Abstract
Aim of the study
Parkinson’s disease is a neurological disorder mostly effecting the elder population of the world. Currently there is no definitive treatment or cure for this disease. Therefore, in this study the composition and constituents of the aqueous extract of B. caapi for monoamine oxidases (MAO) inhibitory and antioxidant activities were assessed, which are relevant to the prevention of neurological disorders, including Parkinsonism.
Materials and methods
The aqueous extract of B. caapi stems was standardized and then fractionated using reversed-phase (RP) chromatography. Pure compounds were isolated either by reversed-phase (RP) chromatography or centrifugal preparative TLC, using a Chromatotron®. Structure elucidation was carried out by 1D and 2D NMR, Mass, IR and Circular Dichroism spectroscopy and chemical derivatization. Chemical profiling of the extract was carried out with RP-HPLC. The inhibitory activity of MAO-A, MAO-B, acetylcholinesterase, butyrylcholinesterase and catechol-O-methyl transferase enzymes, as well as antioxidant and cytotoxic activities of both B. caapi extract and isolated compounds were evaluated.
Results
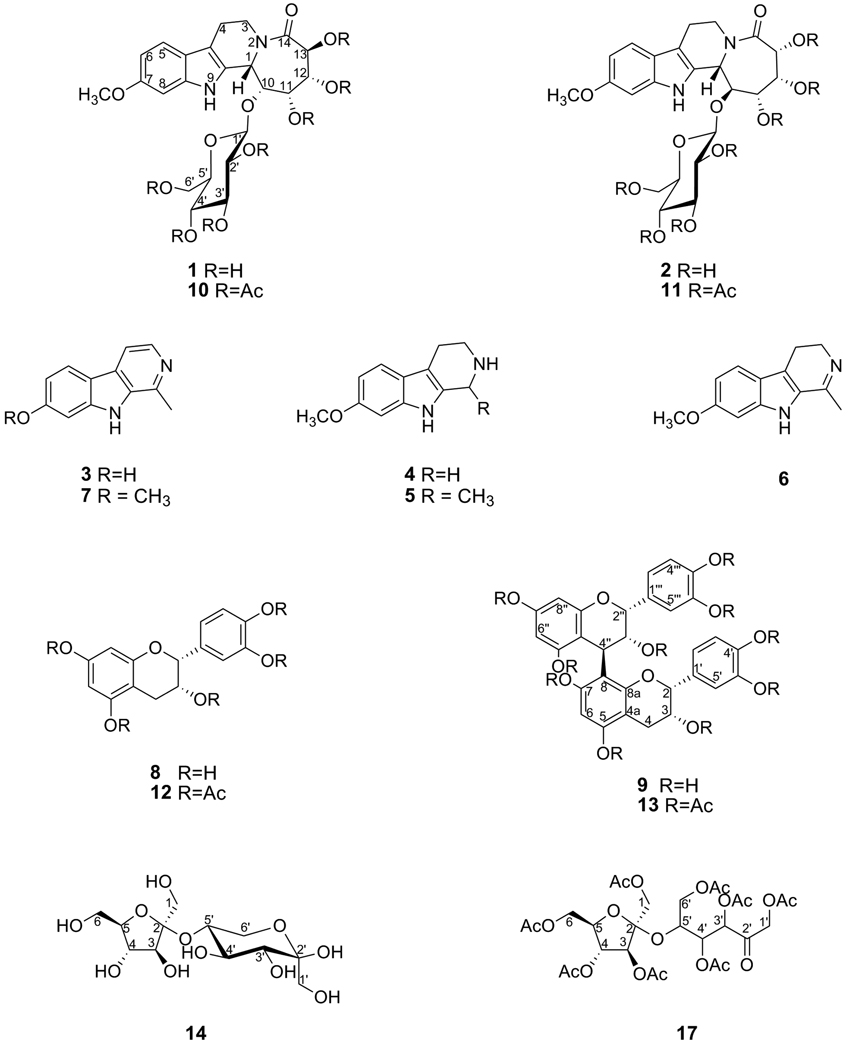
An examination of the aqueous extracts of B. caapi cultivar Da Vine yielded two new alkaloidal glycosides, named banistenoside A (1) and banistenoside B (2), containing “azepino[1,2-a]tetrahydro-β-carboline” unique carbon framework. One additional new natural tetrahydronorharmine (4), four known β-carbolines harmol (3), tetrahydroharmine (5), harmaline (6) and harmine (7), two known proanthocyanidines (−)-epicatechin (8) and (−)-procyanidin B2 (9), and a new disaccharide β-D-fructofuranosyl-(2→5)-fructopyranose (14) together with known sacharose (15) and β-D-glucose (16) were also isolated. In addition, the acetates of 1, 2, 8, 9, 14 and 15 (compounds 10–13, 17, 18) were also prepared. Harmaline (6) and harmine (7) showed potent in vitro inhibitory activity against recombinant human brain monoamine oxidase (MAO) -A and -B enzymes (IC50 2.5 and 2.0 nM, and 25 and 20 µM, respectively), and (−)-epicatechin (8) and (−)-procyanidin B2 (9) showed potent antioxidant and moderate MAO-B inhibitory activities (IC50 <0.13 and 0.57 µg/mL, and 65 and 35 µM). HPLC analysis revealed that most of the dominant chemical and bioactive markers (1, 2, 5, 7–9) were present in high concentrations in dried bark of large branch. Analysis of regular/commercial B. caapi dried stems showed a similar qualitative HPLC pattern, but relatively low content of dominant markers 1, 2, 7, and 9, which led to decreased MAO inhibitory and antioxidant potency.
Conclusion
Collectively, these results give additional basis to the existing claim of B. caapi stem extract for the treatment of Parkinsonism, including other neurodegenerative disorders.
Keywords: Banisteriopsis caapi (Spruce ex Griseb.), β-carboline alkaloids, Proanthocyanidines, Monoamine oxidase inhibitors, Antioxidants, Parkinson’s disease
1. Introduction
Banisteriopsis (Family: Malpighiaceae) is a tropical South American genus with 92 species distributed mainly in Brazil, Bolivia, Colombia, Ecuador, and Peru (Mabberley, 1997; Schultes, 1970). B. caapi (Spruce ex Griseb.) Morton (Vine of the Soul) (Schultes and Raffauf, 1992) is an ingredient of the popular sacred and psychoactive drinks Ayahuasca, also known as Caapi, Pinde, Natema or Yaje, which is widely used for prophecy, divination, and as a sacrament in the northern part of South America (Schultes, 1970; Schultes and Siri von Reis, 1995). However, to the best of our knowledge, no traditional drink prepared only from B. caapi has been consumed for such uses. Earlier chemical investigation have reported the presence of β-carboline alkaloids (β-CA) harmine, harmaline and tetrahydroharmine (THH) as the principal MAO inhibitors, together with other β-CA’s, from B. caapi (Hochstein and Paradies, 1957; Hashimoto and Kawanishi, 1975; ibid 1976; Callaway et al., 2005). In addition, two pyrrolidines, shihunine and (S)-(+)-dihydroshihunine (Kawanishi et al., 1982), and terpenoids (Aquino et al., 1991) were also reported. The alkaloid content of B. caapi was determined previously by GC/MS (Rivier and Lindgren, 1972), LC/MS (Kawanishi et al., 1998), and HPLC (Serrano-Duenas et al., 2001), suggesting the content of harmine is highest among β-CA’s, followed by THH and harmaline.
Parkinson’s disease (PD) is caused by a loss of neurons from substantia nigra of the brain. Once damaged, these neurons stop producing dopamine and compromise the brain's ability to control movement. It is not known what damages certain neurons in PD patients. One reason is that free radicals/ toxic particles normally deactivated in the body are responsible, which can be controlled by antioxidants as adjuvant with dopamine agonist or MAO inhibitors. The usefulness of B. caapi was established for alleviating symptoms of PD (Serrano-Duenas et al., 2001), which contains MAO inhibitor harmine as active constituent used in PD treatment (Sanchez-Ramos, 1991). A double-blind, randomized placebo-controlled trial of B. caapi, using a single dose, revealed a significant improvement in motor function of PD patients (Serrano-Duenas et al., 2001). Tests for MAO inhibition using liver homogenate showed that B. caapi stem extract and harmine showed a concentration-dependent inhibition of MAO-A, and an increase in release of dopamine from rat striatal slices (Schwartz et al., 2003).
It should be noted that the identities of different Banisteriopsis species are incompletely known due to the paucity of fertile collections and lack of detailed taxonomic study. There are at least thirty different B. caapi that natives of Amazon have knowledge of and have different uses (Schultes and Hofman, 1992). During the course of chemical and biological standardization of B. caapi under an NIH funded investigation at the NCNPR for neurological disorders relevant to Parkinsonism, an extract of B. caapi cultivar Da Vine, collected in Oahu, Hawaii, demonstrated potent in vitro MAO-A inhibitory and antioxidant activities. This led to the bioassay guided isolation of two new β-carboline alkaloidal glycosides, banistenoside A (1) and banistenoside B (2), a new tetrahydronorharmine (4), four known β-carbolines harmol (3), tetrahydroharmine (5), harmaline (6) and harmine (7), two proanthocyanidines (−)-epicatechin (8) and (−)-procyanidin B2 (9), and a new disaccharide β-D-fructofuranosyl-(2→5)-fructopyranose (14) (Figure 1), using regular and RP silica gel chromatography. In this paper, we report the isolation, characterization and bioactivities of isolated compounds, and HPLC analysis of B. caapi cultivar Da Vine and two regular/ commercial samples of B. caapi.
Figure 1.
Chemical structures of compounds isolated from Banisteriopsis caapi.
