Abstract
Objectives:
To evaluate the relationship between sensory and cognitive decline, particularly with respect to speed of processing, memory span, and fluid intelligence. Additionally, the common cause, sensory degradation and speed of processing hypotheses were compared.
Methods:
Structural equation modeling was used to investigate the complex relationships among age-related decrements in these areas.
Results:
Cross-sectional data analyses included 842 older adult participants (M = 73 years). After accounting for age-related declines in vision and processing speed, the direct associations between age and memory span and between age and fluid intelligence were nonsignificant. Older age was associated with visual decline, which was associated with slower speed of processing, which in turn was associated with greater cognitive deficits.
Discussion:
The findings support both the sensory degradation and speed of processing accounts of age-related cognitive decline. Further, the findings highlight positive aspects of normal cognitive aging in that older age may not be associated with a loss of fluid intelligence if visual sensory functioning and processing speed can be maintained.
Keywords: Processing Speed, Fluid Intelligence, Memory Span, Visual Function, Aging
Introduction
Persons 65 years of age and older are currently the fastest growing segment of the population within industrialized countries, as evidenced by projections that they will encompass 20.4% of the total US population by the year 2050 (Economics and Statistics Administration, 1996). It has been well-established that with age there is increased risk for declines in a variety of areas including vision, cognition, psychomotor abilities, and health. Examinations of the complex relationship between sensory and cognitive decline, particularly with respect to the cognitive domains of speed of processing, memory span, and intelligence, has generated a large amount of interest in this area. An array of theories and methodologies have been postulated and debated within the literature. However, to date there is no consensus on the causes and mechanisms of age-related cognitive changes. The current study uses structural equation modeling to investigate the complex relationships among age-related declines in visual function and processing speed as they relate to memory span and fluid intelligence.
Cognitive Aging
Many cognitive changes may occur during normal aging apart from those due to pathological aging such as Alzheimer's disease or other dementias. There have been numerous efforts to explain age-related cognitive changes, including several prominent theories such as the common cause theory, the processing speed theory, and the executive function hypothesis (for a review see Luszcz & Bryan, 1999).
The divergence of opinions on the causes and mechanisms of cognitive aging are partly due to disagreement on various statistical, measurement, and theoretical issues that make generalization across studies difficult. Numerous theoretical and statistical models (Hertzog et. al., 2003; Hertzog & Nesselroade, 2003; Salthouse & Czaja, 2000) have been postulated to explain the complex, and often mediational relationships among sensory function and cognitive abilities. Generalization across studies can be difficult due to different methods of measuring and operationalizing constructs such as speed of processing and memory (Anstey, Luszcz, & Sanchez, 2001b; Salthouse, Atkinson, & Berish, 2003). Just one example of contention concerning constructs can be found in a journal issue which devoted four articles to discussions concerning constructs of working memory and intelligence (Ackerman, Beier, & Boyle, 2005; Beier & Ackerman, 2005; Kane, Hambrick, & Conway, 2005; Oberauer et al., 2005). Such discussions illustrate the continuing need for researchers to carefully operationalize and investigate latent variables and constructs with a sound theoretical, as well as, an ecologically valid basis.
Cognitive and Sensory Functioning
There are also many age-related changes that may affect the sensory systems (Owsley & Sloane, 1990; Whitebourne, 1999). The importance of sensory discrimination to theories of intelligence can be traced back over a hundred years to Galton's (1883) hypothesis that differences in cognitive functioning are related to individual differences in sensory functioning. Visual and auditory functioning are two sensory systems that have been found to be associated with intellectual performance (Anstey, Hofer, & Luszcz, 2003a; Anstey, Luszcz, Giles, & Andrews, 2001; Baltes & Lang, 1997; Lindenberger & Baltes, 1994, 1997; Lindenberger, Scherer, & Baltes, 2001). However, there is some evidence that age-related visual changes, as compared to hearing, may be more closely related to changes in cognitive abilities over time. For example, Anstey and colleagues found that while visual declines significantly impacted memory, hearing difficulties were not associated with decline in any cognitive domain (Anstey, Luszcz, & Sanchez, 2001b). The strong connection of sensory and cognitive abilities with age has been explained by the sensory deprivation hypothesis, which states that a prolonged lack of adequate sensory input will result in cognitive deterioration due to neuronal atrophy (Oster, 1976; Valentijn et al., 2005). Another explanation is provided by the common cause hypothesis, which suggests that “correlations between measures of sensory functioning and intellectual ability may increase in old age because both sets of measures are an expression of the physiological architecture… of the brain” (Lindenberger & Baltes, 1994, p. 339).
