SUMMARY
Despite the remarkable regenerative capacity of mammalian skin, an adult dermal stem cell has not yet been identified. Here, we investigated whether skin-derived precursors (SKPs) might fulfill such a role. We show that SKPs derive from Sox2+ hair follicle dermal cells, and that these two cell populations are similar with regard to their transcriptome and functional properties. Both clonal SKPs and endogenous Sox2+ cells induce hair morphogenesis, differentiate into dermal cell types, and home to a hair follicle niche upon transplantation. Moreover, hair follicle-derived SKPs self-renew, maintain their multipotency, and serially reconstitute hair follicles. Finally, grafting experiments show that follicle-associated dermal cells move out of their niche to contribute cells for dermal maintenance and wound-healing. Thus, SKPs derive from Sox2+ follicle-associated dermal precursors and display functional properties predicted of a dermal stem cell, contributing to dermal maintenance, wound-healing, and hair follicle morphogenesis.
HIGHLIGHTS
Hair follicles contain Sox2+ dermal precursors that are cells of origin for cultured SKPs
Sox2+ dermal precursors and SKPs share similar function and transcriptional profile
SKPs function as dermal stem cells, clonally generating dermal cells and inducing hair morphogenesis
SKPs home to a hair follicle niche and this niche maintains their stem cell properties
INTRODUCTION
The skin is a unique organ that undergoes continuous cell-turnover and harbors significant regenerative capacity in order to repair environmentally-mediated insults. At least some of this regenerative capacity is due to somatic tissue stem cells, including basal layer and hair follicle epidermal stem cells (Fuchs, 2009) and melanocyte stem cells (Nishimura et al., 2005). However, a dermal stem cell, responsible for maintaining and repairing the dermis, has not yet been described.
The dermis is a complex tissue comprised of many cell types, including dermal fibroblasts, myofibroblasts, adipocytes, blood vessels, nerves, and sensory receptors such as Merkel cells. The dermis also contributes the inductive mesenchymal cells necessary for regulating hair follicle morphogenesis, a cyclic process that occurs continuously throughout the life of many mammals. During embryogenesis, the dermis develops from mesenchymal precursors that generate dermal fibroblasts and adipocytes, and produce the inductive hair follicle cells. Thus, by analogy to other somatic tissue stem cells, one possibility is that a multipotent embryonic mesenchymal precursor persists into adulthood, thereby providing the dermal stem cell activity necessary for homeostatic maintenance and regeneration of this tissue. Support for the presence of such a multipotent dermal precursor comes from work showing that, in some animals, dermal cells play a key role in limb regeneration (Muneoka et al., 1986; Kragl et al., 2009), and can directly differentiate into the skeletal cells necessary to form an exoskeleton (Vickaryous and Hall, 2008).
In this regard, we previously isolated a multipotent precursor cell from rodent and human dermis that differentiated into mesodermal and peripheral neural progeny including adipocytes, skeletogenic cell types and Schwann cells (McKenzie et al., 2006). These cells, termed SKPs for SKin-derived Precursors, displayed properties similar to embryonic neural crest precursors, and within facial dermis were derived from the neural crest (Fernandes et al., 2004). Interestingly, the dermal papillae (DP) of hair follicles appear to comprise one niche for SKPs, based upon coincident patterns of gene expression, and upon the finding that cells with properties of SKPs can be cultured from adult whisker follicle papillae (Fernandes et al., 2004; Hunt et al., 2007; Joannides et al., 2004). Since DP mesenchymal cells are essential for hair follicle induction (Jahoda et al., 1984; Oliver, 1967), and since it has been suggested that DP cells might be dermal precursors (Gharzi et al., 2003), we asked whether SKPs might represent a previously-unrecognized dermal stem cell. Here, we provide evidence in support of this idea, showing that SKPs derive from Sox2+ follicle-associated precursors, and that they can contribute dermal cells for tissue maintenance, wound-healing, and hair follicle morphogenesis.
RESULTS
Endogenous hair follicle DS and DP cells express the stem cell gene Sox2 and generate SKPs when cultured
To investigate whether SKPs originate from endogenous hair follicle dermal cells, we took advantage of our finding that Sox2 is expressed by SKPs, as detected by RT-PCR and immunostaining of neonatal murine SKP spheres (Fig. 1A,B). Immunostaining for Sox2 in neonatal murine back skin showed its expression in follicle DP and lower dermal sheath (DS) cells (Fig. 1C). We confirmed this localization in a mouse with EGFP knocked-in to the Sox2 locus (Ellis et al., 2004). Within skin, Sox2:EGFP-expressing cells first appeared in the embryonic dermal condensates that precede hair and whisker follicle formation (Fig. 1D; Fig. S1A,B), as recently published (Driskell et al., 2009). At birth, when hair follicles were in the anagen growth phase, Sox2:EGFP was expressed in all awl, auchene and guard hair follicle DS and DP (Fig. 1E,F). In adulthood, Sox2:EGFP was expressed in DP and DS cells of anagen, but not catagen/telogen follicles (Fig. 1G–I), suggesting that expression was dynamically regulated. Sox2:EGFP was also expressed in dermal cells of adult whisker follicles (Fig. S1C), and in a small number of cells in close proximity to the hair follicle bulge (Fig. S1D). These latter cells did not express K17, K15, nestin, PDGFRα, the melanocyte marker DCT or the Schwann cell marker P0 (data not shown). However, a few expressed the epidermal precursor marker K5 (Fig. S1D).
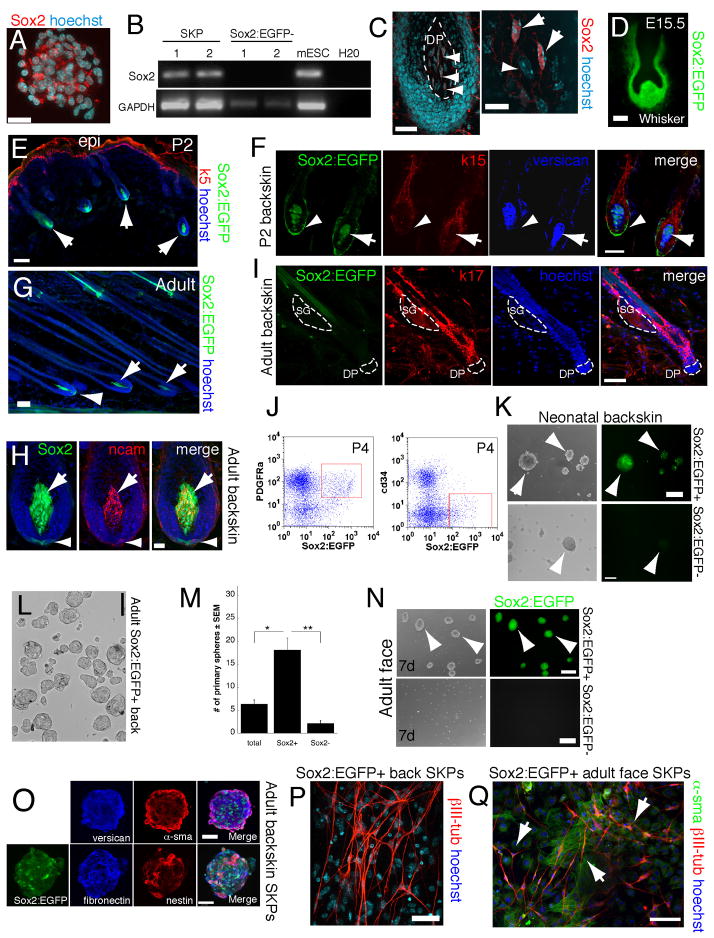
Figure 1. Sox2 is dynamically expressed in the hair follicle DP and DS.