2. Materials and methods
2.1. General experimental procedures
Optical rotations were measured in CHCl3 or MeOH using an AUTOPOL IV® instrument at ambient temperature; IR spectra were obtained using a Bruker Tensor 27 FTIR instrument; Circular Dichroism (CD) spectra were recorded on a Olis DCM 20 CD spectrometer. The HPLC system consisted of a Model 2695 Alliance Separations Module equipped with a 2996 photodiode array detector, and a computerized data station equipped with Waters Empower 2 software (Waters, Milford, MA), using a Gemini C18 110 Å column (Phenomenex, 150 × 4.6 mm I.D.; 5 µm particle size; Phenomenex Inc., Torrance, CA, USA) and operated at 30 °C. The column was equipped with a 2 cm LC-18 guard column (Phenomenex Inc., Torrance, CA, USA). The NMR spectra were acquired on a Bruker Avance DRX-400 instrument at 400 MHz (1H), 100 (13C) in CDCl3 or CD3OD, using the residual solvent as int. standard; multiplicity determinations (DEPT) and 2D NMR spectra (COSY, HMQC, HMBC and NOESY) were obtained using standard Bruker pulse programs; HRMS were obtained by direct injection using a Bruker Bioapex-FTMS with electro-spray ionization (ESI). Plant material was extracted by either Coffee Maker (Mr. Coffee®, ISX-43) or Accelerated Solvent Extractor (Dionex®, ASE-200) using H2O as a solvent. Water extracts were freeze-dried using Freeze Dry System (Labconco®, Freezone 4.5). TLC was carried out on either reversed phase silica gel (Analtech®, RP18 SiO2, 150 µm, UV254) with MeCN-H2O (9:1) or acetone-H2O-NH3·H2O (7:3:0.1), silica gel (EMD® Chemicals Inc., SiO2 60 F254) using CHCl3-MeOH (7:3) or alumina plates (EMD® Chemicals Inc., Al2O3 60 F254) using CH2Cl2-MeOH (9:1) as solvent system; centrifugal preparative TLC (CPTLC) was performed by a Chromatotron® (Harrison Research Inc., model 8924), tagged with a fraction collector (SpectralChrom® CF-1) on a 1, 2 or 4 mm silica gel rotors (Analtech®, SiO2 coated with F254 indicator); flash CC was carried out over reversed phase silica gel (J.T. Baker, Bakerbond® C18 prep LC SiO2, 40 µm). Samples were dried using a Savant Speed Vac Plus SC210A Concentrator. The isolated compounds were visualized by observing under UV light at 254 or 365 nm, followed by spraying separately with Dragendorff’s and/or 1% vanillin-H2SO4 spray reagents. The reference standards of harmol, harmine, harmaline, and (−)-epicatechin were purchased from Sigma-Aldrich (St. Louis, MO), while others were available in our laboratories.
2.2. Plant material
Fresh leaves, stems, and large branches of B. caapi cultivar Da Vine (Miller, 1986) were collected from the island of Oahu, Hawaii, in August and November 2007, and June 2008, as well as from Hilo (Big island), Hawaii, USA, in October, 2007. A reference specimen was collected from Oahu, and a voucher specimen (HLA # 7835) was deposited at the Herbarium of Harold Lyon Arboretum, University of Hawaii. The collector of the voucher specimen of the plant at Lyon Arboretum was Dr. Kenneth M. Nagata (Accession # L-81.0727; collector # 2789; dated 03/13/1984). The regular B. caapi (mature stems) samples analyzed in this study (BCEx-1–BCEx-4) were procured/obtained from commercial (via internet) and NCNPR sources during 2005–2009. In addition, all samples used in this work are preserved using the standard procedures for collection, drying, grinding and packaging at the NCNPR.
2.3. Extraction of plant material
2.3.1. Preparation of extracts from different plant parts
The different plant parts (leaves, young and mature stems, bark and debarked stems, and large branches (diameter: 3–8 cm)) of B. caapi, fresh and dried, were extracted using hot water by two different methods. A portion of each fresh sample was dried at 45 °C for 2–4 days, to get dried material. Before processing, the fresh plant material was cut into small pieces, while dried plant material was ground.
A. “Hot maceration”. Water extracts were prepared by simmering with boiled water using a Coffee Maker. Plant material was placed onto a coffee filter and extracted successively with distilled H2O for three times (30–70 g/ 100 mL; H2O × 3). This gave strong yellow colored extracts.
B. “ASE extraction”. Water extracts were prepared by a semi-automated Accelerated Solvent Extractor (ASE-200). Plant material was packed into a steel 33 mm cells and extracted successively with distilled H2O for four times (15–20 g/ 5 mL H2O × 4; 100 °C, 3.45 MPa of N2, 30 min). Next optimal method was developed and used: preheat time = 0 min, pressure = 3.45 MPa, heat time = 5 min, T = 100 °C, static time = 30 min, flush = 100% vol, purge time = 2 min, cycles = 4. This gave strong yellow-brown colored water extract (ca 20 mL in total).
All water extracts were cooled, frozen at −20 °C and freeze-dried by Freeze Dry System (−44 °C, 1.6 Pa). After freeze-drying all samples were transferred into the vials and sealed with Parafilm®.
2.3.2. Preparation of standardized extract of B. caapi (Da Vine) whole stem
Based on the MAO-A and antioxidant activity of initial extracts of different plant parts, prepared by using different extraction methods, the “most active” extracts were identified (Table 1). These are hot aqueous extracts from dried powdered samples of large branch, whole mature/big stem and mature stem bark prepared by method A (using a coffee maker). In order to proceed for bioassay-guided isolation of active constituents and markers, bulk extraction of dried powdered large branch (471 g) was executed using the standardized method, as described above, which yielded 60 g of dried pale-yellow powder.
Table 1.
MAO-A and MAO-B inhibitory, and antioxidant and cytotoxic activities of representative B. caapi extracts and compounds.
| Sample Name | Samples collected |
Code # | Yielda % | MAO-A IC50 (µg/mL) |
MAO-B | Antioxidant | Cytotoxicity | |
|---|---|---|---|---|---|---|---|---|
| µg/mL | % Inhibition | IC50 (µg/mL) | ||||||
| Fresh stem | August, 2007; Oahu |
fBCDVS-1 | 0.85 | 0.2 – 0.67 | 100 | 43 | NT | NT |
| Dried stem | BCDMS | 12.74 | 0.04 | 100 | 38 | NT | NT | |
| Dried young stem bark | BCBYS | 10.08 | 0.1 | 100 | 46 | 1.6 | NC | |
| Fresh mature stem bark | fBCDVBm-1 | 2.16 | 0.015 – 0.025 | 100 | 23 | NT | NT | |
| Dried mature stem bark | BCBMS | 14.29 | 0.033 | 100 | 45 | 2.2 | NC | |
| Fresh debarked young stem |
fBCDVDB-1 | 2.67 | 0.025 – 0.035 | 100 | 27 | NT | NT | |
| Dried debarked young stem |
DDYS | 9.76 | 0.08 – 0.043 | 100 | 45 | 4.0 | NC | |
| Dried debarked mature stem |
BCDMDS | 10.12 | 0.05 | 100 | 57 | 4.0 | NC | |
| Fresh leaves | fBCDVL-1 | 0.49 | 0.125 | 100 | 41 | NT | NT | |
| Dried leaves | BCDL | 10.06 | 0.6 | 100 | 39 | 5.9 | NC | |
| Fresh stemb | fBCDVS-2 | 11.7 | 0.9 | 100 | 16 | NT | NT | |
| Fresh leavesb | fBCDVL-2 | 5.28 | 0.2 | 100 | 30 | NT | NT | |
| Fresh young stem | October, 2007; Big Islandd |
BCDVFYSO | 0.80 | 0.58 | >100 | ND | NT | NT |
| Dried young stem | BCDVDYSO | 7.28 | 1.13 | >100 | ND | NT | NT | |
| Fresh leaves | BCDVFLO | 0.60 | 1.35 | >100 | ND | NT | NT | |
| Dried leaves | BCDVDLO | 4.08 | 0.33 | >100 | ND | NT | NT | |
| Fresh young stemb | BCDVFYSAO | 6.29 | 0.24 | >100 | ND | NT | NT | |
| Fresh leavesb | BCDVFLAO | 8.35 | 0.22 | >100 | ND | NT | NT | |
| Fresh bark large branch | November, 2007; Oahu |
BCfBS | 3 | 0.045 | 100 | 51 | 0.4 | NC |
| Fresh debarked large branch |
BCfDS | 5.2 | 0.035 | 100 | 55 | 3.0 | NC | |
| Dried bark large branch | BCDBS | 18.1 | 0.03 | 100 | 34 | 1.9 | 23 | |
| Dried debarked large branch |
BCDDS | 7.6 | 0.02 | 100 | 46 | 2.0 | NC | |
| Fresh big stems | July 2008; Big Island |
fBCDVSbig | 2.9 | 0.034 | >100 | ND | NT | NT |
| Dried big stems | BCDVSbig | 9.56 | 0.028 | >100 | ND | NT | NT | |
| Dried matured stemc | BCEx-1 | 15.43 | 0.32 | >100 | ND | 7.8 | NC | |
| Dried matured stemc | BCEx-4 | 9.80 | 0.385 | >100 | ND | 1.0 | 29 | |
| Pure compd/ Standards |
MAO-A IC50 (nM) |
MAO-B IC50 (µM |
Antioxidant | Cytotox. | Pure compd/ Standards |
MAO-A IC50 (nM) |
MAO-B IC50 (µM) |
Antioxidant | Cytotox. |
|---|---|---|---|---|---|---|---|---|---|
| IC50 (µg/mL) | IC50 (µg/mL) | ||||||||
| Banistenoside A (1) |
4.9×103 | >100 | NA | >12.5 | Epicatechin (8) |
51.7×103 | 65 | <0.13 | 6.3 |
| Banistenoside B (2) |
21.5×103 | >100 | NT | 7.8 | Procyanidin B2 (9) |
8.5×103 | 35 | 0.57 | NC |
| Harmol (3) | 18 | NT | NT | NT | Clorgyline | 1.6 | 1.8 | NT | NT |
| THH (5) | 74 | >100 | NA | NC | Deprenyl | 9.0 | 0.040 | NT | NT |
| Harmaline (6) | 2.5 | 25 | NA | 25 | Vitamin C | NT | NT | 1.34 | NT |
| Harmine (7) | 2.0 | 20 | NA | NC | Doxorubicin | NT | NT | NT | 0.2 – 0.35 |
Yield was measured by weight.
Extracted with water by automated ASE-200 extractor.
Regular/Commercial B. caapi sample.
Plants from this collection are 3 years old.
NT = Not tested. ND = Not detected (tested up to maximum concentration of 100 µg/mL). NA = No antioxidant activity upto 31.25 µg/mL. NC = No cytotoxicity to HL-60 cells up to 31.25 µg/mL.