The common cause hypothesis has been investigated by a plethora of studies. As examples, Lindenberger and Baltes (1997) found that sensorimotor variables, such as visual acuity, balance-gait, and auditory acuity, predicted 59% of total reliable variance in general intelligence. Baltes and Lindenberger (1997) found that individual differences in vision and hearing predicted decreases in intellectual ability gradients accounting for 31% of variance in intellectual functioning. Similarly, Anstey et al. (2001a) found that sensory functioning explained nearly 80% of age-related cognitive variation (verbal memory and speed).
A criticism of such research has been that the commonly-cited covariation between sensory and intellectual functioning may be a result of poorer cognitive psychometric performance of persons with sensory difficulties. One possible mechanism is that sensory decline results in less resource allocation to cognitive tasks (Valentijn et al., 2005). Another possibility is that sensory decline results in an appearance of cognitive decline due to psychometric testing methods (Gussekloo, et al., 2005). The latter hypothesis was tested by Lindenberger et al. (2001) who found that simulated sensory deprivation did not directly result in decreased cognitive performances. They concluded that the connection between sensory and intellectual ability was better explained by the common cause hypothesis.
There are very few studies that have empirically evaluated the sensory deprivation hypothesis, or the similar notion that sensory decline results in reduced processing resources. One method used to examine such hypotheses is by evaluating the impact of sensory interventions upon cognitive function. Recent investigations employing this technique have indicated that sensory improvement does not result in cognitive improvement. For examples, Valnetijn and colleagues (2005) did not find either cataract surgery or acquisition of hearing aids to enhance cognition as measured by memory or executive function. Similarly, in two separate studies Anstey, Lord, Hennessy and colleagues (2006) and Hall and colleagues (2005) both found that cataract surgery did not significantly impact cognitive performance as indicated by visual memory, facial recognition, nonverbal reasoning or mental status. These results do not support the sensory deprivation hypothesis.
Processing Speed
Another thoroughly researched theory of cognitive aging and age-related decline in fluid abilities is the processing speed hypothesis (Birren, Woods, & Williams, 1980; Salthouse, 1996). This theory suggests that slower information processing by the central nervous system influences both the qualitative and quantitative aspects of cognitive abilities. This theory is governed by both the limited time mechanism, which postulates that cognitive processes cannot be successfully completed due to slower processing speed, and the simultaneity mechanism, which postulates that there is a reduction in the amount of simultaneous information available for further processing as a result of slower processing abilities. Thus, slower processing speed would impact cognitive skills because there may not be enough time to process all stimuli (limited time mechanism) nor enough availability of previously processed information for further cognitive processing and interpretation (simultaneity mechanism).
Much research evaluating this theory has focused on working memory, attention and processing speed. Luszcz & Bryan (1999) reviewed research on the processing speed hypothesis, the common cause hypothesis, and the executive function hypothesis and concluded that reductions in processing speed were the most parsimonious and fundamental factor in nonpathological age-related memory loss. However, the authors cautioned that other mechanisms, such as working memory, sensory abilities and executive function also play an important role in memory loss under some conditions. Hertzog et al. (2003) found that individual differences in episodic memory change were predicted by speed and working memory changes, thus providing evidence for the speed of processing theory. Verhaeghen and Salthouse (1997) used hierarchical regression analyses and found that processing speed shared from 71% to 79% of age-related variance in cognitive factors. Zimprich and Martin (2002) investigated the influence of processing speed on fluid intelligence using longitudinal data and found a moderate correlation between changes in speed and changes in fluid intelligence over a four-year period. Although this study found less common variance in speed of processing and fluid intelligence than was previously reported in cross-sectional studies, processing speed still remained an important contributing factor. The authors concluded that researchers may be misled if searching for a single factor responsible for cognitive aging.
Memory Span and Fluid Intelligence
Memory span refers to the storage and maintenance of either verbal or nonverbal information for a short period of time. Beginning with Ebbinghaus' pioneering work, several studies have investigated the maximum amount of information that can be retained in memory and immediately reproduced after a brief presentation. Tests of memory span have been considered useful measures of intellectual ability from the inception of intellectual assessment. Several reviews and meta-analyses have revealed a significant moderate correlation between memory span and general cognitive ability (Ackerman et al., 2005; Carroll, 1993; Mukunda & Hall, 1992). Colom and colleagues (2005) used a latent-variable approach to simultaneously examine the relationships among memory span, working memory, and general intelligence. Their full structural model showed that memory span and working memory factors significantly predicted general intelligence (loadings of 0.58 and 0.79, respectively). Using a “best evidence” approach, Beier and Ackerman (2004) reanalyzed data from two prior studies and found that the correlation between memory span and intelligence was between .71 and .83. However, this relationship was reduced by half after accounting for common content variance. Taken together, the forgoing studies demonstrate a moderate to substantial relationship between memory span and general intellectual abilities.