(A) Neonatal murine SKP sphere immunostained for Sox2 (red). (B) RT-PCR showing Sox2 expression in 2 neonatal murine SKP cultures. Embryonic stem cells (mESC) were a positive control. (C) Longitudinal section of a neonatal mouse follicle showing Sox2+ DP cells (red, arrowheads). Right panel shows Sox2+ (arrows) and negative (arrowhead) DP cells at higher magnification. (D) Sox2:EGFP+ cells (green) in an E15.5 whisker follicle. (E,F) Dorsal skin sections from P2 Sox2:EGFP mice immunostained for EGFP (green), the epidermal markers keratin 5 (E) or keratin 15 (F; both red), and the DP marker versican (blue). In (E) arrows denote anagen hair follicles. In (F) arrowheads indicate EGFP+ DS cells and arrows EGFP+ DP cells. (G–I) Dorsal skin sections of adult Sox2:EGFP mice immunostained for EGFP (green) and NCAM (H, red) or keratin 17 (I, red). (G,H) show EGFP+ cells in anagen hair follicles, with arrows denoting the DP and arrowheads the lower DS. (I) shows a telogen hair follicle where the DP (indicated by hatched lines) is negative for Sox2:EGFP expression. (J–Q) Sox2:EGFP+ hair follicle cells proliferate in FGF2 and EGF to generate multipotent SKP spheres. (J) Flow cytometric analysis of neonatal Sox2:EGFP backskin cells sorted for EGFP (x-axis) and PDGFRα or cd34 (y-axis). Red boxes indicate gates for prospective isolation experiments. (K) Sox2:EGFP+ (top panels) and negative (bottom) neonatal backskin cells cultured for 7 days in SKPs conditions. Arrows denote SKP spheres. (L,M) Quantification (M) of SKP spheres generated from sorted adult Sox2:EGFP+ (L) versus Sox2:EGFP− back skin cells in experiments similar to that in (K). Total back skin cells from the same mice were used for comparison (*p<0.05, **p<0.001; n=4). (N) Sox2:EGFP+ (top) and negative (bottom) cells sorted from adult facial skin and cultured in SKPs conditions for 7 days. (O) SKP spheres generated from Sox2:EGFP+ adult back skin cells immunostained for EGFP (green), and the SKP markers fibronectin (blue) and nestin (red; merge is on right) or versican (blue) and α-sma (red; merge is on the right). (P,Q) Sorted adult Sox2:EGFP+ back skin (P) or facial skin (Q) cells were cultured as SKPs, differentiated under neural conditions for 12–18 days and immunostained for the neuronal marker III-tubulin (red in both) and -sma (green in Q). In some panels, cells were counterstained with Hoechst 33258 (blue), as indicated. Scale bars = 25μm in A,C (left panel), 10μm in C (right panel), 50μm in E,F,G,H and 100μm in D,I,K,L,N,O,P,Q. See also Figure S1.
To determine whether Sox2+ follicle cells gave rise to SKPs, we prospectively isolated them using flow cytometry. Approximately 1–3% and 0.1–1% of neonatal and adult back skin cells, respectively, were Sox2:EGFP positive (Fig. S2A,B). Similar results were obtained with facial skin (Fig. S2C,D). Most Sox2:EGFP+ cells expressed PDGFRα, but not CD34, a marker for bulge epidermal cells and dermal fibroblasts (Fig. 1J). Sorted Sox2:EGFP+ cells from neonatal and adult back and facial skin gave rise to EGFP+ SKP spheres when cultured in SKPs conditions, and they were enriched in this ability relative to Sox2:EGFP− cells (Fig. 1K,N). Quantification showed that adult Sox2:EGFP+ back skin cells were enriched 4-fold and 10-fold relative to unsorted and EGFP− cells (Fig. 1L,M). SKP spheres generated from Sox2:EGFP+ cells expressed the SKPs markers fibronectin, nestin, versican, and α-sma (Fig. 1O), and when differentiated for 14 days, generated βIII-tubulin+ neurons and α-sma+ myofibroblasts (Fig. 1P,Q) that had lost their EGFP expression. Thus, anagen hair follicles are a primary niche for SKP-forming dermal precursors.
Prospectively-isolated Sox2:EGFP+ dermal cells home to hair follicles, induce hair follicle morphogenesis, and differentiate into neural and dermal cell types
Since SKPs generate peripheral neural cells, we differentiated sorted Sox2:EGFP+ cells versus Sox2:EGFP− cells under neural conditions. Only Sox2:EGFP+ cells generated nestin+ precursors (data not shown) or βIII-tubulin+ neurons (Fig. 2A). We then asked whether the sorted cells could induce hair follicle morphogenesis, as predicted by their anatomical localization, using an ex-vivo hair reconstitution “patch assay” (Zheng et al., 2005). These experiments showed that Sox2:EGFP+, cd34− cells induced hair morphogenesis when mixed with neonatal C57/Bl6 epidermal cells and transplanted beneath the dermis of adult nude mice for 12 days (Fig. 2B). In addition, Sox2:EGFP− cells were depleted in this inductive ability relative to total dermal cells (Fig. 2C).
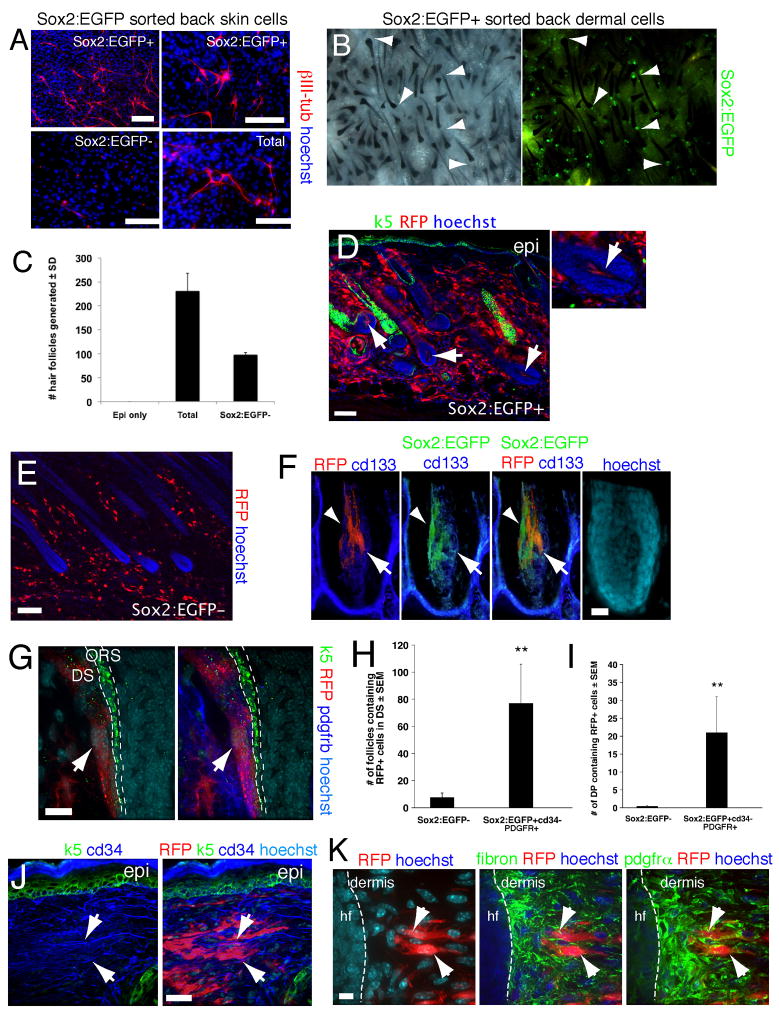
Figure 2. Sox2:EGFP prospectively identifies endogenous dermal precursors that induce hair follicles, home to a hair follicle niche, and differentiate into neural and dermal cell types.
(A) Sorted Sox2:EGFP+ and negative neonatal back skin cells differentiated for 12 days under neural conditions, and immunostained for III-tubulin (red). Total cells were sorted but gated only for live cells. (B) Patch assays using sorted Sox2:EGFP+, cd34− neonatal back skin dermal cells. The left panel shows brightfield and the right fluorescence illumination. Arrowheads denote EGFP+ DP. (C) Number of hair follicles generated in patch assays with total or Sox2:EGFP− neonatal dermal cells (n = 3). (D–K) Sorted, uncultured Sox2:EGFP+, cd34−, PDGFRα+ (D,F–K), or Sox2:EGFP− (E) neonatal back skin cells were transplanted into adult NOD/SCID mouse back skin, and analyzed after 2 weeks. All sorted cells were infected with an RFP-expressing retrovirus. (D) Immunostaining for RFP (red) and keratin 5 (green). Arrows denote follicle DP containing transplanted cells, with the right panel at higher magnification. (E) Immunostaining for RFP (red) on skin transplanted with Sox2:EGFP− cells. (F,G) High magnification confocal images of hair follicles immunostained for RFP (F,G, red) and Sox2:EGFP (F, green) plus the DP marker cd133 (F, blue), or keratin-5 (G, green) plus PDGFRβ (G, blue). In (F) the arrowhead and arrow denote Sox2:EGFP+ and cd133+ cells, respectively. In (G) the arrow denotes RFP+, PDGFRβ+ cells in the DS. ORS = outer root sheath. (H,I) Number of hair follicles (H) or follicle DP (I) containing RFP+ transplanted cells as shown in D–G. **P<0.01. (J,K) Immunostaining of interfollicular dermis for RFP (red) and cd34 (J, blue) plus keratin 5 (J, green) or fibronectin (K, pseudocolored green, center panel) plus PDGFRα (pseudocolored green, right panel). Arrows show cells positive for RFP and cd34 (J), or for RFP, fibronectin and PDGFRα (K). hf = hair follicle. Nuclei were stained with Hoechst 33258 (blue), as indicated. Scale bars = 100μm in A,D,E, 25μm in F,J and 10μm in G,K. See also Figure S2.
