0.0025 one-tailed, uncorrected). Then we employed AFNI’s AlphaSim program (http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf) to control for false positives. With the parameters of our analyses (e.g., FWHM smoothing), we ran 1000 Monte Carlo simulations to estimate the overall significance level (probability of a false detection) for thresholding the 3D functional z-map image over the entire volume regardless of activation within that map. These simulations indicated that a cluster size of 110 in combination with a Z-value of 2.81 (p<.0025) provided an overall family-wise false positive rate of.05, while cluster sizes of 99 and 84 provide a false positive rate of.10 and.20, respectively. Since this is a pilot study, we report all clusters above 50 voxels in prefrontal regions, as these regions are strongly implicated in attention and/or substance abuse, and all in subcortical regions, since these areas compromise a much smaller volume of brain tissue and a cluster thresholding may be overly conservative.
3. Results
3.1. Sample Characteristics
Table 1 presents the sample characteristics. Patients had been in treatment a mean of 82 days (range 20–209) before scanning. Unpaired t-tests revealed no group differences in age (t(22) =.327, p=.438). As expected, the patients reported significantly more symptoms than control of substance use disorder (t(22) = 8.58, p <.005), and conduct disorder (t(22) = 5.25, p <.05). Mean symptom counts on the CIDI-SAM are considerably higher than the substance symptom counts on the CD/SUD Self-Report Questionnaire because CIDI-SAM asks about each dependence symptom, drug-by-drug, while the questionnaire assessed whether symptoms had occurred “because of my drug/alcohol use” across all substances. Of note, the patients’ symptom counts on the CIDI-SAM and those endorsed on the questionnaire correlated significantly (r = 0.820; p < 0.001), as did patients’ CD symptom counts on the DISC and those endorsed on the questionnaire (r = 0.766; p < 0.005). Seven of the 12 patients met full DSM-IV criteria on the DISC for past-year CD, and 9 for lifetime CD. Five of the patients did not have a lifetime diagnosis of CD, despite being referred to, and accepted in, a treatment program focused on youth with serious conduct and substance problems. This sometimes occurs because youths have had strict legal supervision for some time, or because of under-reporting of symptoms at interview. On the CIDI-SAM, all patients had dependence diagnoses on one or more non-tobacco substances (See Table 2). On self-report from the DISC, two patients had diagnoses of attention deficit hyperactivity disorder. However, we previously have shown that self-report for ADHD in patients like these yields suspiciously low prevalence rates (Crowley et al., 2001).
Table 1.
Patient and Control Characteristics
| Age | CD Symptoms | SUD Symptoms | DISC CD Symptoms | CIDI-SAM Dependence Symptoms | |
|---|---|---|---|---|---|
| Patients | 16.7 (1.02) | 5.33 (2.50) | 6.58 (2.61) | 4.58(3.26) | 13.17(5.31) |
| Controls | 16.5 (.99) | .92 (1.51) | .08 (.29) | -- | -- |
Mean value with standard deviation in parentheses. Conduct Disorder (CD) and Substance Use Disorder (SUD) symptoms as assessed by a self-report questionnaire. Diagnostic Interview Schedule for Children (DISC) whole life CD symptom count and the sum of Composite International Diagnostic Interview- Substance Abuse Module (CIDI-SAM) DSM-IV dependence symptoms.
Table 2.
Drug Abuse or Dependence for Patients for Specific Substances
| No Diagnosis | Abuse | Dependence | |
|---|---|---|---|
| Tobacco | 3 | 0 | 9 |
| Alcohol | 5 | 5 | 2 |
| Cannabis | 2 | 2 | 8 |
| Cocaine | 9 | 0 | 3 |
| Amphetamines | 7 | 1 | 4 |
| Hallucinogens | 11 | 0 | 1 |
| Inhalants | 11 | 1 | 0 |
| Opiates | 12 | 0 | 0 |
| PCP | 12 | 0 | 0 |
| Sedatives | 12 | 0 | 0 |
| Club drugs | 11 | 0 | 1 |
3.2. Behavioral data
Due to technical difficulties, we lost behavioral data on one of the patients. A repeated-measures analysis of variance with the factors of TRIAL TYPE (incongruent, congruent, neutral) and GROUP (patients, controls) was performed on data for the remaining 11 patients and 12 controls. Of note, performance between the groups did not differ, as analyses did not yield a main effect of GROUP nor any interaction with the factor of GROUP. As such, performance level does not confound our blocked fMRI comparison across groups.