The present study was designed to examine the relationships between cognition and vision among older adults and included cognitive measures of speed of processing, memory, and fluid intellectual abilities. Structural equation modeling was used to investigate explanations of age-related cognitive decline including the common cause and sensory deprivation hypotheses as well as processing speed theory using a single model that assessed the associations between age, sensory functioning, processing speed, memory span, and fluid intelligence. If the relationships between age and processing speed, age and memory span, and age and fluid intelligence are each mediated by visual function, this would support the sensory deprivation hypothesis. If the relationships between age and memory span as well as age and fluid intelligence are mediated by processing speed, this would support the speed of processing theory. The common cause theory would best be supported by direct relationships of similar magnitude between age and visual function, as well as age and other indicators of cognition.
Method
Participants
Data are reported for 842 participants ranging in age from 62 to 96 years with a mean age of 73.32 years. The sample was 89% Caucasian, 10% African-American and 58% female. Education levels of the participants ranged from 6th grade to Ph.D. with a mean of 14 years. Participants were community dwelling older adults from Bowling Green, Kentucky, Birmingham, Alabama and the surrounding areas. Inclusion criteria included minimal visual function (20/80 or better with corrective lenses) and literacy level (at least 5th grade) in order to view and read study materials, and a Mini-Mental State Exam (MMSE) score of 24 or better, in order to minimize the possibility of undiagnosed dementia. Descriptive statistics for the study variables are reported in Table 1.
Table 1.
Descriptive Statistics for Study Variables
| Variable | M | SD | Minimum | Maximum |
|---|---|---|---|---|
| Age | 73.32 | 5.87 | 62.00 | 95.92 |
| Far Visual Acuity | 71.83 | 11.59 | 20.90 | 90.00 |
| Contrast Sensitivity | 1.69 | 0.15 | 0.90 | 1.95 |
| Digit Symbol | 40.64 | 11.30 | 9.00 | 74.00 |
| Letter Comparison | 39.80 | 9.22 | 13.00 | 69.00 |
| Pattern Comparison | 27.23 | 6.34 | 4.00 | 45.00 |
| UFOV® | 861.23 | 274.95 | 302.00 | 2000.00 |
| Digit Span | 9.62 | 2.13 | 4.00 | 16.00 |
| Spatial Span | 7.47 | 1.75 | 0.00 | 13.00 |
| WASI Matrix Reasoning | 17.48 | 7.32 | 2.00 | 31.00 |
Note: UFOV = useful field of view; WASI = Wechsler Abbreviated Scale of Intelligence.
Measures
Visual Sensory Functioning
Far visual acuity
Far visual acuity was binocularly assessed with the Good-Lite model 600A light box with an ETDRS chart using standard procedures. If usually needed, the participants wore their corrective lenses. The Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) scoring system was utilized which allowed for a total of 10 points for each line correctly read (Ball et al., 2002). Resulting scores may range from 0 (approximate Snellen score of 20/125) to 90 (approximate Snellen score of 20/16).
Contrast sensitivity
Contrast sensitivity was binocularly assessed with participants' usual correction using the Pelli-Robson Constrast Sensitivity Chart at a distance of 40 inches (Pelli, Robson, & Wilkins, 1988). The chart has 8 rows with 2 sets of 3 letters on each row that gradually fade in contrast from left to right and top to bottom. Scores may range from 0.00 to 2.25 log10 with lower scores indicating poorer performance. Although not as commonly used in cognitive aging research, contrast sensitivity is often a better predictor of cognitive (Skeel, et al., 2006) and everyday functioning than visual acuity (i.e., Lord & Lord, 2006).
Processing Speed
Useful Field of View (Edwards, Vance et al., 2005)
The four subtests of the UFOV® test were summed as a measure of speed of processing, and this composite was used in the analyses. For each subtest, the score represents the briefest duration (in ms) at which the individual performs accurately on 75% of trials. The first subtest required the participant to identify a car or truck flashed briefly inside a central fixation box. The second subtest required the identification of the central target and simultaneous localization of a car in the periphery. The third subtest was identical to the second subtest except for the addition of distractors. The final subtest prompted the participant to determine whether two objects presented simultaneously at central fixation were the same (2 cars or 2 trucks) or different (1 car and 1 truck) and at the same time locate the peripheral car surrounded by distractors. Inclusion of this speed of processing measure is beneficial given its demonstrated relationships with everyday performance (i.e., Owsley Sloane, McGwin, & Ball, 2002) and driving in particular (Ball et al., 2006; Clay et al., 2005; Owsley et al., 1998).