2.4. Bioassay-guided fractionation and characterization of isolated compounds
The freeze-dried powdered aqueous extract (53.7 g), obtained by bulk isolation from large branch, was subjected to RP-CC over C-18 silica gel (1:15) and eluted with MeCN (8 L) and then MeCN-H2O mixture with increasing concentrations of H2O (1% → 10%), and 50 mL fractions were collected. Fractions were pooled by TLC, combined, and then evaporated under reduced pressure (total 18.65 g from 14 combined fractions, i.e. fractions A-N). Fraction A (325 mg) was purified by CPTLC, using 2 mm silica gel rotor with CHCl3-MeOH (9:1) as a solvent, which afforded 8 (110 mg), followed by 9 (24 mg), while fraction B (125 mg) yielded 5 (38 mg), followed by 4 (24 mg), by using a similar CPTLC procedure. Fraction D (118 mg) was also separated by CPTLC, using 1 mm silica gel rotor with CHCl3-MeOH (9:1) as a solvent, which afforded 7 (56 mg), followed by 6 (17 mg), while fraction H (276 mg) was separated by a 2 mm silica gel CPTLC rotor, using CHCl3-MeOH (9:1) as solvent, which gave 14 (20 mg), and a mixture H-1 (90 mg), containing 9 and 14. Fraction I (489 mg) was also separated by CPTLC, using 4 mm silica gel rotor [CHCl3-MeOH (9:1) as solvent], which yielded 7 (29 mg), followed by a another mixture I-1 (317 mg), which was further purified by 2 mm silica gel CPTLC rotor [CHCl3-MeOH (9:1) as solvent] to yield compounds 15 (sacharose; 79 mg) and 16 (β-D-glucose; 55 mg). Finally, fraction L (2.0 g) was separated by RP-flash chromatography over C-18 silica gel, eluted with increasing concentrations of H2O in acetone (1:9 → 1:1, 2 L), which afforded 1 (60 mg), followed by 2 (20 mg) as well as fractions L-1 (0.3 g) and L-2 (1.3 g). Fraction L-2 consisted of mixtures of 1, 2 and 15, while fraction L-1 was the mixture of 6 and 7.
Using the above procedures, bulk quantities of 1–9 (i.e. 80–100 mg) were isolated from aqeuous stem extracts of samples collected in 2007 and 2008. Compound 3 was identified by HPLC using direct comparison with an authentic sample of harmol. Compounds 5–9, 15 and 16 were identified by comparison of physical and spectral data with those reported in the literatures, and by direct comparison with their authentic samples as THH, harmaline, harmine, epicatechin and procyanidin B2, sacharose and glucose, respectively.
Banistenoside A (1)
Colorless solid; UV (MeOH) λmax, nm: 226, 269 and 290; IR (film) νmax, cm−1: 3382 (NH), 2920, 2850, 1804, 1752, 1631, 1225, 1037, 733; 1H and 13C NMR data, see Table 2; HRESIMS m/z 367.1522 [MH+H2O-Glucosyl]+ (calcd. for C17H23N2O7, 367.1505); 529.2013 [MH+H2O]+ (calcd. for C23H33N2O12, 529.2033).
Table 2.
1H and 13C NMR data of 1, 10 and 11.
| # | 1 | 1 | 10 | 10 | 11 | 11 | ||
|---|---|---|---|---|---|---|---|---|
| 1H | 13C | 1H | 13C | HMBC | 1H | 13C | HMBC | |
| 1 | 4.44 m | 56.0 (d) | 5.98 d (4.3) | 49.4 | C-3, 4a, 9a, 10, 11, 14 |
5.08 d (10.0) | 52.2 | C-3, 4a, 9a, 10, 11, 14 |
| 3 | 3.31 brs 3.05 m |
40.8 (t) | 4.06 dd (2.0, 12.4), 3.42 m |
41.9 | C-14 | 4.98 dd (5.6, 11.6), 2.81 m** |
38.3 | - |
| 4 | 2.51 m | 16.0 (t) | 2.81 m | 21.8 | C-4a | 2.81 m** | 20.7 | C-4a |
| 4a | - | 109.6 (s) | - | 109.6 | - | - | 110.2 | - |
| 4b | - | 119.9 (s) | - | 120.4 | - | - | 120.0 | - |
| 5 | 6.90* | 118.0 (d) | 7.34 d (8.8) | 118.9 | C-4a, 4b, 7, 8a | 7.34 d (8.4) | 119.1 | C-4a, 4b, 7, 8a |
| 6 | 6.19 brd (8.8) | 108.3 (d) | 6.77 dd (2.0, 8.8) | 109.1 | C-4b, 7, 8 | 6.76 dd (2.4, 8.8) | 109.8 | C-4b, 7, 8 |
| 7 | - | 158.5 (s) | - | 156.8 | - | - | 156.9 | - |
| 8 | 6.35 d (2.8) | 94.6 (d) | 6.81 d (2.0) | 94.8 | C-4b, 6, 7, 8a | 6.92 d (2.4) | 95.2 | C-4b, 6, 7, 8a |
| 8a | - | 137.0 (s) | - | 137.2 | - | - | 137.1 | - |
| 9a | - | 120.0 (s) | - | 126.7 | - | - | 126.7 | - |
| 10 | 4.23 d brs | 73.0 (d) | 4.32 d (4.3) | 72.9 | C-1, 1´, 9a, 12 | 3.98 d (10.0) | 81.2 | C-1, 1´, 9a, 11, 12 |
| 11 | 4.44 m | 79.3 (d) | 5.20 d (8.4) | 77.2 | C-10, 12, 13 | 5.77 d (4.4) | 69.5 | C-10, 12, 13 |
| 12 | 4.09 brs | 68.9 (d) | 5.61 t (8.0,8.4) | 72.5 | C-10, 11, 13 | 5.32 t (4.4, 4.8) | 71.1 | C-10, 11, 13 |
| 13 | 4.04 brs | 70.9 (d) | 5.72 d (8.0) | 71.2 | C-12 | 5.87 d (4.8) | 73.4 | C-12, 14 |
| 14 | - | 175.3 (s) | - | 170.6 | - | - | 164.4 | |
| 1´ | 4.44 m | 94.4 (d) | 4.54 d (4.8) | 96.7 | C-2´, 10 | 4.91 d (3.6) | 101.6 | C-2´, 10 |
| 2´ | 4.36 m** | 71.8 (d) | 4.94–5.0 m** | 71.4 | - | 5.11–5.17 m** | 71.5 | - |
| 3´ | 4.38 m** | 71.7 (d) | 4.94–5.0 m** | 72.1 | - | 5.11–5.17 m** | 73.3 | - |
| 4´ | 4.31 m | 66.1 (d) | 4.94–5.0 m** | 68.6 | C-6´ | 5.11–5.17 m** | 67.4 | C-6´ |
| 5´ | 3.82 m** | 72.3 (d) | 3.55 m | 72.8 | - | 3.81 dd (2.4, xx) | 72.5 | - |
| 6´ | 3.81 m** 3.31 m |
61.5 (t) | 4.30 dd (5.2, 12.0), 4.12 m |
62.2 | C-4´ | 4.38 dd 4.0,12.4), 4.0 dd (1.2, 12.4) |
61.5 | C-4´ |
| 7-OMe | 3.23 s | 54.3 q | 3.83 s | 55.6 q | C-7 | 3.86 s | 55.7 | C-7 |
| OH/NH | - | - | 8.45 s (NH) | - | C-4a, 4b, 8a, 9a |
7.9 brs (NH) | - | C-4a, 4b, 8a, 9a |
| OCOMe | - | - | 2.23, 2.15, 2.14, 2.07, 1.98, 1.94, 1.91 (7 × s) |
22.0, 20.7, 20.5 ×3, 20.4, 20.2 (7q) |
- | 2.21, 2.17, 2.09 × 2, 2.01, 2.0, 1.53, (7 × s) |
20.9,20.6, 20.6 ×3, 20.5 ×2 (7q) |
- |
| CO-Me | - | - | - | 170.7, 170.1, 169.9, 169.5, 169.4, 169.2 ×2 |
- | - | 170.4 ×2, 170.1, 170.0, 169.4 ×2, 169.2 |
- |
NMR Spectra of 1 (CF3COOD/D2O), and 10 and 11 (in CDCl3) were recorded at 400 (1H) and 100 (13C) MHz, using a Varian spectrometer. The coupling constants (J values, in Hz) are in parentheses. The multiplicities of carbon signals were determined by 13C NMR DEPT experiments. Assignments were made using 2D NMR COSY, HMBC and HMQC experiments.
Signals superimposed with solvent.
Signals superimposed on each other.
Banistenoside B (2)
Colorless solid; UV (MeOH) λmax, nm: 226, 269 and 290; IR (film) νmax, cm−1: 3448 (NH), 3021, 2923, 2851, 1749, 1692, 1632, 1221, 1044, 757; HRESIMS m/z 367.1491 [MH+H2O-Glucosyl]+ (calcd. for C17H23N2O7, 367.1505); 529.2028 [MH+H2O]+ (calcd. for C23H33N2O12, 529.2033).
Tetrahydronorharmine (4)
Colorless solid; UV (MeOH) λmax, nm: 226, 269 and 290; 1H NMR (CDCl3, J in Hz) δH 7.10 (1H, d, 7.5, H-5), 6.67 (1H, brs, H-8), 6.49 (1H, brd, 7.5, H-6), 4.15 (2H, m, H-1), 3.58 (3H, s, OCH3), 3.30 (2H, m, H-3), 2.81 (2H, m, H-3); 13C NMR (CDCl3) δC 156.9 (s, C-7), 137.8 (s, C-8b), 124.0 (s, C-9a), 120.8 (s, C-4b), 118.2 (d, C-5), 109.2 (d, C-6), 105.8 (s, C-4a), 94.5 (d, C-8), 54.7 (q, OCH3), 42.6 (t, C-3), 41.0 (t, C-1), 18.5 (t, C-4); HRESIMS m/z 203.1176 [MH]+ (calcd. for C12H15N2O, 203.1184).
(−)-2R,3R–Epicatechin (8)
The physical and spectral data of 8 were identical to those reported in the literature (Sun et al., 2006; Balde et al., 1991). The CD data [(in MeOH); λmax, nm ([θ] in mdeg): 244 (21.58), 269 (−15.83), 290 (−3.46)] was in agreement with those recorded for an authentic sample of (−)-2R,3R-epicatechin, and (Korver and Wilkins, 1971).