3.2.1. Reaction time
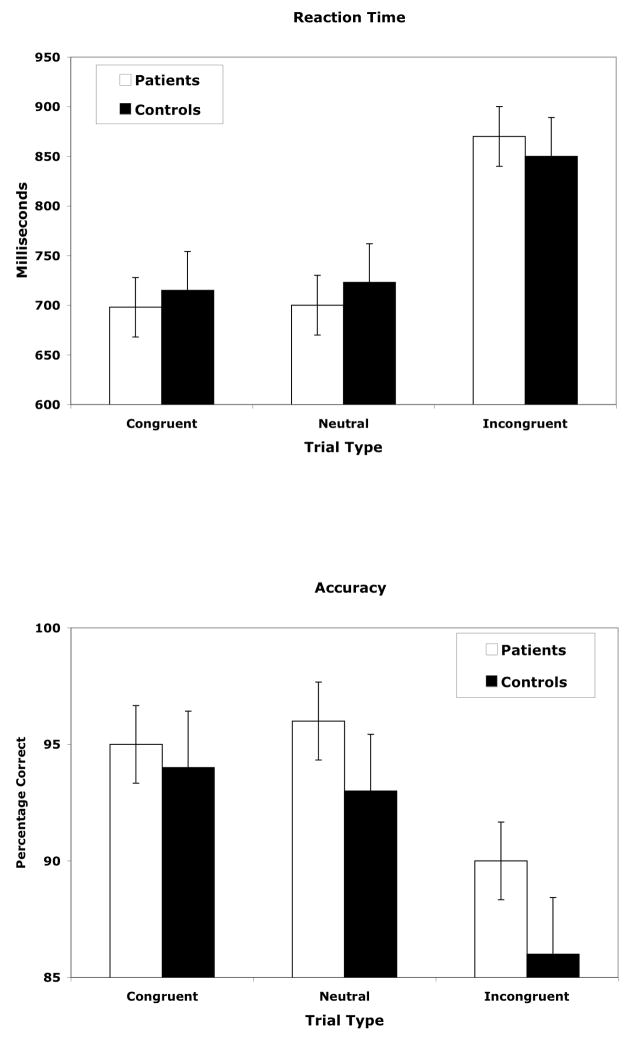
This analysis yielded only a significant main effect of TRIAL TYPE [F(2,42)=81.89, p<.0001] with post-hoc tests indicating that mean RT for incongruent trials (860 ms) was significantly longer (p<.0001 in both cases) than for congruent (707 ms) or neutral trials (712 ms), which did not differ significantly from each other (see Figure 1a).
Figure 1. Behavioral results for the SCP group and controls.
a) Reaction Time b) Accuracy. Error bars indicate + and – one standard error of the mean.
3.2.2. Accuracy
This analysis yielded only a main effect of TRIAL TYPE [F(2,42)=21.03, p<.0001] with post-hoc tests indicating that accuracy for incongruent trials (88%) was significantly less (p<.001) than for either congruent (95%) or neutral trials (94%), which did not differ significantly from each other (see Figure 1b).
3.3. fMRI data
3.3.1. Incongruent & Congruent > Neutral
The only significant group difference for this contrast was greater activity in the left dorsomedial region of the thalamus in patients than controls (see Table 3).
Table 3.