To ask if Sox2:EGFP+ cells could also generate differentiated dermal progeny, we transduced sorted Sox2:EGFP+, PDGFRα+, CD34− backskin cells with an RFP-expressing retrovirus (to allow tracing if Sox2:EGFP expression was lost), and transplanted them into NOD/SCID mouse back skin for 3 weeks. For comparison, we used Sox2:EGFP− cells. Remarkably, the Sox2:EGFP+, but not negative, cells homed back to their DP and DS follicle niche, where they appropriately expressed the DP markers NCAM, cd133 and Sox2:EGFP itself (Fig. 2D–G; Fig. S2E–G). Quantification confirmed this selective homing (Fig. 2H,I). In contrast, Sox2:EGFP+ cells that integrated into the interfollicular dermis expressed the dermal fibroblast markers PDGFRα, PDGFRβ, fibronectin, and cd34 (Fig. 2J,K; Fig. S2I) but no longer expressed EGFP or cd133 (Fig. S2H). Thus, Sox2:EGFP+ follicle dermal cells home to a hair follicle niche, induce hair follicles, generate interfollicular dermal cell types, and differentiate into neural cells that are never normally found within skin.
Global gene expression analysis of SKPs versus Sox2:EGFP+ dermal cells
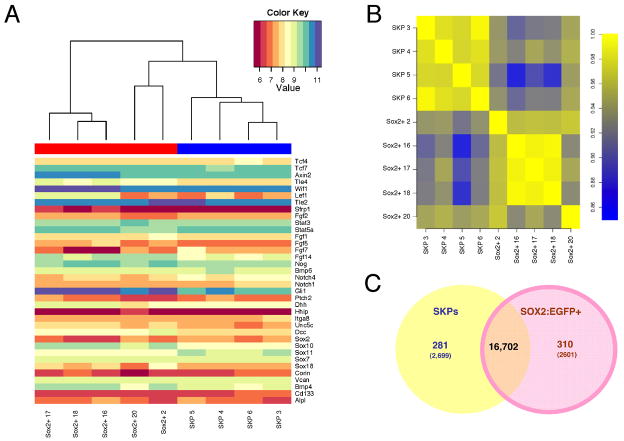
To ask whether SKPs maintain properties of their Sox2+ dermal precursor parents, we performed global gene expression analysis, comparing 4 primary passage neonatal back skin SKP preparations with 5 sorted neonatal Sox2:EGFP+CD34− back skin cell isolates. RNA samples were analyzed on Affymetrix GeneChip Mouse Gene 1.0 ST Array. Spearman rank correlation analysis demonstrated that the two groups were very similar, with intersample correlations ranging from 0.86 to 0.94 (Fig. 3B). For comparison, intrasample analysis demonstrated correlations of 0.91–0.99 and 0.96–0.99 for Sox2:EGFP+ and SKP samples, respectively (Fig. 3B). We also analyzed a subset of 36 genes previously associated with follicle dermal cells including versican, BMP4, cd133, alkaline phosphatase, and corin. Sox2:EGFP+ cells and SKPs expressed these genes at similar levels (Fig. 3A).
Figure 3. Microarray analysis demonstrates that SKPs and Sox2:EGFP+ cells are transcriptionally similar.
Microarray gene expression profiling of sorted Sox2:EGFP+, cd34− neonatal dermal cells versus primary passage neonatal murine SKPs. (A) Heat map showing the relative expression levels of 36 hair follicle dermal genes in 5 independent Sox2:EGFP cell isolates (red bar on the top left) and 4 independent SKP cultures (blue bar on the top right). Relative expression levels are color-coded as per the color key. The cluster analysis on top shows that some Sox2:EGFP+ cell samples are more related to SKPs than they are to each other. (B) Spearman rank correlations were computed for every pair of microarray experiments. The resulting color-coded correlation matrix reveals the similarity of the transcriptomes of SKPs and Sox2:EGFP+ cells. (C) Venn diagram illustrating that 281/22,102 and 310/22,102 genes are upregulated by at least 2-fold in SKPs and Sox2:EGFP cells respectively, while an additional 2418 and 2291 genes are significantly upregulated by less than 2-fold. See also Figure S3.
Some genes were, however, expressed at significantly different levels based on the F-statistic with Benjamini-Hochberg multiple testing correction implemented in the LIMMA Bioconductor package (Smyth, 2004). Of 22,102 non-redundant protein-coding genes assayed by the array, 281 and 310 were significantly upregulated at least two-fold (Fig. 3C). Ingenuity pathway analysis demonstrated that most of these were associated with metabolic pathways or growth factor signaling (Fig. S3A). SKPs were relatively enriched in genes associated with O-glycan, phenylalanine, tyrosine and tryptophan biosynthesis, and the citrate cycle, and Sox2:EGFP+ cells in genes associated with ERK and PI3-kinase-Akt signaling, and glycosphingolipid biosynthesis. These differences were likely due to differences in environment rather than cell identity, since of those transcription factors differentially expressed 2-fold or more (the range was 2 to 4.3-fold), none were cell-type-specific or associated with dermal or stem cell identity (Fig. S3B). Thus, SKPs and Sox2:EGFP+ cells are highly similar at the transcriptional level, arguing that culturing does not fundamentally change the identity of the endogenous dermal precursors.
SKPs differentiate into dermal cell types within intact skin
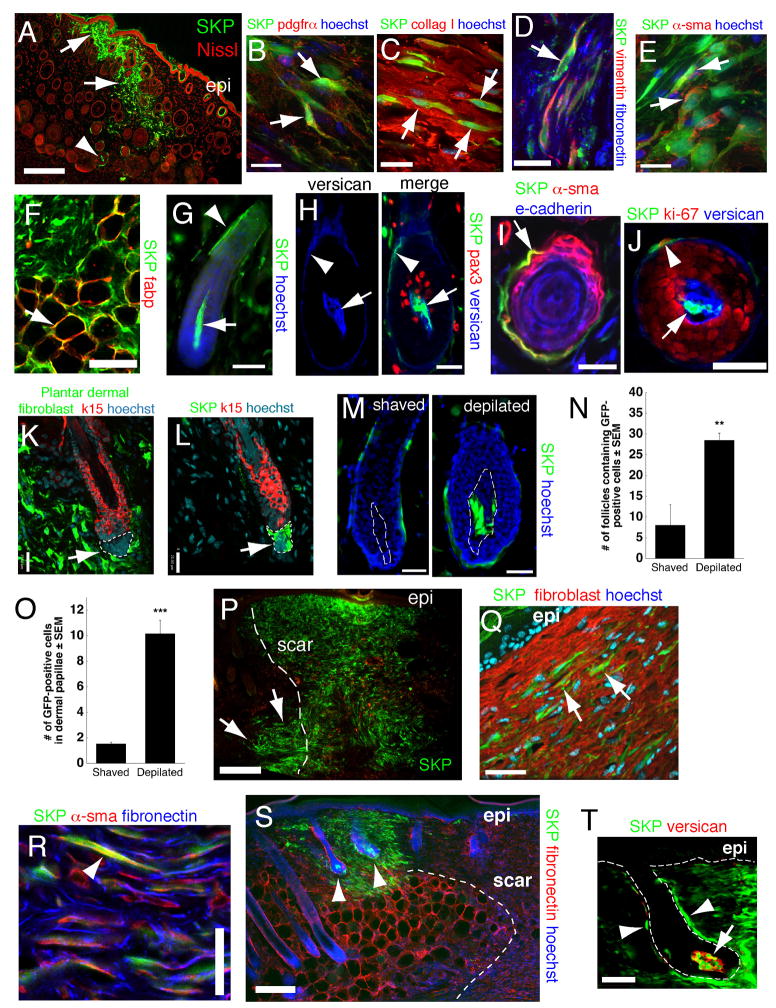
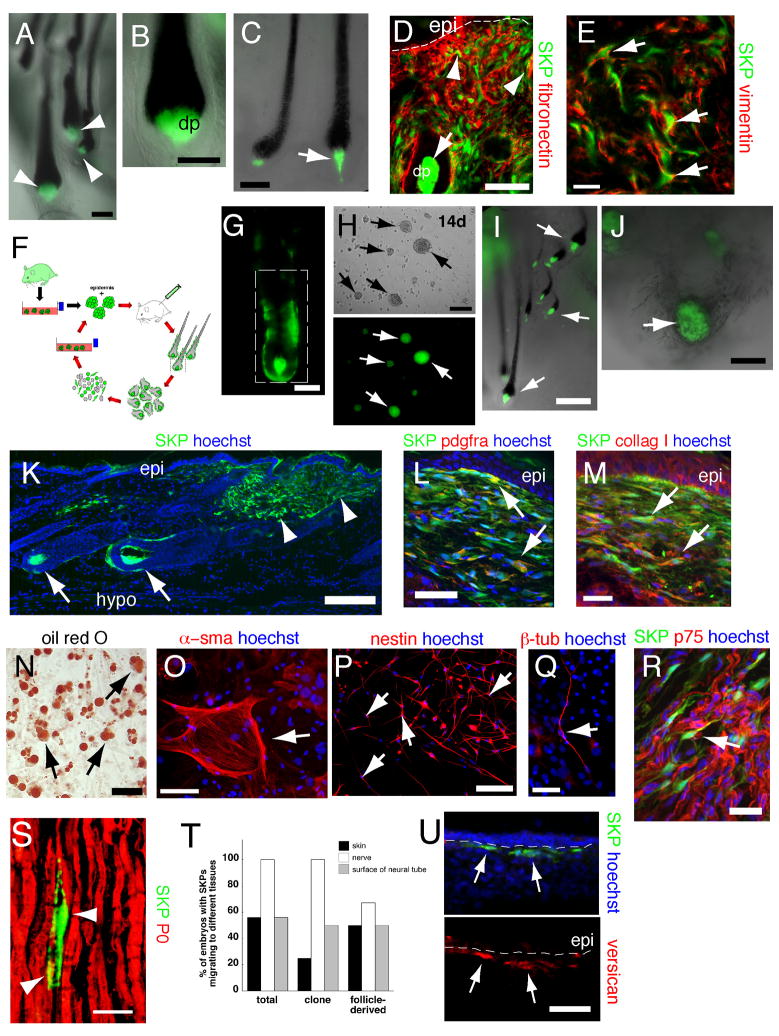
To ask whether SKPs were also functionally similar to Sox2:EGFP+ dermal precursors, we transplanted neonatal YFP-expressing murine or adult GFP-expressing rat SKPs into adult NOD/SCID mouse back skin. At 2–3 weeks post-transplant, both populations of genetically-tagged cells were located throughout the dermis (Fig. 4A). These cells were morphologically similar to the endogenous fibroblasts, and expressed the dermal fibroblast markers PDGFRα, collagen type I, vimentin, fibronectin (Fig. 4B–D), and fibroblast specific antigen (data not shown) (>90% expressed fibroblast specific antigen or collagen type I, ~73% PDGFRα). Some also coexpressed the hyaluronic acid receptor, CD44 or the myofibroblast protein α-sma (Fig. 4E) (~80% and 7% expressed α-sma in the upper and lower dermis, respectively). Some transplanted cells were present in the adipocyte-rich hypodermis, where they expressed fatty acid binding protein and displayed adipocyte morphology (Fig. 4F). Genetically-tagged transplanted cells were never observed within the epidermis. Thus, SKPs, like Sox2+ dermal precursors, differentiate into dermal cell types.