(−)-2R,3R-epicatechin-4β,8-(−)-2R,3R-epicatechin [(−)-procyanidin B2] (9)
The physical and spectral data of 9 were identical to those reported in the literature (Korver and Wilkins, 1971; Barrett et al., 1979; Khan et al., 1997). In addition, the CD data [(in MeOH) λmax, nm ([θ] in mdeg): 221 (−16.24), 243 (52.58), 276 (−3.64)] was also in agreement with those reported in the literatures (Barrett et al., 1979)
(−)-β-D-Fructofuranosyl-(2→5)-fructopyranose (Di-D-Fructose) (14)
Colorless solid; [α]D23.5 −55.8 (c 0.78, MeOH); 1H NMR (Pyridine-D5, J in Hz) δH 5.60 (brs, OH), 5.12 (1H, d, 8.0, H-3), 5.04 (1H, t, 8.0, H-4), 4.82 (1H, brd, 12.0, H-4'), 4.62 (1H, dd, 8.0, 12.0, H-3'), 4.59 (1H, m, H-5), 4.49 (1H, dd, 1.0, 12.0, H-6'), 4.39 (1H, d, 8.0, H-1'), 4.38 (1H, m, H-5'), 4.29a (m, H-1'), 4.29a (m, H-6), 4.24–4.26a (m, H-1), 4.24 (1H, d, 12.0, H-6), 4.11 (1H, dd, 4.0, 12.0, H-6'); 13C NMR (Pyridine-D5) δC 103.6 (s, C-2), 99.3 (s, C-2'), 84.3 (d, C-5), 78.0 (d, C-3), 77.0 (d, C-4), 73.3 (d, C-3'), 71.7 (d, C-5'), 70.0 (d, C-4'), 66.2 (t, C-1'), 64.9 (t, C-1), 64.2 (t, C-6'), 63.8 (t, C-6); 1H NMR (CD3OD, J in Hz) δH 5.51 (brs, OH), 4.62b (m, H-1'), 4.62b (m, H-1), 4.49c (d, 8.0, H-1), 4.49c (d, 8.0, H-1'), 4.06d (m, H-3), 4.06d (m, H-5'), 4.02 (1H, dd, 4.0, 12.0, H-6'), 3.86 (1H, brs, H-3'), 3.78e (m, H-4), 3.78e (m, H-4'), 3.73 (1H, m, H-5), 3.62e (dd, 4.0, 12.0, H-6), 3.62e, (dd, 4.0, 12.0, H-6'), 3.49 (1H, brd, 8.0, H-6) (a–esignals are superimposed on each other); 13C NMR (CD3OD) δC 101.7 (s, C-2), 97.8 (s, C-2'), 81.3 (d, C-5), 76.6 (d, C-5'), 75.4 (d, C-3), 70.4 (d, C-4), 69.8 (d, C-3'), 68.3 (d, C-4'), 64.5 (t, C-6'), 62.9 (t, C-1'), 62.9 (t, C-1), 61.2 (t, C-6).
Acetylation of Fraction L-2
Fraction L-2 (355 mg) containing the mixture 1, 2 and 15 was dissolved in pyridine (1 mL) and treated with acetic anhydride (2 mL). The reaction mixture was stirred overnight at room temperature, and then dried under vacuum to give the solid residue. The residue was subjected to CPTLC over 2 mm silica gel rotor [CHCl3-MeCN (9:1) as solvent], which afforded 10 (28 mg) and 18 (127 mg), together with fraction L-2-1 (127 mg), which was further separated by CPTLC, using 1 mm silica gel rotor with CHCl3-MeCN (19:1) as a solvent to yield compounds 11 (20 mg) and additional amounts of 18 (37 mg). Compound 18 was identified as sacharose octaacetate by physical and NMR data.
Banistenoside A heptaacetate (10)
Colorless solid; [α]D28 −8.7 (c 0.6, CHCl3); CD (in MeOH) λmax, nm ([θ] in mdeg): 316 (13.03), 361 (−0.85), 369 (−0.79); IR (film) νmax, cm−1: 3455 (OH, NH), 3382 (OH, NH), 2920, 2850, 1804, 1752, 1631, 1426, 1371, 1225, 1072, 1037, 910, 733; 1H and 13C NMR data, see Table 2; HRESIMS m/z 805.2635 [MH]+ (calcd. for C37H45N2O18, 805.2667); 827.2454 [MNa]+ (calcd. for C37H44N2O18Na, 827.2487).
Banistenoside B heptaacetate (11)
Colorless solid; [α]D28 +31.1 (c 0.4, CHCl3); CD (in MeOH) λmax, nm ([θ] in mdeg): 311 (21.39); IR (film) νmax, cm−1: 3448 (OH, NH), 3021, 2923, 2851, 1749, 1692, 1434, 1371, 1221, 1044, 757; 1H and 13C NMR data, see Table 2; HRESIMS m/z 805.2693 [MH]+ (calcd. for C37H45N2O18, 805.2667); 827.2518 [MNa]+ (calcd. for C37H44N2O18Na, 827.2487).
Acetylation of (−)-epicetechin (8)
Epicatechin 8 (27 mg, 93 mmol) was dissolved in pyridine (1 mL). Acetic anhydride (1 mL) was added dropwise to the solution while stirring. The reaction mixture was stirred overnight at ambient temperature. The reaction mixture was dried under vacuum to give the solid residue which was further purified by flash chromatography over silica gel to yield compound 12 (39 mg, yield 84 %) as a pale solid. Physical and Spectral data of 12 were identical to those reported in the literature (Castillo et al., 2004).
Acetylation of Fraction H-1
Fraction H-1 (77 mg), containing the mixture 9 and 14, was dissolved in pyridine (1 mL) and treated with acetic anhydride (2 mL). The reaction mixture was stirred overnight at room temperature, and dried under vacuum to give the solid residue, which was subjected to CPTLC, using 1 mm silica gel rotor with CHCl3-MeCN (98:2) as solvent system to afford acetates 13 (29 mg) and 17 (47 mg).
(−)-Procyanidin B2 acetate (13)
Colorless solid; [α]D235 −80 (c 0.025, MeOH); CD (in 1,4-dioxane) λmax, nm ([θ] in mdeg): 233 (−5.14), 245 (73.43), 274 (−12.35), 285 (−10.86); IR (film) νmax, cm−1: 2963, 2917, 2855, 1767, 1621, 1596, 1208, 1122, 1049, 901; 1H and 13C NMR data were identical to those reported in the literature (Korver and Wilkins, 1971; Barrett et al., 1979; Khan et al., 1997); HRESIMS m/z 1016.2859 [MNH4]+ (calcd. for C50H50O22N, 1016.2824); 1021.2346 [MNa]+ (calcd. for C50H46O22Na, 1021.2378); 1037.2160 [MK]+ (calcd. for C50H46O22K, 1037.2118).
β-D-fructofuranosyl(2→5)fructopyranose octaacetate (17)
Colorless solid; [α]D235 +30.4 (c 0.1, CHCl3); IR (film) νmax, cm−1: 2942, 1746, 1433, 1372, 1222, 1050; 1H NMR (CDCl3, J in Hz) δH 5.72 (1H, d, 4.0, H-3), 5.63 (1H, dd, 2.0, 8.8, H-3'), 5.44 (1H, d, 2.0, H-4'), 5.15 (1H, m, H-2'), 5.07 (1H, dd, 4.0, 6.0, H-4), 4.86 (1H, brd, 17.6, H-6'), 4.63 (1H, brd, 17.6, H-6'), 4.55 (1H, d, 9.6, H-1), 4.45 (1H, m, H-5), 4.35 (1H, dd, 2.8, 12.4. H-1'), 4.30 (1H, d, 9.6, H-1), 4.22 (1H, dd, 5.6, 12.4, H-6), 4.10 (1H, dd, 5.2, 12.4, H-1'), 4.05 (1H, dd, 4.8, 12.4, H-6), 2.15, 2.10, 2.05, 2.04 (×3), 2.02, 2.01 (8s, CH3); 13C NMR (CDCl3) δC 197.9 (s, C-2'), 179.9, 170.4, 169.8, 169.6, 169.5, 169.4, 169.02, 169.0 (8s, C=O), 107.9 (s, C-2), 80.6 (d, C-5), 78.4 (d, C-3), 76.1 (d, C-4), 74.2 (d, C-3'), 68.3 (d, C-4'), 68.0 (d, C-5'), 66.6 (t, C-1'), 62.8 (t, C-6'), 61.7 (t, C-6), 61.5 (t, C-1), 20.7 (×2), 20.6 (x2), 20.5, 20.4, 20.3, 20.2 (8q, CH3); HRESIMS m/z 696.2330 [MH2O]+ (calcd. for C28H42O19N, 696.2351); C28H38O19; 701.1903 [MNa]+; (calcd. for C28H38O19Na, 701.1905).
2.5. Inhibition kinetics assay using recombinant human MAO-A and MAO-B
Recombinant human Monoamine Oxidase A (MAO-A) and Monoamine Oxidase B (MAO-B) were obtained from BD Biosciences (Bedford, MA). To investigate the effect of extracts on MAO-A and MAO-B, the kynuramine deamination assay was adapted for 96-well plates. The inhibition kinetics of MAO-A and MAO-B by inhibitors/extracts were determined in a range expected to produce 30% to 90% inhibition. The IC50 values for extracts, pure compounds and reference standards were determined at five concentrations of 1, 0.1, 0.01, 0.001 and 0.0001 µg/mL for MAO-A, where as for MAO-B, percent inhibitions of all crude extracts were evaluated at three concentrations, 100, 10 and 1 µg/mL. All experiments were carried out in duplicate. A fixed substrate concentration and varying inhibitor concentrations were used to determine the IC50 value at the point where 50% inhibition of the catalytic activity of the enzyme occurred. For MAO-A, the substrate concentration of 80 µM kynuramine was chosen because the apparent Km value for substrate binding has been reported previously was approximately 40 µM (Parikh et al., 2002). Since Km is the substrate concentration at half Vmax, therefore, 2 × Km(2×40 = 80 µM), was selected for determining IC50 values. Similarly, for MAO-B, substrate concentration of 50 µM kynuramine was chosen. The assay was performed with the addition of inhibitor. Inhibition was calculated as percent of product formation compared to the corresponding control (enzyme-substrate reaction) without the inhibitors. The reactions were carried out in 0.1 M potassium phosphate buffer at pH 7.4. Incubations mixtures contained 5 µg/mL of MAO-A (50 µL in buffer) and 10 µg/mL of MAO-B (50 µL in buffer). The inhibitor was dissolved in DMSO or in buffer (if not dissolved in DMSO). The total reaction volume was 200 µL yielding a final DMSO concentration of 1.0% in the reaction mixture. The reaction mixtures were pre-incubated for 10 minutes at 37 °C followed by the addition of MAO-A / MAO-B to initiate the reactions. Reactions were incubated for 20 minutes at 37 °C and were stopped immediately by the addition of 75 µL of 2N NaOH. The formation of 4-hydroxyquinoline was determined fluorometrically by SpectraMax M5 fluorescence plate reader (Molecular Devices, Sunnyvale, CA) with an excitation and emission wavelength of 320 nm and 380 nm, respectively, using the SoftMax Pro program.