Clusters within regions that exhibit significant group differences for the contrast of incongruent & congruent trials > neutral trials.
| Region | BA | max Z | Coordinates | # of voxels | Source |
|---|---|---|---|---|---|
| Controls>Patients | |||||
| (None) | |||||
| Patients>Controls | |||||
| Thalamus (L) | 3.85 | −8 −18 0 | 41 | Patient activation |
BA=Brodmann area; max Z=maximum Z value of activation for peak; Coordinates= x,y,z MNI coordinates of the peak; # of voxels = number of voxels in the cluster; Source = Source of group difference
3.3.2. Incongruent >Congruent
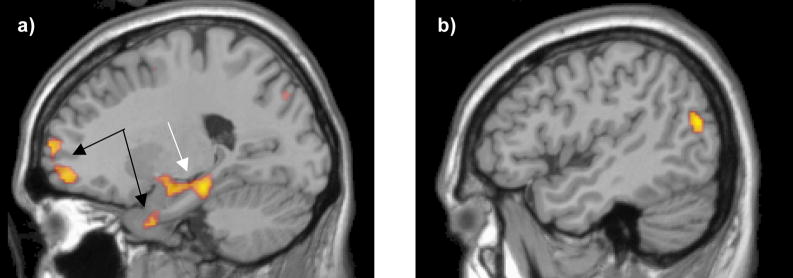
All group differences for this contrast also occurred because of greater activation in patients than controls (See Table 4; Figure 2).
Table 4.
Clusters within regions that exhibit significant group differences in activation for the contrast of incongruent > congruent trials.
| Region | BA | maxZ | Coordinates | # of voxels | Source |
|---|---|---|---|---|---|
| Controls>Patients | |||||
| (None) | |||||
| Patients>Controls | |||||
| Temporal Regions | |||||
| Uncus/Superior Temporal Gyrus (L) | 38 | 3.95 | −24, 2, −32 | 120 | Control deactivation |
| Parahippocampal Gyrus (L) | 35/36 | 3.75 | −18, −28, −16 | 79 | Patient activation Control non-sig deact |
| Parahippocampal Gyrus (L) | 34 | 3.3 | −18, −8, −18 | 68 | Patient non-sig activation Control non-sig deact |
| Parahippocampal Gyrus (R) | 35 | 3.52 | 20, −18, −16 | 105 | Patient activation Control non-sig deact |
| Middle Temporal Gyrus (L) | 39 | 3.84 | −44, −72, 18 | 80 | Patient activation |
| Frontal Regions | |||||
| Superior Frontal Gyrus (L) | 10 | 3.75 | −19, 66, 10 | 54 | Control deactivation |
| Superior Frontal Gyrus (L) | 11 | 3.39 | −18, 54, −10 | 55 | Control deactivation |
| Subcortical Regions | |||||
| Caudate (L) | 3.39 | −8, 20, 12 | 54 | Control deactivation |
BA=Brodmann area; max Z= maximum Z value of activation for peak; Coordinates= x,y,z MNI coordinates of the peak; # of voxels=Number of voxels in the cluster Source = Source of group difference
Figure 2. Areas yielding significantly greater activation for the SCP group than controls.
a) Slice shown at x = −21 (MNI space). Prefrontal regions and regions of the uncus denoted by the black arrows yielded a group differences because these regions were deactivated in controls but not patients. Parahippocampal and amygdalar regions denoted by the white arrow yielded a group difference because they were activated in patients b) Slice shown at x = −47 (MNI space). This lateral left temporal region yielded a group difference because of activation by patients but not controls.
3.3.2.1 Temporal regions
The most notable group difference was observed for parahippocampal regions. These differences were quite extensive across the entire length of the left hippocampus and included portions of the left amygdala as well. There were two peaks within this band of activation, one more anterior and one more posterior. In contrast, group differences in activation in right parahippocampal regions were not as extensive. These parahippocampal regions are typically considered to be involved in memory processing (e.g., Schon et al. 2004). These group differences, as well as the separate peak in the left middle temporal gyrus were driven by activation for the patients but not the controls.
3.3.2.2 Frontal regions
Two regions of the left medial frontal cortex yielded group differences, although the cluster sizes for both were somewhat small and below threshold for a high degree of protection against a false positive. The group difference arose because these regions were deactivated in the controls but not the patients.
3.3.2.3 Subcortical regions
Differences were noted in the caudate nucleus bilaterally but with a greater spatial extent in the left hemisphere. Like the frontal regions, these differences resulted from deactivation in controls but not patients.