Figure 4. SKPs regenerate the dermis and integrate into a hair follicle niche upon transplantation into adult skin.
(A,B) Back skin transplanted with YFP-tagged neonatal mouse SKPs 2 weeks earlier and immunostained for YFP (green) and PDGFRα (B, red). Arrows and arrowheads in (A) show transplanted cells in the interfollicular dermis and the DP and DS of follicles, respectively. Arrows in (B) denote double-labeled cells. (C–E) GFP-expressing adult rat SKPs transplanted into depilated adult NOD/SCID mouse dermis 21 days earlier, and immunostained for GFP (green) and collagen type 1 (C, red), vimentin and fibronectin (D, red and blue, respectively), or α-sma (E, red). Arrows denote double-labeled (C,E) or triple-labeled (D) cells. (F) GFP-tagged cells (green) within the hypodermis expressing the adipocyte marker fatty acid binding protein (red, arrow). (G–J) Hair follicles containing neonatal murine YFP+ SKPs 2–4 weeks post-transplantation as in (A). (G) Hair follicle with YFP-labeled cells (green) in the DP (arrow) and DS (arrowhead). (H) Follicle triple-labeled for YFP (green), the DP marker versican (blue) and the melanoblast marker pax3 (red). The arrow and arrowhead indicate the DP and DS. (I,J) Cross-sections of follicles showing transplanted cells (green) in the DS (arrow in I, arrowhead in J) expressing -sma (I, red) or Ki67 (J, red), but not e-cadherin (I, blue, an epidermal marker). In (J), DP cells (arrow) are positive for versican (blue). (K,L) Telogen follicles in skin transplanted 4 weeks earlier with GFP-tagged rat plantar dermal fibroblasts (K) or SKPs (L), immunostained for GFP (green) and keratin 15 (red). Only SKPs are in the DP (arrows, denoted by hatched lines). (M) Representative hair follicles containing neonatal murine YFP+ SKPs (green) 3 weeks after skin was shaved or depilated. Hatched lines denote the DP. (N,O) Number of follicles containing GFP+ cells (N) or GFP+ cells within the DP of individual follicles (O) following transplantation of adult rat GFP+ SKPs into depilated (n=4) versus shaved (n=4) skin. **p<0.01, ***p<0.001. (P–T) Skin 3 (P–R) or 4 (S,T) weeks after transplantation of neonatal YFP-tagged murine SKPs (green in all panels) adjacent to a back skin punch wound. In (P), transplanted cells are present at the site of injection (arrows), and within the regenerated tissue (denoted by dashed lines). (Q,R) Transplanted cells immunostained for fibroblast-specific antigen (Q, red, arrows), or for α-sma and fibronectin (R, arrowhead, red and blue, respectively). (S,T) Transplanted cells are also present in peg-like hair follicles at the boundary of the wound (S, arrowheads) within the DP and DS (T, arrow and arrowheads). Cells in the DP express versican (T, red). Some sections were counterstained with Hoechst 33258 (blue) or fluorescent Nissl (red), as indicated. Epi = epidermis. Scale bars = 200μm in A,P,S, 16μm in B–E, 25 μm in G-M,Q, 50μm in F,R,T. See also Figure S4.
SKPs actively integrate into a hair follicle niche
Intriguingly, some transplanted SKPs homed back to the follicle DP and DS (Fig. 4A,G), where they appropriately expressed the DP markers versican (Fig. 4H; Fig. S4A), p75NTR, and NCAM (data not shown), or the DS marker α-sma (Fig. 4I). Some transplanted cells in the DS also expressed the proliferation marker Ki67 (Fig. 4J), consistent with the fact that DS but not DP cells proliferate. Transplanted cells did not differentiate into Pax3+ or tyrosinase+ melanocytes (Fig. 4H; Fig. S4B). The specificity of this integration was shown by transplanting neonatal murine YFP+ forebrain neurospheres (NSCs), adult rat GFP+ bone marrow MSCs, and dermal fibroblasts from non-hairy plantar skin. The NSCs survived poorly, and never associated with follicles, while MSCs and plantar skin dermal fibroblasts survived, but were never found within the DP (Fig. 4K,L; Fig. S4C–E).
To determine whether follicle cycling alters this homing behavior, we depilated or shaved back skin of NOD/SCID mice (depilation induces follicles to enter anagen), prior to transplantation. Depilation caused adult rat SKPs to integrate into 2–3-fold more hair follicles than did shaving (28.5±4.9 versus 8.0±1.6 follicles), with the DP of each positive follicle containing 6-fold more transplanted cells (10.16±1.0 versus 1.52±0.14) (Fig. 4N,O). Similar results were obtained with neonatal murine SKPs (Fig. 4M).
Finally, we asked whether SKPs hair follicle integration was modified by skin wounding. Neonatal murine YFP+ SKPs were transplanted adjacent to punch wounds on NOD/SCID mouse back skin. 3–4 weeks later, many transplanted cells were present within the scar, where they expressed fibroblast-specific antigen, collagen type 1, fibronectin and the myofibroblast protein α-sma (Fig. 4P–R; Fig. S4F,G) (16% expressed high α-sma). Interestingly, transplanted cells were also present in DP and DS of immature-appearing hair follicles (Fig. 4S,T), with the DP cells appropriately expressing versican (Fig. 4T) and p75NTR (data not shown), suggesting that SKPs contribute to new follicle formation in wounded skin.
SKPs regulate hair follicle morphogenesis
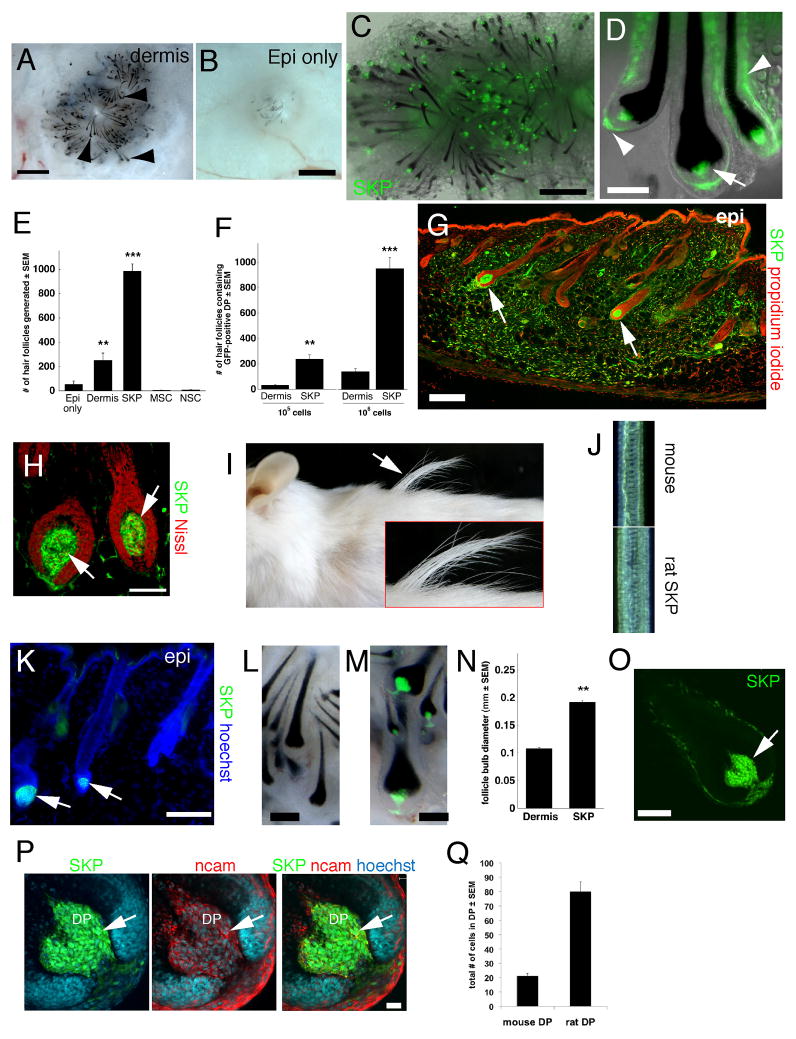
To ask if SKPs induce hair follicle morphogenesis, as do Sox2:EGFP+ cells, we performed patch assays. As positive controls, we used neonatal dermal cells, which generated many hair follicles (Fig. 5A,B), and as negative controls MSCs or NSCs, which generated no or very few follicles (Fig. S5A,B). Interestingly, adult rat GFP+ SKPs were highly enriched for hair follicle inductive ability (Fig. 5E,F), generating follicles where the entire DS and DP was comprised of genetically-tagged cells (Fig. 5C,D). Neonatal YFP-expressing murine SKPs also induced follicle formation (data not shown).