2.6. In vitro cytotoxicity assay
The in vitro cytotoxic activity was determined against four human cancer cell lines (SK-MEL, KB, BT-549 and HepG2), monkey kidney fibroblasts (VERO) and pig kidney epithelial cells (LLC-PK11). All cell lines were obtained from the American Type Culture Collection (ATCC, Rockville, MD). The assay was performed in 96-well tissue culture-treated microplates. Cells were seeded at a density of 25,000 cells/well and incubated for 24 h. Samples at different concentrations were added and plates were again incubated for 48 h. The number of viable cells was determined using Neutral Red according to a modification of the procedure of Borenfreund et al (1990). IC50 values were determined from dose response curves of percent growth inhibition against test concentrations. Doxorubicin was used as a positive control, while DMSO was used as the negative (vehicle) control.
2.7. Cell Based Assay for Antioxidant Activity (Rosenkranz et al., 1992; Scudiero et al., 1988)
The effect of samples on the generation of ROS in Myelomonocytic HL-60 cells is determined by the DCFH-DA (2',7'-dichlorofluorescin diacetate) method. HL-60 cells (ATCC) were cultured in RPMI-1640 medium with 10% fetal bovine serum, penicillin (50 units/mL) and streptomycin (50 µg/mL). 125 µL of the cell suspension (1×106 cells/mL) was added to the wells of a 96-well plate. After treatment with different concentrations (i.e., six concentrations of 62.50, 31.25, 15.63, 7.82, 3.91 and 1.95 µg/mL were used) of the test samples for 30 min, cells were exposed to 100 ng/mL phorbol 12-myristate-13-acetate (PMA, Sigma) for 30 min. DCFH-DA (Molecular Probes, 5 µg/mL) was added and cells were further incubated for 15 min. Levels of fluorescent DCF (produced by ROS catalyzed oxidation of DCFH) was measured on a PolarStar Galaxy plate reader with excitation wavelength at 485 nm and emission at 530 nm. Inhibition of ROS generation by test samples is determined in terms of % decrease in DCF production compared to the vehicle control. Vitamin C and trolox were used as the positive control in each assay. DCFH-DA is a non-fluorescent probe that diffuses into cells, where cytoplasmic esterases hydrolyse the DCFH-DA to 2',7'- dichlorofluorescin (DCFH). The ROS generated within HL-60 cells oxidize DCFH to the fluorescent dye 2',7'-dichlorofluorescein (DCF). The ability of the test materials to inhibit exogenous cytoplasmic ROS-catalysed oxidation of DCFH in HL-60 cells is measured in comparison to PMA treated vehicle control. The cytotoxicity of samples to HL-60 cells was also determined after incubating the cells (2×104 cells/well in 225 µL) with test samples for 48 hours by XTT method. Briefly, 25 µL of XTT-PMS solution (1 mg/ml XTT solution supplemented by 25 µM of PMS) was added to each well. After incubating for four hours at 37 °C, absorbance was measured at a dual wavelength of 450 – 630 nm on a Bio-Tek plate reader.
2.8. Inhibition assay for acetylcholinesterase, butyrylcholinesterase and catechol-O-methyl transferase enzymes activity
Acetylcholinesterase, butyrylcholinesterase and catechol-O-methyl transferase enzymes assays were carried out as described previously (Ellman et al., 1961; Learmonth et al., 2004).
3. Results and discussion
The hot aqueous extracts of fresh and dried large branches of B. caapi demonstrated significant MAO-A inhibitory and antioxidant activity (Table 1). A bioassay-guided fractionation of crude aqueous extract of B. caapi by reversed-phase column chromatography (RPCC) afforded a polar non-alkaloidal fraction by eluting with MeCN, which showed potent antioxidant and moderate MAO-B inhibitory activities. This was followed by an alkaloidal fraction, eluted with MeCN-H2O as solvent, which showed potent MAO-A and moderate MAO-B inhibitory activites. Subsequent RPCC of polar alkaloidal fraction yielded two new alkaloidal glycosides 1 and 2, as major markers, while polar non-alkaloidal fractions afforded the potent antioxidants 8 and 9. Furthermore, centrifugal preparative TLC (CPTLC) of alkaloidal fractions yielded the tetrahydronorharmine (4), and four known MAO inhibitors β-CA’s 3 and 5–7.
3.1. Structure elucidation
Compound 1 was analyzed for C23H30N2O11 by ESIHRMS. The UV spectrum of 1 demonstrated absorption bands at λmax 226, 269 and 290 nm, typical of THH chromophore (Hashimoto and Kawanishi, 1975; ibid 1976), and IR spectrum showed absorption bands at νmax 3550 and 1740 cm−1, attributed to hydroxyl and amide (δC-14 175.3) groups, respectively. The NMR spectrum of 1, recorded in CF3COOD+D2O, revealed the presence of three aromatic protons δH-5 6.90, δH-6 6.19 and δH-8 6.35, a methine proton at δH-1 4.44, two methylene groups at δH-3 3.05 and 3.31 (each 1H), and δH-4 2.51 (2H), and a methoxyl group at δH 3.23, suggested the presence of a C-1 substituted THH nucleus. It also showed four oxymethine protons at δH-10 4.23, δH-11 4.44, δH-12 4.09 and δH-13 4.04, either as broad singlets or multiplets, which showed 2D NMR COSY correlations for the system [-CH(OH)-CH(OH)-CH(OH)-CH(OH)-]. The 13C NMR spectrum showed the presence of a glucose moiety (δC-1´ 94.4, δC-2´ 71.8, δC-3´ 71.7, δC-4´ 66.1, δC-5´ 72.3 and δC-6´ 61.5), and a methoxyl group (δC-7-OMe 54.3). In addition, the 13C NMR showed signals for C-1 – C-9a of THH base skeleton, four oxymethine carbons and a quaternary amide carbonyl, which were assigned by using 2D NMR HMQC and HMBC experiments of 1. Compound 2 was also analyzed for C23H30N2O11 by ESIHRMS and its UV spectrum demonstrated absorption bands at λmax 226, 267 and 294 nm, as observed for 1. The presence of amide carbonyl and hydroxyl groups was suggested from the IR spectrum. Compound 2 was highly insoluble in most of the NMR solvents, like 1, and therefore, the spectroscopic analyses were performed on the acetates for both the compounds.
Acetylation of a fraction (L-1) containing mixture of 1 and 2 afforded the corresponding heptaacetates 10 and 11, which were subsequently separated and purified by CPTLC (see Materials and Methods Section). Compound 10 was analyzed for C37H44N2O18 by ESIHRMS, and found to be levorotatory; [α]D −8.7 (c 0.6, CHCl3). It’s 1H and 13C NMR spectra displayed signals for seven acetyl groups, a methoxyl and a glucose moiety (Table 2). The 1H NMR spectrum of 10, in CDCl3, demonstrated multiplicities for all the protons, previously not observed for 1 in CF3COOD+D2O, and structure 1 was fully substantiated by 2D NMR COSY, HMQC, HMBC and NOESY experiments on 1-Ac (10). The presence of tetra-O-acetyl-β-D-glucose moiety (Baddeley et al., 2005) was suggested from its 13C and 1H NMR [δH-12 96.7, 71.4, 72.1, 68.6, 72.8, 62.2; C-1´-C-6´, respectively; δH-1´ 4.54 (d, J=4.8 Hz)]. A 2D NMR COSY spectrum showed correlations between four acetoxy protons at δH-10 4.32, δH-11 5.20, δH-12 5.61 and δH-135.72, together with the correlation between δH-104.32 (brd, J=4.3 Hz) and δH-1 5.98 (d, J=4.3 Hz), suggesting C-10 of the system [−1CH-10CH(OR)-11CH(OAc)-12CH(OAc)-13CH(OAc)-] was connected with C-1 of THH base skeleton. The structure was confirmed by a 2D NMR HMBC experiment (Table 2), which showed key correlations between H-1 and C-3, C-4a, C-9a, C-10, C-11 and C-14, and H-10 and C-1, C-1' (i.e., anomeric carbon), C-9a and C-12, confirming the formation of a seven- member ring between C-1 and N-2 of THH skeleton (-1C-10C-11C-12C-13C-14C-2N-), and the linkage of C-1´ of glucose moiety at C-10. HMBC further showed cross peaks between N9-H (δN-H 8.45) and C-4a, C-4b, C-8a and C-9a, and between C-7-OMe (δH-7-OMe 3.84) and C-7 (δC 156.8), confirming the presence of a free indole N-H proton and -OMe group at C-7. Finally, the configuration of compound 10 was assigned by a Circular Dichroism spectra and NOESY experiment (vide infra).