Digit Symbol Substitution (Wechsler, 1981)
Participants were presented with a paper containing a grid of empty boxes with numbers above each box and a key that paired a particular symbol with each number at the top. Participants were asked to correctly substitute as many symbols associated with each of the numbers as possible within 90 s. The number of correctly paired symbols was used in these analyses.
Letter Comparison (adapted from Salthouse & Babcock, 1991)
Participants were presented with a paper containing two columns of paired letter sets containing strings of varying length (3, 6, or 9 letters). Participants were then asked to determine whether or not each paired set contained the same or different letters within 20 s. The sum of the correctly completed comparisons across all items was used in these analyses.
Pattern Comparison (adapted from Salthouse & Babcock, 1991)
Participants were presented with a paper containing two columns of paired line pattern sets consisting of 3, 6, or 9 abstract line drawings. Participants were then asked to determine whether or not each pair contained the same or different patterns within 20 s. The sum of the correctly completed comparisons across all items was used in these analyses.
Memory Span
Wechsler Memory Scale (WMS®-III) Digit Span forward (Wechsler, 1997)
Digit span forward requires that the participant listen to a set of numbers and then verbally repeat those numbers back to the tester. The number set continues to grow increasingly longer until the participant either completes the most difficult set, or fails two consecutive number sets of equal length. The number of correctly repeated series was used in these analyses.
WMS®-III Spatial Span forward (Wechsler, 1997)
This measure assesses spatial memory and requires that the participant to first pay close attention to the specific order in which the tester touches a series of blocks and then repeat this same order. The complexity and series length continue to increase until the participant either successfully completes the series, or fails a specific preset number of trials to trigger discontinuation. The number of correct trials completed was used in these analyses.
Fluid Intelligence
Mini-Mental State Exam (Folstein, Folstein, & McHugh, 1975)
The MMSE was used to screen for possible dementia or other types of cognitive impairment. Only participants with scores of 24 or better were included in analyses.
WASI Matrix Reasoning (Psychological Corporation, 1999)
This measure requires that the participant review a series of picture puzzles and select the piece that is missing from the target picture from five possible choices at the bottom of each page. This is a nonverbal assessment that taps fluid intelligence, which has been shown to decline with age (Horn, 1982).
Procedures
The Staying Keen in Later Life (SKILL) study was designed to investigate both the interrelationships among cognitive, functional, and sensory abilities in older adults, and the potential impact of cognitive training on persons with processing speed impairment. The study was conducted between 2000 and 2004. Participants completed a 1 ½-hour screening visit and a 2-hour baseline visit during which measures of sensory function, speed of processing, memory span, and intelligence were administered. Only these pre-training data are included in the present analyses. Further details of this study are available elsewhere (Edwards, Wadley, et al., 2005; Wood et al., 2005).
Analyses
Structural Equation Modeling (SEM) was used to examine the interrelations among age, visual function, and cognitive abilities. A two-step approach was utilized to examine the data structure (Anderson & Gerbing, 1988). Initially, a confirmatory measurement model was tested using the Proc Calis procedure of SAS software Version 9 (SAS, 2002) to determine if the latent constructs of sensory functioning, processing speed, memory span, and fluid intelligence could be represented with the measures selected. Next, a full structural model was constructed to test the strength of the associations between measures. The effect of age on the latent factors of sensory functioning, processing speed, memory span, and fluid intelligence was evaluated. The effect of visual function on processing speed, memory span, and fluid intelligence was also assessed. Processing speed was evaluated as a predictor of memory span and fluid intelligence. Finally, memory span was assessed as a predictor of fluid intelligence. Tests for these effects were necessary to examine our hypotheses involving mediation (Baron & Kenny, 1986; Frasier, Tix, & Barron, 2004). After evaluating the full model, associations between variables that were not significant were trimmed in an attempt to construct a model that adequately represented the data.
For each model estimated by SAS Version 9, the observed variance-covariance matrix was compared with the model-reproduced matrix using the conventional chi-square goodness-of-fit test. Models that fit very well will yield nonsignificant chi-square statistics. Because the chi-square is sensitive to sample size, additional fit indices are informative and indicate the absolute degree of fit. Four such indices are reported here: the goodness of fit index (GFI), the adjusted goodness of fit index (AGFI), the normed fit index (NFI), and the root mean square error of approximation (RMSEA). The GFI, AGFI, and NFI all range from 0 to 1, with higher scores representing better fit. For the RMSEA, scores closer to 0 represent better fit. Kline (1998) suggests that values for the GFI, AGFI, and NFI above .90, and values of the RMSEA below .10 are considered as “favorable” fit statistics.