Figure 5. SKPs, but not other adult stem cells, reconstitute a hair follicle niche and instruct epidermal cells to generate hair follicles.
(A,B), Patch assays at 12 days, combining neonatal C57/Bl6 murine epidermal cells with (A) and without (B) 106 neonatal rat dermal cells, visualized by phase illumination (arrowheads denote hair follicles, which are black). (C,D) Combined phase and fluorescence illumination of similar patch assays with 106 GFP-expressing adult rat SKPs (green) (arrow and arrowheads in D denote the DP and DS, respectively). (E) Number of hair follicles with GFP+ or YFP+ DP as shown in (C,D), using 106 adult rat GFP+ SKPs or MSCs, or neonatal murine YFP+ dermal cells or NSCs. ** p<0.001 relative to epidermis only, ***p<0.001 relative to epidermis only and to dermis (n=6 for adult rat SKPs, and 3 for other groups). (F) Quantification of hair follicle numbers as in (E), where adult rat SKPs were compared to neonatal rat dermal cells. ***p<0.001 relative to dermal cells (n=3 and 4 each for 105 and 106 cells, respectively). (G–K) Adult GFP-expressing rat SKPs (green) were injected into the back skin of adult NOD/SCID mice for 8 weeks. (G,H) Immunostaining for GFP (green). Arrows indicate transplanted cells in hair follicle anagen DP. (I,J) Chimeric rat/mouse hairs were longer (I) and thicker (J) than endogenous pelage hairs. (K) Some transplanted GFP+ cells comprised the DP of telogen hair follicles (arrows). (L,M) Hair follicles from patch assays where 106 mouse neonatal dermal cells (L) or dissociated adult rat GFP-expressing SKPs (M, green) were used. (N) Hair bulb diameter in follicles similar to those in (L,M) (n=2 independent experiments). **p=0.0074. (O–Q) Hair follicles were generated in patch assays using either YFP+ mouse or GFP+ rat SKPs. (O,P) A single hair follicle derived from rat SKPs at low (O) and high (P) magnification, immunostained for GFP (green), NCAM (red), which marks DP and DS cells, and Hoechst to delineate cell nuclei. (Q) Number of cells in the DP of follicles generated from mouse versus rat SKPs, as shown in (P). Nuclei were stained with propidium iodide (red), fluorescent Nissl (red) or Hoechst 33258 (blue), as indicated. epi=epidermis, hypo=hypodermis. Scale bars = 500μm in A,C, 1mm in B, 50μm in D,H, 100μm in G,K,L,M,O and 20μm in P. See also Figure S5.
We next performed in vivo experiments, taking advantage of the finding that the size of the DP determines follicle size (Ibrahim and Wright, 1982). GFP+ adult rat SKPs were injected into depilated adult NOD/SCID mouse back skin and analyzed after 8 weeks. Many transplanted cells differentiated into interfollicular dermal fibroblasts (Fig. 5G), and many comprised the entire DP and DS of correctly-oriented follicles (Fig. 5G,H). Remarkably, relative to endogenous murine hairs, hairs induced by rat SKPs were longer (10.41 mm ± 0.23 versus 7.96 mm ± 0.11; p < 0.0001) and had increased follicle bulb diameter (107.24 μm ± 4.99 versus 82.27 μm ± 2.51; p <0.01) and hair fiber width (49.26 μm ± 0.87 versus 44.60 μm ± 0.83; p < 0.001) (n=8, with 3 animals quantified) (Fig. 5I,J). Although many of these follicles were in anagen (Fig. 5H), some were in catagen/telogen phase (Fig. 5K), indicating that follicle-associated SKPs cycle with their newly-induced hairs.
To confirm that rat SKPs induced larger follicles, we performed patch assays. Adult rat SKPs instructed mouse epidermal cells to generate larger follicles than did mouse dermal cells, with an almost 2-fold increase in bulb diameter (Fig. 5L–N). Confocal microscopy demonstrated that the increased rat SKP follicle size correlated with almost 4 times more NCAM+ DP cells (Fig. 5O–Q) (88% of these cells were GFP+).
SKPs clonally differentiate into dermal cell types and induce hair follicle morphogenesis
To ask if single SKPs could both induce follicle morphogenesis and differentiate into dermal cell types, as expected for a dermal stem cell, we generated clones of adult rat SKPs. Patch assays showed that, of 7 clonally-derived rat SKP lines passaged at least 6 times (approximately 8–12 weeks in culture), 5 induced hair follicle formation (Fig. 6A,B). Indeed, when 50 spheres from one clone were mixed with 5 × 105 total neonatal murine skin cells, 30 ± 2 hair follicles had DP entirely comprised of GFP+ SKPs. This activity was persistent; one clone induced follicle formation after 11 months in culture, albeit inefficiently (Fig. 6C). Consistent with these results, 3 of 3 clones transplanted into adult NOD/SCID mouse skin reconstituted hair follicle DP and DS in vivo (Fig. 6D). Transplanted clone cells also differentiated into fibronectin− and vimentin+ interfollicular dermal fibroblasts and α-sma+ myofibroblasts (Fig. 6D,E). Thus, single SKPs were multipotent with regard to these dermal activities.
Figure 6. (A–E) Clonally-derived SKPs reconstitute the dermis and induce hair follicle formation.
Analysis of one adult rat GFP+ SKP clone in patch assays (A–C), or by transplantation into adult mouse dermis (D,E). After 2–4 (A,B) or 11 months (C) of culturing, clonal SKPs (green) induced the formation of hair follicles, where they comprised the DS and DP (arrowheads in A, arrow in C). (D,E) After 12 weeks in culture, cells from the same clone were transplanted and skin immunostained for GFP (green) and fibronectin (D, red, arrowheads) or vimentin (E, red, arrows). Arrow in (D) denotes the DP. (F–U) SKPs isolated from their hair follicle niche self-renew, serially reconstitute hair follicles and remain multipotent. (F) Schematic showing the serial reconstitution assay of hair morphogenesis. (G,H) A follicle isolated from a patch assay using adult rat GFP-labeled cells. Tagged cells isolated from the boxed area generated GFP+ SKP spheres (H, arrows) after 12 days in culture as seen by phase (top) and fluorescence (bottom) illumination. (I) Cells from these follicle-derived spheres induced formation of secondary hair follicles in the patch assay (arrows). (J) In tertiary follicle reconstitutions, follicle-derived SKPs generated hair follicles, but many also aggregated into DP-like structures surrounded by black melanocytes (arrow). (K–M) Skin transplanted with GFP+ follicle-derived SKPs for 4 weeks. Transplanted cells (green) integrated into follicle DS and DP (K, arrows) and contributed to the dermis (K, arrowheads), where they expressed PDGFRα (L, red) and collagen type 1 (M, red). Arrows indicate double-labeled cells. (N,O) Follicle-derived SKPs generated adipocytes, as indicated by the lipophilic dye oil red O (N, arrows), and sma+ cells, potentially myofibroblasts (O, arrow) in mesodermal differentiations. (P,Q) When differentiated under neurogenic conditions, they generated nestin+ cells after 5 days (P, red, arrows), and III-tubulin positive cells after 14 days (Q, red, arrow). (R,S) Sciatic nerve sections 6 weeks after transplant of follicle-derived SKPs, showing that some transplanted cells expressed the Schwann cell markers p75NTR (R, arrow) and P0 (S, arrowheads). (T,U) Transplantation of follicle-derived SKPs into the chick neural crest migratory stream (H.H. stage 18). (T) Quantification after 3 days in ovo showed follicle-derived (n=6) and clonal (n=8) SKPs behaved like total SKPs (n=9), migrating to the ventral nerve or DRG and skin, with some remaining close to the neural tube. (U) After 8 days in ovo, some of the transplanted cells that were in the dermis (green) were versican+ (red, arrows). Nuclei were stained with Hoechst 33258 (blue), as indicated. epi = epidermis. Scale bars = 100μm in A,B,C,D,H,P, 50μm in E,G,N,O,Q,U, 25μm in L,M,R,S, 200um in I,K, and 250μm in J.