Compound 11 was also dextrorotatory {[α]D +31.1 (c 0.4, CHCl3)}, and analyzed for C37H44N2O18 by ESIHRMS. The 1H and 13C NMR (Table 2) showed the presence of seven acetyl groups, and its THH carbon skeleton fused with a seven-member ring, via five oxygenated carbons C-10 – C-14, was found to be similar to those of acetate 10. The 1H NMR spectrum of 11 was generally similar to those observed for 10 except for significant shieldings of chemical shift values for protons H-1, H-10 and H-12, and deshieldings of H-11, H-13 and H-1', compared to those observed in 10 (δH-1 5.08, δH-10 3.98, δH-11 5.77, δH-12 5.32, δH-13 5.87, δH-1´ 4.91 vs. δH-1 5.98, δH-104.32, δH-11 5.20, δH-12 5.61, δH-135.72 and δH-1´ 4.54, respectively, for 10). A COSY spectrum showed correlations between δH-10 3.98, δH-11 5.77, δH-12 5.32 and δH-13 5.87, and between δH-10 3.98 (brd, J=10.0 Hz) and δH-1 5.08 (d, J=10.0 Hz), suggesting a similar system [−CH-CH(OR)-CH(OAc)-CH(OAc)-CH(OAc)-] as observed for 10, although the chemical shift values for these protons were different to those of 10. Likewise, the 13C NMR also displayed significant deshielding of C-10 and C-1' (δC-10 81.2, δC-1' 101.6 vs. δC-10 72.9, δC-1' 96.7), shielding of C-11 and C-14 (δC-11 69.5, δC-14 164.4 vs. δC-11 77.2, δC-14 170.6, respectively, for 10), compared to those observed for 10, suggesting compound 11 appeared to be a diastereoisomer of 10. The gross structure was established by HMBC experiments, which revealed 2J and 3J-correlation of protons and carbons of ring A – C, and glucose moiety involving H-1 and C-3, C-4a, C-9a, C-10, C-11 and C-14, and H-10 and C-1, C-1', C-9a and C-12. The presence of a free indole N-H proton and C-7-OMe group was also confirmed by HMBC, which showed correlation between N9-H (δN-H 8.45) and C-4a, C-4b, C-8a and C-9a, and between C-7-OMe (δH-7-OMe 3.84) and C-7 (δC 156.8), respectively, as observed for 10.
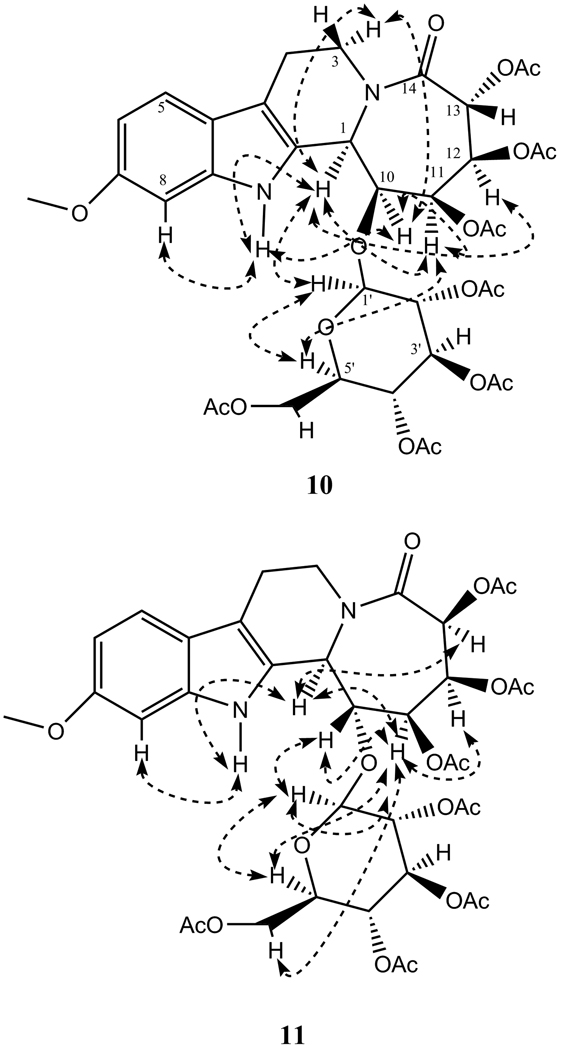
The absolute stereochemistry of C-1 stereocenter of compound 10 and 11 was established by Circular Dichroism spectra and NOESY correlations. The CD spectra of the two compounds displayed a high amplitude strong positive Cotton effect in the 310–320 nm regions, which is explicable to C-1(R) chiral center with α-orientation of H-1. These data was in agreement with C-1(R) mitragynine and analogs (Lee et al., 1967) with strong positive Cotton effect at 270–300 nm regions, while C-1(S)-speciogynine (Lee et al., 1967) and C-1(S)-cadambine (Brown and Fraser, 1974) exhibited strong negative Cotton effect in these regions in their CD spectra. In addition, the 1H NMR spectrum showed small coupling constant (J = 4.3 Hz) of 10, compared to large value (J = 10 Hz) for 11, suggesting cis- and trans-relationships between H-1 and H-10 protons in these two compounds, respectively. Based on this assumption, the spatial orientation of the relevant protons was confirmed by NOESY spectrum of 10 and 11 (Figure 2). Compound 10 showed correlation between H-1, H-3a, H-11 and H-12, and H-10 and H-3a, indicating that these protons are α-cofacially oriented, thereby C-10-glucosyl, and C-11 and C-12-acetoxy groups were on the β-face of the molecule. In addition, NOESY spectra also showed cross peaks between H-1' and H-1 and H-5', and H-5' and H-11, suggesting they are in close spatial proximity among themselves. A close comparison of the NOESY spectra of 11 with those of 10 revealed clear differences for the stereocenters C-10 and C-13, which was earlier indicated by the differences in 1H NMR chemical shift values for H-10 – H-13 between the two compounds. A NOESY experiment of 11 (Figure 2) showed correlation between H-1, and H-11 and H-13, as well as H-11 and H-12, suggesting these protons are cis and placed at the α-face of the molecule, since H-1 had α-disposition due to C-1(R) absolute configuration, like 10, determined by CD spectra. Therefore, the acetyl groups at C-11, C-12 and C-13 were placed on the β-face of the molecule. The placement of C-10 glucosyl moiety at the α-face of the molecule was evident from the large trans coupling (J1,10 = 10 Hz) between H-1 and H-10, as well as NOESY correlations between H-10 and H-1', suggesting their close spatial relationships in 11. Analyses of the molecular models further confirmed the above observations. In addition, NOESY spectra also showed cross peaks between the protons H-1', H-5', and H-11, and H-6'a and H-11, suggesting their close spatial proximity. Collectively, these data permitted the assignment of configuration for compound 10 and 11, and unequivocally confirmed the structures of banistenoside A (1) and banistenoside B (2).
Figure 2.
2D NMR NOESY correlations of 10 and 11.
Compound 4 was isolated as gum and analyzed for C12H14N2O by ESIHRMS. This compound was homogenous on TLC [Rf 0.40, silica gel/solvent: CHCl3-MeOH (2:8)] and not reactive to Dragendorff’s reagant. The 1H and 13C NMR spectra of 4 was generally similar to those observed for THH (5), except for the differences associated with the presence of a methylene group at C-1 (δH-1 4.15, 2H, m; δc 41.1, t), instead of a methine substituted with a methyl group (δH-1 1H, m; δc 50.9, d; δH-10 1.69, d, J=6.4 Hz; δc 18.1) of 5. In addition, a close comparison between the NMR spectra of 4 and other THH analogs (Faizi and Naz, 2002), led to the conclusion that 4 contained a methylene group instead of the methine group at C-1 of THH, suggesting 4 was a C-1 nor- derivative of THH (5). Complete assignment of the spectral data was carried out using 2D NMR experiments, due to unavailability of spectral data in the literatures. The HMBC experiment showed three bond correlations between H2-1, C-3, C-4a and two bond correlations between H2-1 and C-9a. It also showed correlations between H-5, C-6 and C-7, and the H7-OMe group showed cross peak with C-7, suggesting the substitution of the OMe was at C-7. Based on the foregoing data compound 4 was established as THNH.
During the course of the investigation, three sugars, β-D-fructofuranosyl (2→5) fructopyranose (D-difructose) (14), sucrose and β-D-glucose were isolated as major primary metabolites. Compound 14 was isolated as gum and homogenous on TLC [Rf 0.35, silica gel/solvent: CHCl3-MeOH (3:7)]. Its 13C NMR spectrum was generally similar to those observed for fructan-type disaccharide with two fructofuranosyl units (Timmermans et al., 2001), which displayed 12 major carbon signals consisting four triplets, six doublets and two singlets, the latter assigned to anomeric carbons at C-2 and C-2' (iC-2 101.7, δC-2' 97.8 in CD3OD). Comparison of the 13C NMR data of 14 with those of β-D-fructofuranosyl(2→2)β-D-fructofuranose, isolated from Morinda citrifolia (Lin, 2004), suggested the presence of a non-symmetrical dimmer for 14, instead of a symmetrical dimmer, which reported only six 13C NMR signals due to symmetrical nature of the molecule. On acetylation fraction (H-1) containing 14 as a major compound afforded the corresponding octaacetate 17 by using CPTLC (see Materials and Method section). Compound 17 was analyzed for C28H38O19 by positive ion ESIHRMS, and found to be dextrorotatory. Its 1H and 13C NMR spectra (in CDCl3) displayed signals for eight acetyl groups, in addition to 12 carbon signals consisting four triplets, six doublets and two singlets, the latter assigned to one anomeric carbon (δC-2 107.9) and a carbonyl (δC-2 197.9), suggesting one of the anomeric center of 14 was opened during acetylation. The 1H NMR spectrum of 17 demonstrated multiplicities for all the protons, previously not observed for 14, and structure 14 was fully substantiated by 2D NMR COSY, HMQC, HMBC and NOESY experiments on 14-Ac (17). The HMBC experiment showed key 2J and 3J- correlations between C-2 and H2-1, H-3 and H-4, and C-5 and H-3 and H2-6 for the fructofuranose unit. It also showed correlations between C-2' and H2-1', H-3' and H-4', and C-5' and H-4', and H2-6', attributable to acetylated acyclic fructose unit of 17. Based on the foregoing data compound 14 was established as β-D-fructofuranosyl-(2→5)-fructopyranose.