4. Discussion
4.1. General Discussion
The results of this pilot study provide preliminary evidence that the neural systems engaged by adolescents with severe substance and conduct problems during an attentionally demanding task differ from demographically-similar controls, even when their behavioral performance does not differ. Hence, the observed differences in brain activation cannot simply be attributed to unequal abilities to perform the task.
The contrast between conflicting and non-conflicting color information (incongruent > congruent) showed that patients needed to engage prefrontal brain regions outside of DLPFC to a greater extent than controls to obtain the same level of performance. The present results differ from those of Gruber and Yurgelen-Todd (2005) who reported differences in regions of activation in DLFPC between heavy adult users of marijuana and controls. In an attempt to understand this discrepancy, we inspected the maps for each group individually. These revealed that both groups significantly activated dorsolateral prefrontal (DFLPC) and inferior parietal regions, which are consistently engaged during performance of the Stroop task (e.g., Banich et al., 2000a,b). Thus, it appears that both groups can activate the relevant neural machinery.
Yet other evidence in our data suggests that this neural machinery may not exert its effect as efficiently or as strongly for patients compared to controls. In particular, patients exhibited extensive activation of parahippocampal regions. These regions were not activated by controls, and we have found in numerous prior studies in our laboratory with neurologically-intact individuals that these regions tended to be deactivated at subthreshold levels (Banich et al., unpublished observations). Hippocampal regions bind together or conjoin information from different processing streams in the brain (O’Reilly & Rudy, 2001). Hence, deactivation of these regions is likely to be advantageous on incongruent trials by keeping the conflicting word from being linked with the ink color.
Unlike the increased hippocampal activity observed by Jacobsen and colleagues in adolescents who abuse drugs (Jacobsen et al. 2004 a,b), ours cannot be explained by the patients having difficulty and/or exerting more effort during information encoding. The incongruent and congruent conditions require information to be encoded to an equivalent degree, yet we observe differences in parahippocampal activity. Thus, we speculate that in the face of conflicting information, prefrontal regions that exert attentional control do a poor job of modulating the activity of parahippocampal regions (See Anderson, et al., 2004 for an example of such top-down prefrontal modulation of hippocampal activity).
Other data in our study is suggestive of greater processing of task-irrelevant material in the patients as compared to controls. In particular, the patients showed activation of brain regions involved in linguistic processing for incongruent than congruent trials. Such activation is not advantageous because greater processing of the word on incongruent trials would increase its potential to interfere with the correct response.
Group differences also arose because the patients, unlike controls, did not deactivate orbitofrontal regions or the caudate. These structures are implicated in emotion and reward-related processes. The reason for these group differences are not clear, but they are interesting as these regions are often thought to be dysfunctional in individuals with substance use disorders (see Volkow and Fowler, 2000). It may be that the conflict in the incongruent trials engenders activation of emotional circuitry and/or the interplay between regions involved in attention and emotion are disregulated in adolescents with severe SCP.
It is unlikely that the group differences in the patterns of brain activation we observed are generated either by a functional immaturity of the brain in the patients or by group differences in brain morphology. The one study of developmental patterns of brain activation in the Stroop task assessed by fMRI (Adelman et al., 2002) yielded patterns of activation for younger children distinct from what we observed for either group. Furthermore, optimized voxel based morphometry on this same sample of patients and controls did not yield significant group differences in grey matter volume in any of the regions in which we observed activation differences (Milham et al. submitted). Hence it does not appear that greater activation observed in the patients is a compensatory mechanism of a lesser volume of brain tissue working harder to achieve the same end.
4.2. Clinical Implications
Our results provide preliminary evidence that brain systems involved in attentional control are functioning atypically in youth with SCP. It is not clear from our data whether these neural irregularities are a predisposing factor in patients with severe, comorbid conduct and substance problems, or whether they result from the prior ingestion of illicit substances. Regardless, our findings suggest that adolescents with SCP may have difficulty ignoring potent information that should be overlooked. If so, these youth may be especially poor at disregarding salient drug-related information. Said differently, when information that should be ignored is within “sight”, the attentional system of these youth may be unable to keep that information out of mind.