SKPs maintain their multipotency and capacity to self-renew within the hair follicle niche and serially reconstitute hair follicles
One of the most striking assays of in vivo stem cell functionality is serial repopulation by hematopoietic stem cells. We therefore asked whether follicle-derived DS and DP cells could self-renew as SKPs and sequentially induce and repopulate hair follicles (Fig. 6F). Hair follicles were generated in patch assays with adult rat GFP+ SKPs, and cells from these follicles (Fig. 6G) were cultured in SKPs conditions; 10–14 days later, genetically-tagged spheres were observed (Fig. 6H). When these spheres were passaged, and put back into patch assays, they again induced follicle formation (Fig. 6I). Using this approach, we serially generated SKP spheres and reinduced hair follicles up to three times (Fig. 6J).
To ask whether these follicle-derived SKPs also maintained their multipotentiality, we took four different approaches. First, we transplanted them into adult NOD/SCID mouse back skin for 4 weeks. Many transplanted cells differentiated into PDGFRα and collagen type I+ interfollicular dermal fibroblasts (Fig. 6K–M), and some integrated into the DS and DP of endogenous follicles (Fig. 6K), where they expressed versican and NCAM in the DP and fibronectin in the DS (data not shown). Second, we differentiated cells in culture conditions that support neural and mesodermal differentiation of neonatal SKPs (Fernandes et al., 2004). Follicle-derived SKPs generated adipocytes, α-sma+ myofibroblasts or smooth muscle cells, nestin+ precursors and βIII-tubulin+ neurons (Fig. 6N–Q). Third, we transplanted follicle-derived SKPs distal to a sciatic nerve crush in NOD/SCID mice, an environment that instructs naïve SKPs to differentiate into Schwann cells (McKenzie et al., 2006). Immunocytochemistry of the distal nerve 6 weeks post-transplant identified GFP+ cells closely aligned with axons expressing the Schwann cell markers P0 and p75NTR (Fig. 6R,S). Finally, we transplanted follicle-derived SKPs into the embryonic chick neural crest migratory stream at HH stage 18, as we have done for total neonatal SKPs (Fernandes et al., 2004). Analysis 3 days later showed that follicle-derived SKPS migrated out of the neural tube into neural crest targets such as the spinal nerve and DRGs, similar to murine SKPs and adult rat clonal SKPs (Fig. 6T). Intriguingly, some total and follicle-derived SKPs migrated to the presumptive dermis, and at late timepoints, some of these expressed the DP marker versican (Fig. 6U). Thus, follicle-associated SKPs display properties of dermal stem cells.
Lineage tracing of transplanted follicles demonstrates that follicular DP and DS cells contribute to dermal maintenance and wound healing
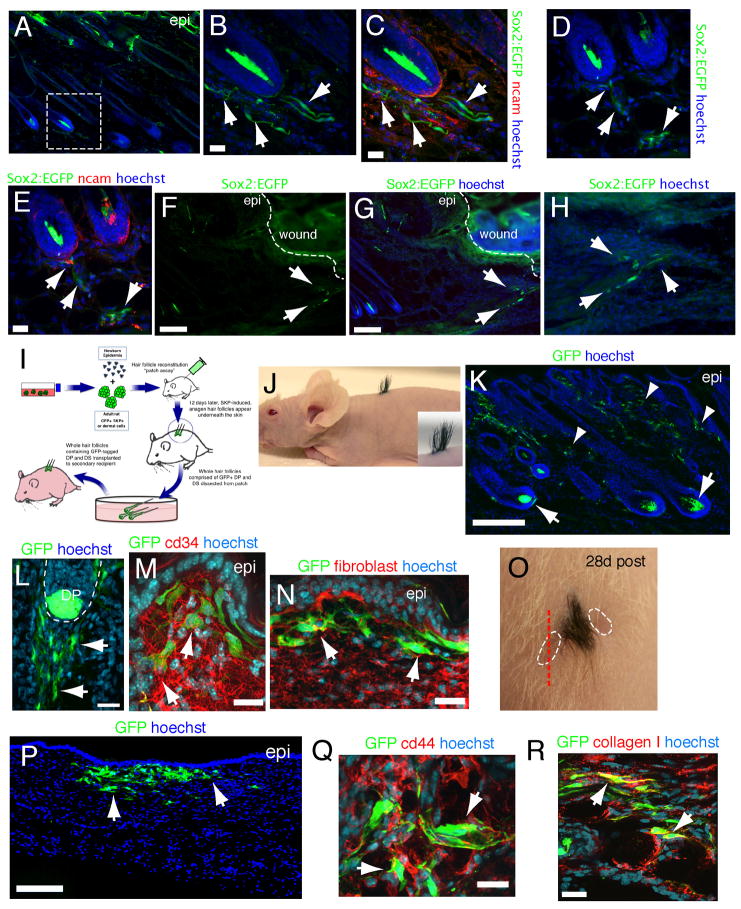
To ask if the endogenous Sox2+ dermal precursors are recruited out of their niche to contribute differentiated dermal cells, we performed punch wounds on Sox2:EGFP mice. At 36 hours, Sox2:EGFP+ cells were limited to hair follicles. However, by 72 hours EGFP+ cells were seen exiting hair follicles and streaming into the interfollicular dermis toward the wound site (Fig. 7A–H). Some of these emigrating cells coexpressed the DP/DS marker NCAM, but many were negative (Fig. 7C,E), indicating that they were losing their follicle-associated phenotype.
Figure 7. (A–H) Sox2:EGFP+ cells migrate out of hair follicles following punch wounds.
Immunostaining for EGFP (green) and NCAM (C,E, red) in Sox2:EGFP skin 3 days after a punch wound. (B–E) show hair follicles in the vicinity of the injury, with (B,C) being higher magnification images of the boxed area in (A), while (D,E) show the same field. (F––H) show the region directly adjacent to the wound, with (H) being a higher magnification image. Arrows show Sox2:EGFP+ cells outside of hair follicles. (I–R) Lineage tracing demonstrates that follicular DP and DS cells contribute to dermal maintenance and wound healing. (I) Schematic depicting lineage tracing procedure. Hair follicles were generated in patch assays using GFP-tagged rat SKPs or uncultured neonatal rat dermal cells, and 40–60 newly-formed genetically-tagged follicles were carefully dissected and grafted on to the back skin of a recipient mouse. Skin was analyzed 4–8 weeks later. (J) Photograph of an adult nude mouse that had received a transplant of chimeric hair follicles as in (I) 3 weeks earlier. (K–N) Back skin of a mouse as in (I) 4 weeks post-transplant immunostained for GFP (green) and cd34 (M, red, arrows) or fibroblast-specific antigen (N, red, arrows). Arrows and arrowheads in (K) denote GFP-tagged cells in follicle DP and DS and interfollicular dermis, respectively. Arrows in (L) denote GFP+ cells leaving the hair follicle. (O) Photograph of a transplant similar to (J), except that two adjacent punch wounds (hatched lines) were made 3 weeks after the initial hair follicle graft, and skin was analyzed 4 weeks later. (P–R) Skin sectioned as shown by the red hatched line in (O), and immunostained for GFP (green) and cd44 (red, Q) or collagen type I (red, R). Arrows denote transplanted cells in P and double-labeled cells in Q,R. Nuclei were stained with Hoechst 33258 (blue). Scale bars = 200um in K,P and 25μm in B,C,E,L,M,N,Q,R. See also Figure S7.
To further define this phenomenon, we used a transplantation-based lineage tracing approach. Genetically-tagged hair follicles were made in patch assays with either rat GFP+ SKPs or neonatal dermal cells (Fig. 7I) and transplanted under adult nude mouse back skin. Over the ensuing 4 weeks, tagged follicles integrated and grew to generate small patches of hair (Fig. 7J). Analysis at this timepoint showed that many GFP-tagged cells were retained within engrafted hair follicles, but that many had also migrated into the adjacent interfollicular dermis where they expressed fibroblast-specific antigen, cd34 and collagen type I (Fig. 7K–N; Fig. S7). Similar results were obtained with both follicle populations. In some animals, we also injured the skin adjacent to transplanted SKP-derived hairs with a punch wound (Fig. 7O). Analysis 4 weeks later showed that GFP+ cells had migrated into the regenerated, newly-healed dermis where they expressed cd44 and collagen type I (Fig. 7P–R). Thus, DP/DS cells can contribute differentiated cells to the dermis.