Results
Measurement Model
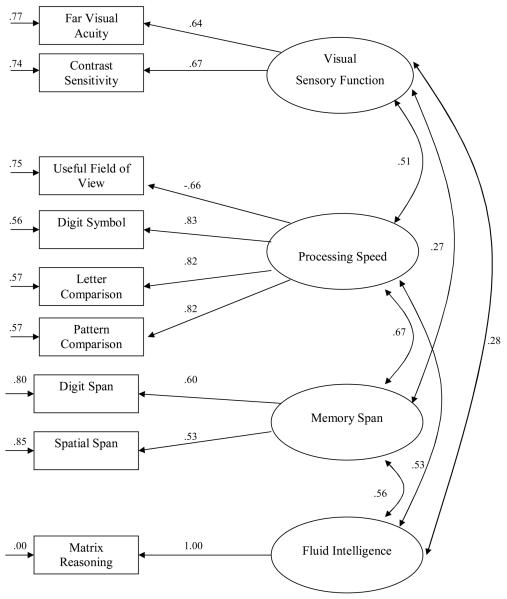
A confirmatory factor analysis was conducted to evaluate the fit of a measurement model with four factors: sensory functioning, processing speed, memory span, and fluid intelligence (see Table 2 for Pearson's correlation matrix). Nine observed measures were allowed to load on the 4 correlated latent factors. A reference variable was chosen for each factor to indicate if higher or lower scores on a measure indicated better functioning. Far visual acuity was the reference for sensory functioning with higher scores representing better sensory performance. Digit symbol substitution number correct was the reference of processing speed with higher scores representing better performance. Digit span and matrix reasoning were the references for memory span and fluid intelligence, respectively, with higher scores representing better functioning.
Table 2.
Correlation Matrix of Study Variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | 1.00 | |||||||||
| 2. Far Visual Acuity | −0.29 | 1.00 | ||||||||
| 3. Contrast Sensitivity | −0.33 | 0.43 | 1.00 | |||||||
| 4. Digit Symbol | −0.25 | 0.24 | 0.24 | 1.00 | ||||||
| 5. Letter Comparison | −0.28 | 0.23 | 0.27 | 0.69 | 1.00 | |||||
| 6. Pattern Comparison | −0.33 | 0.27 | 0.27 | 0.66 | 0.72 | 1.00 | ||||
| 7. UFOV® | 0.39 | −0.33 | −0.40 | −0.55 | −0.49 | −0.52 | 1.00 | |||
| 8. Digit Span | −0.11 | 0.09 | 0.10 | 0.36 | 0.33 | 0.30 | −0.31 | 1.00 | ||
| 9. Spatial Span | −0.08 | 0.09 | 0.11 | 0.33 | 0.22 | 0.24 | −0.31 | 0.31 | 1.00 | |
| 10. Matrix Reasoning | −0.13 | 0.20 | 0.17 | 0.47 | 0.37 | 0.44 | −0.42 | 0.32 | 0.31 | 1.00 |
Note: UFOV = useful field of view. All correlations significant, p<.05.
The model provided favorable fit to the variance-covariance structure for the measures, χ2 (22) = 164.1394, p <.0001, GFI = .9577, AGFI = .9135, NFI = .9369, RMSEA = .0876. The standardized maximum likelihood estimates for the measurement model are presented in Figure 1. All estimates are significantly different from zero, p's < .05 and the latent variables were found to have significant correlations with each other. Fit was also examined for a nested model that forced digit span, spatial span, matrix reasoning, and vocabulary to load on a single “intelligence” factor allowing the same errors to correlate as in the original four-factor model. The fit statistics were found for this model, χ2 (24) = 174.6044, p < .0001, GFI = .9551, AGFI = .9159, NFI = .9328, RMSEA = .0864, and the difference in chi-square statistics indicated that the original measurement model with separate memory span and fluid intelligence factors fit significantly better, χ2 (2) = 10.4650, p < .01.
Figure 1.