The structures of harmol (3), THH (5), harmaline (6) (Coune et al., 1980; Atta-ur-Rehman and Tayyaba, 1972), harmine (7), (−)-2R,3R–epicatechin (8) (Balde et al., 1991; Sun et al., 2006) and (−)-2R,3R–epicatechin-4β8-(−)-2R,3R–epicatechin (procyanidin B2) (9) (Barrett et al., 1979; Korver and Wilkins, 1971; Khan et al., 1997) were determined by physical and spectroscopic data (1H and 13C NMR, 2D NMR experiments, and MS) as well as by direct comparison, except for 9, with their respective authentic samples. The 1H and 13C NMR spectra of harmaline (6) in CD3OD exhibited an equilibrium between imine-enamine tautomeric forms (Coune et al., 1980; Atta-ur-Rehman and Tayyaba, 1972). The imine was predominant in DMSO-d6 (aprotic solvent), while both the tautomers were detected in CD3OD/ trifluroacetic acid (1 drop) as mixtures in the NMR spectra. The structures of 8 and 9 were confirmed by chemical derivatization to their corresponding peracetate 12 and 13. Compound 9 and 13 adopt two conformations in solution, in which the interflavan dihedral angles are roughly ±90°. The preferred conformation of the pyran ring is a half-chair with the 3,4-dihydroxyphenyl substituents in pseudo-equatorial positions (Khan et al., 1997). The absolute configuration of 8 and 9 was confirmed by optical rotation and Circular Dichroism experiments, which is consistent with the CD spectra of reference (−)-2R,3R-epicatechin (for 8) and literature data (for 9) (Korver and Wilkins, 1971; Barrett et al., 1979).
3.2. Biological activity
The hot aqueous extracts of fresh and dried large branches of B. caapi and isolated compounds 1–9 were evaluated for MAO inhibitory and antioxidant activities, as well as cytotoxicity against selected human cancer and mammalian (VERO) cells. Assays for MAO-A and MAO-B inhibitions were carried out fluorometrically at 460 nm emission wavelength using recombinant human brain MAO-A and B enzymes, which showed strong inhibitory activities against MAO-A (IC50 ∼0.01–0.4 µg/mL), and weak activities against MAO-B (i.e., ∼25% inhibition at 5 µg/mL) for most of the extracts. During the measurement of MAO-B activity, strong fluorescence interferences at 460 nm emission wavelength were observed at a concentrations of >5 µg/mL, which interfered with IC50 determinations. To resolve fluorescence interference, a modified standardized protocol with an emission wavelength of 380 nm was employed (Parikh et al., 2002). Using this protocol, percent inhibitions were determined at 1, 10 and 100 µg/mL for MAO-B, while the MAO-A inhibitions remained closely similar to those measured at 460 nm. The extracts of fresh and dried samples of bark/ debarked large branch demonstrated potent MAO-A inhibition (IC50 0.02–0.05 µg/mL) and modest MAO-B inhibitory activities (IC50 ∼100 µg/mL) (Table 1). MAO-A activity of extracts of fresh/ dried stem or bark/ debarked large branch was found to be 2500-fold more potent than MAO-B. The activity of most potent MAO-A inhibitors harmine (7) or harmaline (6) was found to be >10,000-fold more potent than MAO-B (IC50 2.0 nM/ 2.5 nM vs. 20 µM/ 25 µM), which was proportionately consistent with those observed for the crude extract. The activity of 3 and 5 was found to be much weaker than 6 or 7 against MAO-A, while compounds 1, 2 and 4 were inactive (Table 1).
In addition, strong antioxidant activity (Rosenkranz et al., 1992; Scudiero et al., 1988) for inhibition of cellular Reactive Oxygen Species (ROS) generation by phorbol-12-myristate-13-acetate (PMA) was observed for large branches (IC50 0.4–3.0 µg/mL) (Table 1). (−)-Epicatechin (8) and (−)-procyanidin B2 (9) demonstrated potent antioxidant activity; both found to be more potent than vitamin C (IC50 <0.13 and 0.57µg/mL vs. 1.34 µg/mL), while 8 was also more effective than trolox (IC50 0.14 µg/mL). Both the compounds also showed MAO-B inhibitory activity (IC50 65 and 35 µM, respectively) and weak MAO-A inhibitions (IC50 51.7 and 8.5 µM for 8 and 9, respectively). However, epicatechin and catechin had previously been reported as MAO-B inhibitors (IC50 58.9 µM) (Hou et al., 2005). Evaluation of regular sample of B. caapi dried stem (BCEx-1 and BCEx-4) showed consistent MAO-A inhibitory and antioxidant activity, but was relatively less potent than bark of large branch of Da Vine for MAO-A inhibitory (IC50 0.32–0.39 vs. 0.02–0.03 µg/mL) and antioxidant (IC50 1.0–7.8 µg/mL vs. 1.9–2.0 µg/mL) activity. Finally, extracts from different parts of large branch, together with regular/ commercial samples, were tested against acetylcholinesterase (AChE), butylcholinesterase (BuChE) and catechol-O-methyl transferase (COMT) enzymes (100 µg/mL), and cytotoxic activities (Borenfreund et al., 1990) against human breast, melanoma, skin and prostate cancer, and mammalian VERO and kidney cell lines (at 10 µg/mL), and were found to be inactive.
3.3. HPLC analyses and quantification
The isolated markers (1–9) were employed for chemical profiling of B. caapi Da Vine, thereby markers 1–9 were consistently assigned and quantified from aqueous extracts using a reverse phase C-18 column by HPLC according to published methods for β-carboline alkaloids (Abourashed et al., 2003) (using harmine, harmaline, harmol and harmane as standards) and green tea catechins/ proanthocyanidines (Khokhar et al., 1997) (using catechin and epicatechin as standards). HPLC analysis revealed that most of the dominant chemical and bioactive markers were present in high concentrations in dried bark of large branch [1 (0.71%), 2 (1.93%), 5 (0.10%), 7 (0.67%), 8 (0.43%) and 9 (0.16%)]. Analysis of regular/commercial B. caapi dried stems showed a similar qualitative HPLC pattern, but relatively low- content of dominant markers 1 (0.15, 0.17%), 2 (0.07, 0.15%), 7 (0.04, 0.13%) and 9 (0.03, 0.38%) in samples BCEx-1 and BCEx-4, respectively, which led to decreased MAO and antioxidant potency in these samples. However, two other regular/ commercial samples (BCEx-2 and BCEx-3) were found to be inactive and devoid of markers 1–9.
4. Conclusion
This appears to be the first report of banistenoside A (1) and banistenoside B (2), containing an unique “azepino[1,2-a]tetrahydro-β-carboline” carbon framework, either from natural or synthetic sources. Presumably 1 and 2 appears to be biogenetically analogous to mitragynine-type alkaloid dihydrocadambine (Brown and Fraser, 1974) with the unusual N-4 –C-18 bond (i.e., using mitragynine carbon numbering system) in a seven-membered ring. Therefore, the biogenetic pathway for 1(R)-banistenoside A and −B can be envisioned from the precursor of indole alkaloids 3α-H strictosidine via 3α-H epoxystrictosidine, followed by trioxoaglycone (Szabó, 2008), which undergoes extensive decrboxylation, cyclyzation, oxidation and glycoxylation to 1 and 2. Interestingly, a synthetic azepinotetrahydro-β-carboline [CAS 610782-531] related to the compounds 1 or 2 demonstrated potent α2-adrenoceptor activity (Din Belle et al., 2003), therefore, further biological work on 1 and 2 is warranted. In addition, THNH (4) and β-D-fructofuranosyl-(2→5)-fructopyranose (14) were isolated for the first time from a natural source, and (−)-epicatechin (8) and (−)-procyanidin B2 (9) for the first time from the genus Banisteriopsis. During the course of the investigation, 6-methoxy-THH (pinoline) or its corresponding C-5 oxygenated tryptamine analogs, which have potential hallucinogenic or behavioral properties (Rothchild, 2005; Pahkla et al., 1996), were neither identified nor isolated from this cultivar, or regular/ commercial samples. It is also noteworthy that all the isolated β-carbolines 1–7 are oxygenated at C-7 position, suggesting they are biogenetically derived from corresponding C-6 oxygenated tryptamine precursor.
Inhibition of MAO-B activity by β-carbolines harmine (7) and harmaline (6), in addition to potent MAO-A inhibition responsible for antidepressant activity, provide protection against neurodegeneration, and has a potential therapeutic value for the treatment of Parkinson's diseases. In addition, oxidative stress induced by ROS has been strongly associated with the pathogenesis of neurodegenerative disorders, including Parkinson’s and Alzheimer’s disease (Barnham et al., 2004). Therefore, the presence of two potent antioxidants, (−)-epicatechin (8) and (−)-procyanidin B2 (9), have significant added value for the protection of neuronal cells damage by oxidative free radicals. Their selective MAO-B inhibitory activity (Hou et al., 2005) benefits them even more. Usefulness of compounds 8 and 9 was reported for amyloid diseases (Castillo et al., 2002 and 2004), as well as protection against PC12 cells from Aβ-induced neurotoxicity by compound 8 (Cho et al., 2008; Heo and Lee, 2005). In addition, proanthocyanidin dimers, including 9, were recently reported for treating amyloid, α-synuclein or NAC fibrillogenesis in a mammalian subject (Castillo et al., 2004). Collectively, these results give additional basis to the existing claim of B. caapi stem extract for the treatment of Parkinsonism, including other neurodegenerative disorders.