4.3. Limitations
The results of the present study should be viewed as preliminary. The number of individuals in each group was relatively small and replication is needed. The symptomatology of these patients was rather severe with regards to substance use and conduct problems. Hence, it is not clear whether similar results would be obtained in a group of adolescents whose substance use is not as long-standing or as frequent. Because these patients do exhibit severe problems, abuse multiple substances, and in some cases may have co-morbidity with other psychiatric disorders, it is not possible to isolate the source of the pattern we observe to any one of these factors. Nonetheless, these patients represent a population that is often seen in treatment settings, and for that reason knowing about the neural systems underlying their attentional abilities is of importance.
4.4. Conclusion
In conclusion, the present results suggest that even when performing at an equivalent level on a classic task of attentional control, adolescents with SCP engage brain regions that vary substantially from controls. They show extensive bilateral hippocampal activation and also increased activation in brain regions processing task-irrelevant information. Finally, although our task did not involve emotional stimuli, patients also showed increased activation in regions involved in emotion and reward-related processing. Our results are suggestive of a diffuse disregulation of attentional control, which may be associated with the disinhibitory syndrome often observed in adolescents with severe substance and conduct problems. In addition, this pilot study demonstrates that it is feasible to image teenage patients with severe substance and conduct problems in order to provide greater insight into the brain bases of their disorder.
Acknowledgments
This research was supported by NIDA grants DA-011015, DA-012845, DA-009842 to the second author.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. References
- Adleman NE, Menon V, Blasey CM, White CD, Warsofsky IS, Glover GH, Riess AL. A developmental fMRI study of the Stroop color-word task. Neuroimage. 2002;16:61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- Anderson MC, Ochsner KN, Kuhl B, Cooper J, Robertson E, Gabrieli SW, Glover GH, Gabrieli JD. Neural systems underlying the suppression of unwanted memories. Science. 2004;303:232–235. doi: 10.1126/science.1089504. [DOI] [PubMed] [Google Scholar]
- Aytaclar S, Tarter RE, Kirisci L, Lu S. Association between hyperactivity and executive cognitive functioning in childhood and substance use in early adolescence. J Am Acad Child and Adolesc Psychiatry. 1999;38:173–178. doi: 10.1097/00004583-199902000-00016. [DOI] [PubMed] [Google Scholar]
- Banich MT, Milham MP, Atchley RA, Cohen NJ, Webb A, Wszalek T, Kramer AF, Liang Z-P, Wright A, Shenker J, Magin R, Barad V, Gullett D, Shah C, Brown C. fMRI studies of Stroop tasks reveal unique roles of anterior and posterior brain systems in attentional selection. J Cogn Neurosci. 2000a;12:988–1000. doi: 10.1162/08989290051137521. [DOI] [PubMed] [Google Scholar]
- Banich MT, Milham MP, Atchley RA, Cohen NJ, Webb A, Wszalek T, Kramer AF, Liang Z-P, Barad V, Gullett D, Shah C, Brown C. Prefrontal regions play a predominant role in imposing an attentional “set”: Evidence from fMRI. Cogn Brain Res. 2000b;10:1–9. doi: 10.1016/s0926-6410(00)00015-x. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock WM. Subtypes of family history and conduct disorder: effects on P300 during the stroop test. Neuropsychopharmacology. 1999;21:51–62. doi: 10.1016/S0893-133X(98)00139-0. [DOI] [PubMed] [Google Scholar]
- Caldwell LC, Schweinsburg AD, Nagel BJ, Barlett VC, Brown SA, Tapert SF. Gender and adolescent alcohol use disorders on BOLD (Blood Oxygen Level Dependent) response to spatial working memory. Alcohol Alcohol. 2005;40:194–200. doi: 10.1093/alcalc/agh134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley TJ, Mikulich SK, Ehlers KM, Whitmore EA, Macdonald MJ. Validity of structured clinical evaluations in adolescents with conduct and substance problems. J Am Acad Child Adolesc Psychiatry. 2001;40:265–273. doi: 10.1097/00004583-200103000-00005. [DOI] [PubMed] [Google Scholar]