Four-Factor Measurement Model for Sensory Functioning, Processing Speed, Memory Span and Fluid Intelligence
Structural/Mediation Model
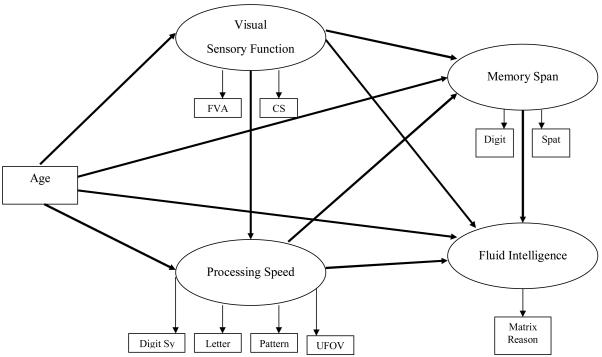
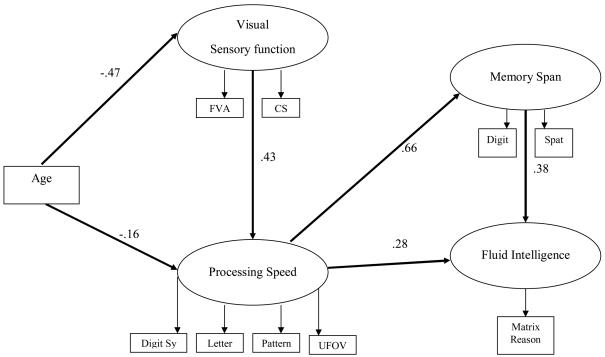
Our structural model examined specific predictive relationships between age, sensory function, speed, memory span, and fluid intelligence (see Figure 2 for all pathways assessed). The common cause hypothesis was evaluated by testing the effects of age on sensory functioning, processing speed, memory span, and fluid intelligence. Next, the sensory deprivation hypothesis was examined by assessing the effects of sensory function on processing speed, memory span, and fluid intelligence. In order to test the processing speed theory, processing speed was evaluated as a predictor of memory span and fluid intelligence. Nonsignificant paths from age to memory span, age to fluid intelligence, sensory function to memory span, and from sensory function to fluid intelligence were trimmed to reveal a final structural model that illustrated favorable fit, χ2 (31) = 205.6528, p < .0001; GFI = .9542; AGFI = .9187; NFI = .9268; RMSEA = .0818. The results of the overall trimmed structural model revealed that (1) after controlling for the relationships between age, sensory functioning, and processing speed, age was found to have no direct effect on memory span and fluid intelligence; (2) sensory functioning partially mediated the effect of age on speed of processing, (3) speed of processing mediated the association between sensory functioning and memory span and the relationship between sensory functioning and general intellectual abilities; and (4) although the direct association between processing speed and fluid intelligence was still significant, this effect was partially mediated by memory span (see Figure 3).
Figure 2.
Full Structural/Mediation Model with All Tested Paths
Note: FVA = far visual acuity; CS = contrast sensitivity; Digit Sy = digit symbol substitution; Letter = letter comparison; Pattern = pattern comparison; UFOV = useful field of view; Digit = digit span; Spat = spatial span; Matrix Reason = matrix reasoning;
Figure 3.
Trimmed Structural/Mediation Model with Significant Paths
Note: FVA = far visual acuity; CS = contrast sensitivity; Digit Sy = digit symbol substitution; Letter = letter comparison; Pattern = pattern comparison; UFOV = useful field of view; Digit = digit span; Spat = spatial span; Matrix Reason = matrix reasoning;
Discussion
Results suggest that the association between age and declines in some cognitive abilities such as memory span and fluid intelligence can be explained by age-related declines in visual functioning and processing speed. The conceptual model tested in this investigation allowed us to investigate the common cause hypothesis, as well as, alternative theories (e.g., sensory deprivation and processing speed theory). Proponents of the common cause theory might examine our structural model and point out that age was either directly or indirectly associated with sensory functioning, processing speed, memory span, and fluid intelligence. They would say that changes associated with aging simultaneously affected the other domains. However, given that the relationships between age and memory span, as well as between age and fluid intelligence, were mediated by visual sensory function and processing speed, we assert that the findings provide better support for the sensory deprivation and processing speed theories. This interpretation is consistent with other recent investigations concluding that the common cause cannot completely explain the association between sensory and cognitive impairment among older adults (i.e., Lovden & Wahlin, 2005; Tay et al., 2006), but that specific factors underlie decline as well (Anstey, Hofer, & Luszcz, 2003b).
Supporters of the sensory deprivation theory would focus on sensory functioning partially mediating the relationship between age and processing speed. Older age was found to be associated with declines in sensory functioning, and the positive relationship between sensory functioning and processing speed indicated that worse sensory functioning was associated with slower processing speed. One explanation would be that as we age, declines in sensory abilities are the central cause of declines in the speed at which we process information. These results are similar to those published by Anstey and colleagues who found that decreased visual contrast sensitivity resulted in slower speed of processing, but was not directly associated with higher order cognitive performance in covariate adjusted models (Anstey, Butterworth, et al., 2006). Similarly, Lovden and Wahlin (2005) found that vision did not predict cognition independent of age, except for cognitive speed of processing. Thus, the sensory deprivation hypothesis may particularly apply to changes in cognitive speed of processing that occur with age, but only indirectly to higher-order cognitive functions.