DISCUSSION
These studies support a number of major conclusions. First, our studies identify a Sox2+ endogenous dermal precursor within the follicle DP and DS that, when prospectively isolated, homes back to a hair follicle niche, induces hair follicle morphogenesis, and displays neural and dermal potential. Second, we show that these endogenous precursors generate SKPs when cultured, and that these two cell populations are very similar with regard to their transcriptome and their functional properties when transplanted into the dermis. Third, we demonstrate that SKPs can clonally reconstitute the dermis and induce hair follicle morphogenesis, properties predicted of a dermal stem cell. Fourth, the serial reconstitution experiments show that SKPs maintain their multipotentiality and their ability to self-renew within their hair follicle niche, and that they can serially induce hair follicle formation. Finally, our follicle graft/lineage tracing studies argue that Sox2+ dermal precursors can be recruited out of their niche to contribute differentiated dermal cells. Together, these experiments provide evidence for a hair follicle-associated dermal precursor that functions to regulate hair morphogenesis, and to maintain the intact or injured dermis and that, when cultured, generate SKPs that display all the predicted properties of multipotent dermal stem cells.
On the basis of these findings, we propose that these adult dermal precursors derive from a subset of embryonic mesenchymal precursors within the presumptive dermis that interact with epidermal precursors and undergo a transition to a Sox2+ precursor state, as recently suggested (Driskell et al., 2009). We also propose that hair follicles provide a niche for maintenance of the mesenchymal precursor phenotype, and that in adult life, these Sox2+ follicle cells both regulate follicle morphogenesis, and serve as a reservoir of dermal precursor activity, contributing dermal cells for the normal and injured dermis. The broader mesenchymal potential displayed by SKPs (Lavoie et al., 2009) and whisker follicle papillae (Jahoda et al., 2003; Wojciechowicz et al., 2008) suggests that they may also serve as precursors for adipocytes and/or blood vessel smooth muscle cells.
That DP mesenchymal cells induce hair follicle morphogenesis is well-established (Jahoda et al., 1984; Oliver, 1967). A precursor function for DP cells has also been previously suggested based upon their ability to contribute cells to healing wounds upon transplantation, and to generate non-dermal cell types when cultured (Waters et al., 2007). The data we present here provide strong evidence that this is indeed the case. Moreover, our finding that both DP and DS cells express Sox2 suggests that these anatomically-distinct cells may in fact represent a common precursor population. In support of this idea, previous studies showed that cells move between the DS and DP (Tobin et al., 2003), and that lower DS cells can regenerate the DP (McElwee et al., 2003). We suggest that since DP cells rarely divide, they may represent a quiescent stem cell population, with DS cells functioning as a transit amplifying population that ultimately provides differentiated cell types. Alternatively, DS cells may represent dermal stem cells, and provide cells both for dermal differentiation, and for the DP, where the cells acquire their inductive properties and function to regulate follicle morphogenesis.
One of the surprising findings reported here is the ability of transplanted Sox2:EGFP+ cells and the SKPs they generate to home back to their hair follicle niche. Interestingly, a hint of this activity was previously obtained in experiments transplanting GFP+ DP/DS cells into ear skin (McElwee et al., 2003). These findings may reflect a previously-undescribed mechanism for recruiting DS cells into the DP and/or for allowing re-establishment of the DS/DP when hair follicles re-enter the hair growth cycle. Clearly, SKPs are attracted to cells that are similar, as seen in patch assays where single dissociated SKP cells aggregated to form structures that nucleated hair follicle formation. While the relevant attractive signal is unknown, evidence that it is cell-intrinsic comes from our data showing that more rat than mouse cells aggregate to form the DP, even within the same tissue environment.
A second surprising finding is that Sox2:EGFP expression was regulated during the hair cycle; DP and DS cells did not express Sox2 during catagen or telogen. While some cells exit the DP during hair follicle regression (Tobin et al., 2003), many are retained, implying that at least some precursors regulate Sox2 expression in response to their external micro-environment. Since Sox2 is associated with maintenance of a stem cell state (Avilion et al., 2003) then this may suggest that the niche regulates the “stemness” of these dermal precursors. However, a recent study also showed Sox2:EGFP expression in the DP of developing hair follicles, and provided evidence that the level of expression was associated with follicle type, with small zig zag hairs expressing low or no Sox2:EGFP (Driskell et al., 2009). Thus, Sox2 expression may be associated with multiple functional properties, and it will be important to determine whether Sox2 is a readout for different functional states and/or whether it actually regulates the properties of these dermal precursors.
One key question in the stem cell field has been whether multipotent precursors like SKPs actually reflect endogenous precursor cells, or whether they are cells that dedifferentiate in culture. Data presented here showing that prospectively-isolated Sox2+ follicle DP and DS cells give rise to SKPs, and that these Sox2+ cells are very similar to SKPs with regard to their transcriptome and functional properties, argue that SKPs accurately reflect this endogenous precursor population. This finding, together with our data showing that SKPs display all of the properties predicted of dermal stem cells, provide strong support for the idea that the Sox2+ precursors we have identified here are endogenous dermal stem cells that serve to induce hair morphogenesis and maintain the dermis.
Experimental Procedures
Tissue culture
SKPs were generated from dorsal back skin of neonatal (P1-7) YFP-expressing mice (Hadjantonakis et al., 1998) (Jackson Laboratory) or P0–P3 and adult (5–10 week) GFP-expressing Sprague Dawley rats (SLC, Japan) and cultured as described (Fernandes et al., 2004) at 1,000–20,000 cells/ml. Spheres were passaged at least twice for transplants. For clones, secondary SKPs were grown at 1,000 cells/ml, and single clonal spheres isolated and expanded at least 5 weeks. MSCs were isolated from adult GFP-expressing rat bone marrow (generous gift of Fabio Rossi, U.B.C.), and cultured at 50,000 cells/ml in Mesencult human MSC medium plus 10% FBS (Stem Cell Technologies). Neurospheres were made from P1 lateral ventricles as described (Reynolds and Weiss, 1992). SKPs were differentiated in vitro under previously-defined conditions for neurons, Schwann cells, and SMA+ cells (Biernaskie et al., 2006; McKenzie et al., 2006). Adipocytes were differentiated in DMEM-F12 containing 1% penicillin streptomycin, 10% FBS, dexamethasone (1μM, Sigma), isobutylmethylxanthine (1mM, Sigma), and insulin (20μg/mL, Gibco/Invitrogen).
In vivo experiments
For transplants, 2×105 to 106 passaged, dissociated murine (n=8) or rat (n=12) SKPs were injected into depilated (n=11) or shaved (n=10) back skin of adult NOD/SCID mice (Charles River Laboratories). Alternatively, a full thickness 4 mm wide biopsy punch was made, and SKPs were injected adjacent to the wound (n=10). Controls were performed with MSCs (n=4) or NSCs (n=4). For in vivo hair follicle morphogenesis experiments, adult rat SKPs were transplanted (n=8), and quantification was performed with 30–50 hairs from each of 3 experiments. Awl hairs were used for width measurements. Punch wounds were performed on Sox2:EGFP mice (n=4) as for transplants. Nerve transplants were performed as described (McKenzie et al., 2006). In ovo transplants were performed as described (Fernandes et al., 2004) at Hamilton/Hamburger stage 18 with analysis at Stage 30–35. All procedures were approved by the Hospital for Sick Children Animal Care Committee and were within the guidelines of the Canadian Council of Animal Care.
Hair follicle induction assay
106 SKPs (n=6 adult rat, 4 neonatal murine), neonatal (n=2) or adult (n=3) rat dermal cells, neonatal murine NSCs (n=3) or adult rat MSCs (n=3) were mixed with 10,000 neonatal epidermal aggregates (5 × 105 to 2 × 106 single cells), and injected into adult nude mouse (nu/nu; Charles River) back skin for 10–12 days as described (Zheng et al., 2005). SKPs, MSCs and NSCs were passaged 1–5 times. For sorted cells, 2–5 × 105 cells were mixed with 10,000 epidermal cells. Individual bulb diameters (50 follicles/graft; n=2 grafts each) were measured using Volocity acquisition software (Improvision) and a Leica MZ16F stereomicroscope. To quantify DP cell number, confocal image stacks spanning the entire DP were collected and all NCAM+ and NCAM+, GFP+ cells were counted. For serial reconstitutions, patches were digested in collagenase (Type XI) at 37 °C for 1 hour or follicles with GFP+ DP (n=40–60 hairs/experiment) were individually dissected and digested with 0.25% trypsin-EDTA; similar results were obtained with both approaches (n=3 adult, 2 neonate rat SKPs isolations). Single cells were liberated by gentle trituration and cultured in SKPs medium at 2,000 to 20,000 cells/ml for 14 days.
Follicle grafting experiments
Follicles generated in patch assays were dissected as above, transplanted into a back skin incision on adult Nude or NOD/SCID mice and those that grew were analyzed at 3–4 weeks (n=4 adult rat SKPs, 2 neonatal rat dermal cells). Alternatively, punch wounds were made adjacent to the graft at 3 weeks, and skin analyzed 4 weeks later (n=3).