Acknowledgement
The authors sincerely thank Dr. Larry A. Walker, NCNPR, University of Mississippi and Professor Keith Tipton, University of Dublin, Ireland, for their valuable advise, suggestions and comments on the project; Mr. Raymond F. Baker, Harold Lyon Arboretum, University of Hawaii, for his kind collection and supply of fresh plant materials from Oahu, HI, and Mr. Frank Wigger and Dr. Bharati Avula, NCNPR, UM, for NMR support and Mass spectra, respectively. This work was supported by the National Center for Complementary and Alternative Medicine (NCCAM), National Institute of Health, Grant No. 5R21AT003409-02.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abourashed EA, Vanderplank J, Khan IA. High-speed extraction and HPLC fingerprinting of medicinal plants II. Application to harman alkaloids of genus Passiflora. Pharmaceutical Biology. 2003;41:100–106. [Google Scholar]
- Aquino R, De Crescenzo S, De Simone F. Constituents of Banisteriopsis caapi. Fitoterapia. 1991;62:453. [Google Scholar]
- Atta-ur-Rehman, Tayyaba B. Imine-enamine tautomerism in harmaline. Pakistan Journal of Scientific and Industrial Research. 1972;15:9–10. [Google Scholar]
- Baddeley TC, Howie RA, Skakle JMS, Wardell JL. 1,2,3,4-Tetra-O-acetyl-beta-D-glucopyranuronic acid monohydrate at 120 K and anhydrous 1,2,3,4-tetra-O-acetyl-beta-D-glucopyranose at 292 K. Acta Crystallographica, Section C: Crystal Structure Communications C61. 2005:o711–o714. doi: 10.1107/S0108270105034979. [DOI] [PubMed] [Google Scholar]
- Balde AM, Pieters LA, Gergely A, Kolodziej H, Claeys M, Vlietinck AJ. A-type proanthocyanidins from stem bark of Pavetta owariensis. Phytochemistry. 1991;30:337–342. [Google Scholar]
- Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nature Reviews Drug Discovery. 2004;3:205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- Barrett MW, Klyne W, Scopes PM, Fletcher AC, Porter LJ, Haslam E. Plant proanthocyanidines Part 6. Chiroptical studies. Part 95. Circular dichroism of procyanidines. Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry. 1979:2375–2377. [Google Scholar]
- Borenfreund E, Babich H, Martin-Alguacil N. Rapid chemosensitivity assay with human normal and tumor cells in vitro. In vitro cellular and developmental biology. Journal of the Tissue Culture Association. 1990;26:1030–1034. doi: 10.1007/BF02624436. [DOI] [PubMed] [Google Scholar]
- Brown RT, Fraser SB. Anthocephalus alkaloids: cadambine and 3α-dihydrocadambine. Tetrahedron Letters. 1974;23:1957–1959. [Google Scholar]
- Callaway JC, Brito GS, Neves ES. Phytochemical analyses of Banisteriopsis caapi and Psychotria viridis. Journal of psychoactive drugs. 2005;37:145–150. doi: 10.1080/02791072.2005.10399795. [DOI] [PubMed] [Google Scholar]
- Castillo GM, Choi PY, Cummings JA, Nguyen BP, Snow AD. Catechins for the treatment of fibrillogenesis in Alzheimer's disease, Parkinson's disease, systemic AA amyloidosis, and other amyloid disorders. 2002 US 2002151506. [Google Scholar]
- Castillo GM, Nguyen BP, Choi PY, Larsen L, Lorimer SD, Snow AD, inventors. Proanthocyanidines for the treatment of amyloid and alpha-synuclein diseases. WO 2004033448. Patent. 2004
- Cho ES, Lee KW, Lee HJ. Cocoa procyanidins protect PC12 cells from hydrogen-peroxide-induced apoptosis by inhibiting activation of p38 MAPK and JNK. Mutation Research, Fundamental and Molecular Mechanisms of Mutagenesis. 2008;640(1–2):123–130. doi: 10.1016/j.mrfmmm.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Coune CA, Angenot LJG, Denoel J. Carbon-13 NMR of Strychnos alkaloids: harmane and usambarensine derivatives Phytochemistry. 1980;19:2009–2011. [Google Scholar]
- Din Belle D, Jokela R, Tolvanen A, Haapalinna A, Karjalainen A, Sallinen J, inventors. Polycyclic compounds as potent alpha2-adenoceptor antagonist. WO 03082866. Patent. 2003
- Ellman GL, Courtney KD, Andres V, Jr, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical Pharmacology. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Faizi S, Naz A. Jafrine, a novel and labile β-carboline alkaloid from the flowers of Tagates patula. Tetrahedron. 2002;58:6185–6197. [Google Scholar]
- Hashimoto Y, Kawanishi K. New organic bases from amazonian Banisteriopsis caapi. Phytochemistry. 1975;14:1633–1635. [Google Scholar]
- Hashimoto Y, Kawanishi K. New alkaloids from Banisteriopsis caapi. Phytochemistry. 1976;15:1559–1560. [Google Scholar]
- Heo HJ, Lee CY. Epicatechin and catechin in cocoa inhibit amyloid β-protein induced apoatosis. Journal of Agricultural and Food Chemistry. 2005;53(5):1445–1448. doi: 10.1021/jf048989m. [DOI] [PubMed] [Google Scholar]
- Hochstein FA, Paradies AM. Alkaloids of Banisteria caapi and Prestonia amazonicum. Journal of the American Chemical Society. 1957;79:5735. [Google Scholar]
- Hou WC, Lin RD, Chen CT, Lee MH. Monoamine oxidase B (MAO-B) inhibition by active principles from Uncaria rhynchophylla. Journal of Ethnopharmacology. 2005;100:216–220. doi: 10.1016/j.jep.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Kawanishi K, Saiki K, Tomita H, Tachibana Y, Farnsworth NR, Bohike M. Chemical components of the Brazilian shamanistic drink “Ayahuaska”. Advances in Mass Spectrometry. 1998;14 D053560/1-D053560/12. [Google Scholar]
- Kawanishi K, Uhara Y, Hashimoto Y. Shihunine and dihydroshihunine from Banisteriopsis caapi. Journal of Natural Products. 1982;45:637–639. [Google Scholar]
- Khan ML, Haslam E, Williamson MP. Structure and conformation of the procyanidin B2 dimer. Magnetic Resonance in Chemistry. 1997;35(12):854–858. [Google Scholar]
- Khokhar S, Venema D, Hollman PC, Dekker M, Jongen W. A RP-HPLC method for the determination of tea catechins. Cancer Letters. 1997;114:171–172. doi: 10.1016/s0304-3835(97)04653-3. [DOI] [PubMed] [Google Scholar]
- Korver O, Wilkins CK. Circular dichroism spectra of flavanols. Tetrahedron. 1971;27:5459–5465. [Google Scholar]
- Learmonth DA, Palma PN, Vieira-Coelho MA, Soares-da-Silva P. Synthesis, biological evaluation and molecular modeling of a novel, peripherally selective inhibitor catechol-O-methyltransferase. Journal of Medicinal Chemistry. 2004;47:6207–6217. doi: 10.1021/jm040848o. [DOI] [PubMed] [Google Scholar]
- Lee CM, Trager WF, Beckett AH. Corynantheidine-type alkaloids. II. Absolute configuration of mitragynine, speciociliatine, mitraciliatine and speciogynine. Tetrahedron. 1967;23:375–385. doi: 10.1016/s0040-4020(01)83323-8. [DOI] [PubMed] [Google Scholar]
- Lin L, inventor. Compound-morindin A and its preparation and use. Faming Zhuanli Shenqing Gongkai Shuomingshu. CN 1486987. Patent. 2004
- Mabberley DJ. A Portable Dictionary of the Higher Plants. Second Edition. Cambridge: Cambridge Univ. Press; 1997. The Plant Book; p. 858. [Google Scholar]
- Miller LS. Reexamination Certificate US PP5751. 1986. Banisteriopsis caapi (cv) ‘Da Vine’ US PP5751; Appl. #669745, 1984; Int Class A01H 005/00; ibid, US PP5751; p. 2001. [Google Scholar]
- Pahkla R, Harro J, Rago L. Behavioural effects of pinoline in rat forced swimming, open field and elevated plus-maze test. Pharmacological Research. 1996;34:73–78. doi: 10.1006/phrs.1996.0066. [DOI] [PubMed] [Google Scholar]
- Parikh S, Hanscom S, Gagne P, Crespi C, Patten C. A. fluorescent-based, high-throughput assay for detecting inhibitors of human Monoamine Oxidase A and B. BD Bioscience Discovery Labware. 2002 S02T081R2. [Google Scholar]
- Rivier L, Lindgren JE. “Ayahuasca”, the South American hallucinogenic drink. Ethnobotanical and chemical investigation. Economic Botany. 1972;26:101–129. [Google Scholar]
- Rosenkranz AR, Schmaldienst S, Stuhlmeier KM, Chen W, Knapp W, Zlabinger GJ. A microplate assay for the detection of oxidative products using 2’, 7’-dichloroflurescin-diacetate. Journal of Immunological Methods. 1992;156:39–45. doi: 10.1016/0022-1759(92)90008-h. [DOI] [PubMed] [Google Scholar]
- Rothchild R. Proton and carbon-13 NMR studies of some tryptamines, precursors, and derivatives: Ab initio calculations for optimized structures. Spectroscopy letters. 2005;38:521–537. [Google Scholar]
- Sánchez-Ramos JR. Banisterine and Parkinsons disease. Clinical Neuropharmacol. 1991;14:391–402. doi: 10.1097/00002826-199110000-00002. [DOI] [PubMed] [Google Scholar]
- Schultes RE. The Botanical and Chemical Distribution of Hallucinogens. Annual Review of Plant Physiology. 1970;21:571–598. [Google Scholar]
- Schultes RE, Hofmann A. Plants of the Gods Their Sacred, Healing and Hallucinogenic Powers. Rochester, VT: Healing Arts Press; 1992. pp. 124–135. [Google Scholar]
- Schultes RE, Raffauf RF. Vine of the Soul: Medicine Men, Their Plants and Rituals in the Colombian Amazon. Oracle, AZ: Synergetic Press; 1992. [Google Scholar]
- Schultes RE, Siri von Reis. Ethnobotany, Evolution of a Discipline. Portland, OR: Dioscorides Press; 1995. [Google Scholar]
- Schwarz MJ, Houghton PJ, Rose S, Jenner P, Lees AD. Activities of extract and constituents of Banisteriopsis caapi relevant to Parkinsonism. Pharmacology, Biochemistry and Behavior. 2003;75:627–633. doi: 10.1016/s0091-3057(03)00129-1. [DOI] [PubMed] [Google Scholar]
- Scudiero DA, Shoemaker RH, Paull KD, Monks A, Tierney S, Nofziger TH. Evalualtion of a soluble tetrazolium/ formazan assay for cell growth and drug sensitivity in culture using human and other cell lines. Cancer Research. 1988;48:4827–4833. [PubMed] [Google Scholar]
- Serrano-Dueñas M, Cardozo-Pelaez F, Sanchez-Ramos JR. Effects of Banisteriopsis caapi extract on Parkinson’s disease. The Scientific Review of Alternative Medicine. 2001;5:127–132. [Google Scholar]
- Sun J, Jiang Y, Wei X, Shi J, You Y, Liu H, Kakuda Y, Zhao M. Identification of (−)-epicatechin as the direct substrate for polyphenol oxidase isolated from litchi pericarp. Food Research International. 2006;39:864–870. [Google Scholar]
- Szabó LF. Rigorous biogenetic network for a group of indole alkaloids derived from strictosidine Molecules. 2008;13:1875–1896. doi: 10.3390/molecules13081875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermans JW, Slaghek TM, Lizuka M, Van den Ende W, De Roover J, Van Laere A. Isolation and structural analysis of new fructans produced by chicory. Journal of Carbohydrate Chemistry. 2001;20:375–395. [Google Scholar]