Our results also support the generalized slowing theory (Birren et al., 1980). Processing speed was the strongest predictor of memory span and fluid intelligence after controlling for the other associations in the structural model. Speed also mediated the relationship between sensory functioning and memory span, and the relationship between sensory functioning and fluid intelligence. A combination of both generalized slowing and the sensory deprivation hypothesis may also be suggested due to the pathways indicating that older age is associated with declines in sensory functioning which leads to slower processing speed, which in turn leads to poorer intellectual function.
The current investigation is limited by the use of cross-sectional data, which prevents a determination of the time sequence of changes in the multiple domains. The fit of our cross-sectional model is acceptable, but not ideal, and it is possible that additional alternative models exist that would provide equivalent or even better fit. Competing models should be constructed based on theoretical concepts, and comparisons between these models on longitudinal data would provide greater clarity on the causal linkages over time. Hertzog and Nesselroade (2003) provided a review of how analytic approaches such as latent growth curve modeling and multilevel, or random effects, models can be used to study intra-individual change (or stability) over time. The combination of these statistical techniques with well implemented longitudinal studies using psychometrically sound measures should enable researchers to obtain a clearer view of the predictive nature of the relationship between sensory functioning, processing speed, memory span, and fluid intelligence in older adults.
Nevertheless, the present results are of interest and useful for sorting out differential findings of the causes of age-related cognitive declines and explanations for the strong associations between sensory and cognitive decline. Strengths of these analyses include the large and relatively diverse sample of older adults due to use of minimal exclusion criteria. Also, this study involved ecologically valid measures of vision and speed of processing. These results are among the first to provide empirical support for the sensory deprivation hypothesis. In particular, results highlight that lower levels of sensory functioning are associated with age-related cognitive speed of processing decline. Furthermore, the finding that if relationships between age, vision, and speed of processing are accounted for, age is not associated with declines in memory span or fluid intelligence highlights the ability for individuals to age successfully if deficits in vision and processing speed can be avoided.
It may be that successful aging is characterized by an individual's ability to cope with declines in sensory function and processing speed. Some older adults have developed elaborate reading, analytic, and organizational skills and acquired knowledge structures that are highly detailed. These strategies enable them to continue to acquire relevant information and evade the usual age-linked declines involved in learning new information (Burke & MacKay, 1997). Considering that there are interventions targeted toward improving speed of processing (Ball et al., 2002; Ball et al., 2007; Edwards, Wadley et al., 2005; Roenker, et al., 2003) and sensory function (e.g. cataract surgery which may help with long-term maintenance of speed of processing), the results of this investigation provide an optimistic view of preventing age-related decline.
Acknowledgements
The project described was funded by an NIH Merit Award to Karlene Ball (SR37AG05739).
Footnotes
No other authors have a financial disclosure or conflict of interest.
References
- Ackerman PL, Beier ME, Boyle MO. Working memory and intelligence: The same or different constructs. Psychological Bulletin. 2005;131(1):30–60. doi: 10.1037/0033-2909.131.1.30. [DOI] [PubMed] [Google Scholar]
- Anderson JC, Gerbing DW. Structural equation modeling in practice: A review and recommended two-step approach. Psychological Bulletin. 1988;103:411–423. [Google Scholar]
- Anstey KJ, Butterworth P, Borzycki M, Andrews S. Between- and within-individual effects of visual contrast sensitivity on perceptual matching, processing speed, and associative memory in older adults. Gerontology. 2006;52(2):124–130. doi: 10.1159/000090958. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Hofer SM, Luszcz MA. Cross-sectional and longitudinal patterns of dedifferentiation in late-life cognitive and sensory function: The effect of age, ability, attrition, and occasion of measurement. Journal of Experimental Psychology: General. 2003a;132(3):470–487. doi: 10.1037/0096-3445.132.3.470. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Hofer SM, Luszcz MA. A latent growth curve anaylsis of late-life sensory and cognitive function over 8 years: Evidence for specific and common factors underlying change. Psychology & Aging. 2003b;18(4):714–726. doi: 10.1037/0882-7974.18.4.714. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Lord SR, Hennessy M, Mitchell P, Mill K, Von Sanden C. The effect of cataract surgery on neuropsychological test performance: A randomized controlled trial. Journal of the International Neuropsychological Society. 