Cell sorting
Back and facial skin from neonatal (n=15) or adult (n=9) Sox2:EGFP mice (Ellis et al., 2004) were dissociated to single cells, sorted for EGFP expression on a MoFlo (Dako) or FACsAria (Becton Dickinson) with viable cells identified by propidium iodide exclusion. In some experiments, cells were stained with APC-conjugated anti-PDGFRα (1:100; eBioscience) and/or biotin conjugated anti-cd34 (1:100; eBioscience) followed by a PECy7 conjugated streptavidin secondary antibody (1:1000; BD Biosciences). Gates were set according to single stained positive controls and negative (secondary only/isotype) controls. For transplants, sorted cells were incubated in medium containing RFP retrovirus and 2 ug/ml polybrene for 18 hours (a gift from Drs. Akitsu Hotta and James Ellis, HSC), washed extensively, and 300,000 cells injected (n=4 Sox2:EGFP+PDGFRα+cd34− cells and 5 Sox2:EGFP− cells).
Microarray and bioinformatics
P4 Sox2:EGFP+, cd34− cells were sorted directly into RNAlater (Ambion). RNA was extracted and quality assessed from sorted cells and primary passage P4 SKPs using Trizol (Invitrogen), and an Agilent BioAnalyzer. RNA samples were analyzed on Affymetrix GeneChip Mouse Gene 1.0 ST Arrays, and background was corrected and normalized using standard RMA procedure implemented in the Affymetrix Expression Console software. Preprocessed data were analysed for significant differential expression using the LIMMA Bioconductor package, with the F-statistic with Benjamini-Hochberg (BH) multiple testing correction implemented in the eBayes function. Genes with BH-corrected p-value < 0.01 were considered statistically significant (Smyth, 2004). Differentially expressed genes were analyzed using Ingenuity Pathway Analysis software (www.ingenuity.com) for functional enrichment. Microrray data are deposited in the NIH GEO repository (accession number GSE18690): http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE18690.
Immunocytochemistry and histology
Antibodies are listed in Supplementary Experimental Procedures. Immunocytochemistry was performed as described (Fernandes et al., 2006; Fernandes et al., 2004; McKenzie et al., 2006), and acquisition and co-localization was confirmed by collecting z-stacks comprised of 0.2μm to 1μm optical slices using a Hamamatsu spinning disk confocal microscope fitted to a Zeiss Axiovert 200 inverted microscope (Quorum Technologies).
Statistics
All data are represented as mean ± SEM. Data (with the exception of the microarray analyses) were analyzed using two-tailed t-tests or one-way ANOVA where appropriate. All experiments were done at least in triplicate, unless otherwise noted.
Supplementary Material
Acknowledgments
This work was funded by grants from CIHR, the Canadian Stem Cell Network, and HHMI. FDM is an HHMI International Research Scholar and CRC chairholder, and JAB was funded by a CIHR fellowship during the course of this work. We thank Fabio Rossi (U.B.C.) for supplying MSCs, Ben Alman and Sophia Cheon (H.S.C) for help with skin wounding assays, Akitsu Hotta and James Ellis for supplying RFP retrovirus, and Richard LeBaron (U.T.S.A.), and Pierre Coulombe (Johns Hopkins) for providing antibodies. We also thank Dennis Aquino and Sherry Zhao for valuable technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003;17:126–140. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biernaskie J, McKenzie IA, Toma JG, Miller FD. Isolation of skin-derived precursors (SKPs) and differentiation and enrichment of their Schwann cell progeny. Nat Protocols. 2006;1:2803–2812. doi: 10.1038/nprot.2006.422. [DOI] [PubMed] [Google Scholar]
- Driskell RR, Giangreco A, Jensen KB, Mulder KW, Watt FM. Sox2-positive dermal papilla cells specify hair follicle type in mammalian epidermis. Development. 2009;136:2815–2823. doi: 10.1242/dev.038620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis P, Fagan BM, Magness ST, Hutton S, Taranova O, Hayashi S, McMahon A, Rao M, Pevny L. SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Dev Neurosci. 2004;26:148–165. doi: 10.1159/000082134. [DOI] [PubMed] [Google Scholar]
- Fernandes KJ, Kobayashi NR, Gallagher CJ, Barnabe-Heider F, Aumont A, Kaplan DR, Miller FD. Analysis of the neurogenic potential of multipotent skin-derived precursors. Exp Neurol. 2006;201:32–48. doi: 10.1016/j.expneurol.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Fernandes KJL, McKenzie IA, Mill P, Smith KM, Akhavan M, Barnabe-Heider F, Biernaskie J, Junek A, Kobayashi NR, Toma JG, et al. A dermal niche for mutipotent adult skin-derived precursor cells. Nature Cell Biol. 2004;6:1082–1093. doi: 10.1038/ncb1181. [DOI] [PubMed] [Google Scholar]
- Fuchs E. Finding one’s niche in the skin. Cell Stem Cell. 2009;4:499–502. doi: 10.1016/j.stem.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharzi A, Reynolds AJ, Jahoda CA. Plasticity of hair follicle dermal cells in wound healing and induction. Exp Dermatol. 2003;12:126–136. doi: 10.1034/j.1600-0625.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- Hadjantonakis AK, Gertsenstein M, Ikawa M, Okabe M, Nagy A. Generating green fluorescent mice by germline transmission of green fluorescent ES cells. Mech Dev. 1998;76:79–90. doi: 10.1016/s0925-4773(98)00093-8. [DOI] [PubMed] [Google Scholar]
- Hunt DP, Morris PN, Sterling J, Anderson J, Joannides A, Jahoda C, Compston A, Chandran S. A highly enriched niche of precursor cells with neuronal and glial potential within the hair follicle dermal papilla of adult skin. Stem Cells. 2007 doi: 10.1634/stemcells.2007-0281. [DOI] [PubMed] [Google Scholar]
- Ibrahim L, Wright EA. A quantitative study of hair growth using mouse and rat vibrissal follicles. I. Dermal papilla volume determines hair volume. J Embryol Exp Morphol. 1982;72:209–224. [PubMed] [Google Scholar]
- Jahoda CA, Horne KA, Oliver RF. Induction of hair growth by implantation of cultured dermal papilla cells. Nature. 1984;311:560–562. doi: 10.1038/311560a0. [DOI] [PubMed] [Google Scholar]
- Jahoda CA, Whitehouse J, Reynolds AJ, Hole N. Hair follicle dermal cells differentiate into adipogenic and osteogenic lineages. Exp Dermatol. 2003;12:849–859. doi: 10.1111/j.0906-6705.2003.00161.x. [DOI] [PubMed] [Google Scholar]
- Joannides A, Gaughwin P, Schwiening C, Majed H, Sterling J, Compston A, Chandran S. Efficient generation of neural precursors from adult human skin: astrocytes promote neurogenesis from skin-derived stem cells. Lancet. 2004;364:172–178. doi: 10.1016/S0140-6736(04)16630-0. [DOI] [PubMed] [Google Scholar]
- Lavoie JF, Biernaskie JA, Chen Y, Bagli D, Alman B, Kaplan DR, Miller FD. Skin-derived precursors differentiate into skeletogenic cell types and contribute to bone repair. Stem Cells Dev. 2009;18:893–906. doi: 10.1089/scd.2008.0260. [DOI] [PubMed] [Google Scholar]
- McElwee KJ, Kissling S, Wenzel E, Huth A, Hoffmann R. Cultured peribulbar dermal sheath cells can induce hair follicle development and contribute to the dermal sheath and dermal papilla. J Invest Dermatol. 2003;121:1267–1275. doi: 10.1111/j.1523-1747.2003.12568.x. [DOI] [PubMed] [Google Scholar]
- McKenzie IA, Biernaskie J, Toma JG, Midha R, Miller FD. Skin-derived precursors generate myelinating Schwann cells for the injured and dysmyelinated nervous system. J Neurosci. 2006;26:6651–6660. doi: 10.1523/JNEUROSCI.1007-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura EK, Granter SR, Fisher DE. Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Science. 2005;307:720–724. doi: 10.1126/science.1099593. [DOI] [PubMed] [Google Scholar]
- Oliver RF. The experimental induction of whisker growth in the hooded rat by implantation of dermal papillae. J Embryol Exp Morphol. 1967;18:43–51. [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3 doi: 10.2202/1544-6115.1027. Article3. [DOI] [PubMed] [Google Scholar]
- Tobin DJ, Gunin A, Magerl M, Handijski B, Paus R. Plasticity and cytokinetic dynamics of the hair follicle mesenchyme: implications for hair growth control. J Invest Dermatol. 2003;120:895–904. doi: 10.1046/j.1523-1747.2003.12237.x. [DOI] [PubMed] [Google Scholar]
- Waters JM, Richardson GD, Jahoda CA. Hair follicle stem cells. Semin Cell Dev Biol. 2007;18:245–254. doi: 10.1016/j.semcdb.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Wojciechowicz K, Markiewicz E, Jahoda CA. C/EBPalpha identifies differentiating preadipocytes around hair follicles in foetal and neonatal rat and mouse skin. Exp Dermatol. 2008;17:675–680. doi: 10.1111/j.1600-0625.2007.00689.x. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Du X, Wang W, Boucher M, Parimoo S, Stenn K. Organogenesis from dissociated cells: generation of mature cycling hair follicles from skin-derived cells. J Invest Dermatol. 2005;124:867–876. doi: 10.1111/j.0022-202X.2005.23716.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.