- Cottler LB, Robins LN, Helzer JE. The reliability of the CIDI-SAM: A comprehensive substance abuse interview. Br J Addict. 1989;84:801–814. doi: 10.1111/j.1360-0443.1989.tb03060.x. [DOI] [PubMed] [Google Scholar]
- Filmore MT, Rush GR. Impaired inhibitory control of behavior in chronic cocaine users. Drug Alcohol Depend. 2002;66:265–273. doi: 10.1016/s0376-8716(01)00206-x. [DOI] [PubMed] [Google Scholar]
- Giancola PR, Mezzich AC, Tarter RE. Disruptive, delinquent and aggressive behavior in female adolescents with a psychoactive substance use disorder: Relation to executive cognitive functioning. J Stud Alcohol. 1998;59:560–567. doi: 10.15288/jsa.1998.59.560. [DOI] [PubMed] [Google Scholar]
- Giancola PR, Shoal GD, Mezzich AC. Constructive thinking, executive functioning, antisocial behavior, and drug use involvement in adolescent females with a substance use disorder. Exper Clin Psychopharmacol. 2001;9:215–227. doi: 10.1037//1064-1297.9.2.215. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Yurgelun-Todd DA. Neuroimaging of marijuana smokers during inhibitory processing: A pilot investigation. Cogn Brain Res. 2005;23:107–118. doi: 10.1016/j.cogbrainres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Herba CM, Tranah T, Rubia K, Yule W. Conduct problems in adolescence: three domains of inhibition and effect of gender. Dev Neuropsychol. 2006;30:659–95. doi: 10.1207/s15326942dn3002_2. [DOI] [PubMed] [Google Scholar]
- Hicks BM, Krueger RF, Iacono WG, McGue M, Patrick CJ. Family transmission and heritability of externalizing disorders: A twin-family study. Arch Gen Psychiatry. 2004;61:922–928. doi: 10.1001/archpsyc.61.9.922. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Mencl WE, Westerveld M, Pugh KR. Impact of cannabis use on brain function in adolescents. Ann N Y Acad Sci. 2004a;1021:384–390. doi: 10.1196/annals.1308.053. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Mencl WE, Pugh KR, Skudlarski P, Krystal JH. Preliminary evidence of hippocampal dysfunction in adolescent MDMA (“ecstasy”) users: possible relationship to neurotoxic effects. Psychopharmacology. 2004b;173:383–390. doi: 10.1007/s00213-003-1679-4. [DOI] [PubMed] [Google Scholar]
- Liu X, Banich MT, Jacobson BL, Tanabe JL. Common and distinct neural substrates of attentional control in an integrated Simon and spatial Stroop task as assessed by event-related fMRI. Neuroimage. 2004;22:1097–106. doi: 10.1016/j.neuroimage.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Liu X, Banich MT, Jacobson BL, Tanabe JL. Functional dissociation of attentional selection within PFC: response and non-response related aspects of attentional selection as ascertained by fMRI. Cereb Cortex. 2006;16:827–34. doi: 10.1093/cercor/bhj026. [DOI] [PubMed] [Google Scholar]
- MacLeod CM. The Stroop task: The “gold standard” of attentional measures. J Exp Psychol. 1992;121:12–14. [Google Scholar]
- McGue M, Pickens RW, Svikis DS. Sex and age effects on the inheritance of alcohol problems: a twin study. J Abnorm Psychol. 1992;101:3–17. doi: 10.1037//0021-843x.101.1.3. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT. Anterior cingulate cortex: an fMRI analysis of conflict specificity and functional differentiation. Hum Brain Mapp. 2005;25:328–335. doi: 10.1002/hbm.20110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Barad V. Competition for priority in processing increases prefrontal cortex’s involvement in top-down control: an event-related fMRI study of the Stroop task. Cogn Brain Res. 2003a;17:212–222. doi: 10.1016/s0926-6410(03)00108-3. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Claus E, Cohen N. Practice-related effects demonstrate complementary roles of anterior cingulate and prefrontal cortices in attentional control. Neuroimage. 2003b;18:483–493. doi: 10.1016/s1053-8119(02)00050-2. [DOI] [PubMed] [Google Scholar]
- Milham MP, Crowley TJ, Thompson LL, Raymond KM, Claus ED, Banich MT. Reduction in orbitofrontal brain volume in adolescents with severe substance and conduct problems submitted. [Google Scholar]
- Milham MP, Banich MT, Webb A, Barad V, Cohen NJ, Wszalek T, Kramer AF. The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Cogn Brain Res. 2001;12:467–473. doi: 10.1016/s0926-6410(01)00076-3. [DOI] [PubMed] [Google Scholar]
- Milham MP, Erickson KI, Banich MT, Kramer AF, Webb A, Wszalek T, Cohen NJ. Attentional control in the aging brain: Insights from an fMRI study of the stroop task. Brain Cogn. 2002;49:277–296. doi: 10.1006/brcg.2001.1501. [DOI] [PubMed] [Google Scholar]
- Mintzer M, Stitzer M. Cognitive impairment in methadone maintenance patients. Drug Alcohol Depend. 2002;67:41–51. doi: 10.1016/s0376-8716(02)00013-3. [DOI] [PubMed] [Google Scholar]
- Oosterlaan J, Scheres A, Sergeant JA. Which executive functioning deficits are associated with AD/HD, ODD/CD and comorbid AD/HD+ODD/CD? J Abnorm Child Psychol. 2005;33:69–85. doi: 10.1007/s10802-005-0935-y. [DOI] [PubMed] [Google Scholar]
- O’Reilly RC, Rudy JW. Conjunctive representations in learning and memory: principles of cortical and hippocampal function. Psychol Rev. 2001;108:311–345. doi: 10.1037/0033-295x.108.2.311. [DOI] [PubMed] [Google Scholar]
- Schon K, Hasselmo ME, Lopresti ML, Tricarico MD, Stern CE. Persistence of parahippocampal representation in the absence of stimulus input enhances long-term encoding: a functional magnetic resonance imaging study of subsequent memory after a delayed match-to-sample task. J Neurosci. 2004;24:11088–11097. doi: 10.1523/JNEUROSCI.3807-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Cheung EH, Brown GG, Brown SA, Tapert SF. FMRI to spatial working memory in adolescents with comorbid marijuana and alcohol use disorders. Drug Alcohol Depend. 2005;79:201–210. doi: 10.1016/j.drugalcdep.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Simon SL, Domier C, Carnell J, Brethen P, Rawon R, Ling W. Cognitive impairment in individuals currently using methamphetamine. Am J Addict. 2000;9:222–231. doi: 10.1080/10550490050148053. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown SA. Neuropsychological correlates of adolescent substance abuse: four year outcomes. J Int Neuropsychol Soc. 1999;5:475–487. doi: 10.1017/s1355617799566010. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown SA. Substance dependence, family history of alcohol dependence and neuropsychological functioning in adolescence. Addict. 2000;95:1043–1053. doi: 10.1046/j.1360-0443.2000.95710436.x. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Baratta MV, Abrantes AM, Brown SA. Attention dysfunction predicts substance involvement in community youth. J Am Acad Child Adolesc Psychiatry. 2002;41:680–686. doi: 10.1097/00004583-200206000-00007. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Barlett VC, Brown GG, Brown SA, Frank LR, Meloy MJ. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcoh Clin Exp Res. 2004;28:1577–1586. doi: 10.1097/01.alc.0000141812.81234.a6. [DOI] [PubMed] [Google Scholar]
- van den Bree MBM, Johnson EO, Neale MC, Pickens RW. Genetic and environmental influences on drug use and abuse/dependence in male and female twins. Drug Alcohol Depend. 1998;52:231–241. doi: 10.1016/s0376-8716(98)00101-x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–25. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Vollm B, Richardson P, Stirling J, Elliott R, Dolan M, Chaudhry I, Del Ben C, McKie S, Anderson I, Deakin B. Neurobiological substrates of antisocial and borderline personality disorder: preliminary results of a functional fMRI study. Crim Behav Mental Health. 2004;14:39–54. doi: 10.1002/cbm.559. [DOI] [PubMed] [Google Scholar]