2006;12(5):632–639. doi: 10.1017/S1355617706060954. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Luszcz MA, Giles LC, Andrews GR. Demographic, health, cognitive, and sensory variables as predictors of mortality in very old adults. Psychology and Aging. 2001;16(1):3–11. doi: 10.1037/0882-7974.16.1.3. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Luszcz MA, Sanchez L. A reevaluation of the common factor theory of shared variance among age, sensory function, and cognitive function in older adults. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences. 2001a;56B(1):P3–P11. doi: 10.1093/geronb/56.1.p3. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Luszcz MA, Sanchez L. Two-year decline in vision but not hearing is associated with memory decline in very old adults in a population-based sample. Gerontology. 2001b;47(5):289–293. doi: 10.1159/000052814. [DOI] [PubMed] [Google Scholar]
- Ball KK, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, et al. Effects of cognitive training interventions with older adults. A randomized controlled trial. JAMA. 2002;288:2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball KK, Edwards JD, Ross LA. The impact of speed of processing training on cognitive and everyday functions. The Journals of Gerontology: Psychological Sciences. 2007;62(Special Issue 1):19–31. doi: 10.1093/geronb/62.special_issue_1.19. [DOI] [PubMed] [Google Scholar]
- Ball KK, Roenker D, McGwin G, Wadley VG, Edwards JD, Raleigh R, et al. Can high-risk older drivers be identified through performance-based measures in a Department of Motor Vehicles setting? Journal of the American Geriatrics Society. 2006;54:77–84. doi: 10.1111/j.1532-5415.2005.00568.x. [DOI] [PubMed] [Google Scholar]
- Baltes MM, Lang RF. Everyday functioning and successful aging: The impact of resources. Psychology and Aging. 1997;12(3):433–443. doi: 10.1037//0882-7974.12.3.433. [DOI] [PubMed] [Google Scholar]
- Baltes PB, Lindenberger U. Emergence of a powerful connection between sensory and cognitive functions across the adult life span: a new window to the study of cognitive aging? Psychology and Aging. 1997;12(1):12–21. doi: 10.1037//0882-7974.12.1.12. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator mediator variable distinction in social psychological research: Conceptual, strategic and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Beier ME, Ackerman PL. A reappraisal of the relationship between span memory and intelligence via “best evidence synthesis”. Intelligence. 2004;32:607–619. [Google Scholar]
- Beier ME, Ackerman PL. Working memory and intelligence: Different constructs. Reply to Oberauer et al. (2005) and Kane et al. (2005) Psychological Bulletin. 2005;131(1):72–75. doi: 10.1037/0033-2909.131.1.30. [DOI] [PubMed] [Google Scholar]
- Birren JE, Woods AM, Williams MV. Behavioral slowing with age: Causes, organization, and consequences of slowing. In: Poon LW, editor. Aging in the 1980's: Psychological Issues. American Psychological Association; Washington, DC: 1980. pp. 293–308. [Google Scholar]
- Burke DM, MacKay DG. Memory, language, and ageing. Philosophical Translations: Biological Sciences. 1997;352:1845–1856. doi: 10.1098/rstb.1997.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll RB. Human cognitive abilities: A survey of factor-analytic studies. Cambridge University Press; New York: 1993. [Google Scholar]
- Clay OJ, Wadley VG, Edwards JD, Roth DL, Roenker DL, Ball KK. Cumulative meta-anaysis of the relationship between useful field of view and driving performance in older adults: Current and future implications. Optometry & Vision Science. 2005;82(8):724–731. doi: 10.1097/01.opx.0000175009.08626.65. [DOI] [PubMed] [Google Scholar]
- Colom R, Abad FJ, Rebollo I, Shih PC. Memory span and general intelligence: A latent-variable approach. Intelligence. 2005;33:623–642. [Google Scholar]
- Economics and Statistics Administration Sixty-five plus in the United States. United States Department of the Census: Current Population Reports. 1996 Retrieved May 5, 2005, 2005, from http://www.census.gov/prod/1/pop/p23-190/p23-190.pdf.
- Edwards JD, Vance DE, Wadley VG, Cissell GM, Roenker DL, Ball KK. The reliability and validity of the Useful Field of View Test as administered by personal computer. Journal of Clinical and Experimental Neuropsychology. 2005;27:529–543. doi: 10.1080/13803390490515432. [DOI] [PubMed] [Google Scholar]
- Edwards JD, Wadley VG, Vance DE, Roenker DL, Ball KK. The impact of speed of processing training on cognitive and everyday performance. Aging & Mental Health. 2005;9:262–271. doi: 10.1080/13607860412331336788. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frasier PA, Tix AP, Barron KE. Testing moderator and mediator effects in counseling psychology research. Journal of Counseling Psychology. 2004;51:115–134. [Google Scholar]
- Galton F. Inquiries into human faculty. Dent; London: 1883. [Google Scholar]
- Gussekloo J, de Craen AJM, Oduber C, Van Boxtel MPJ, Westerndorp RGJ. Sensory impairment and cognitive functioning in the oldest old. American Journal of Geriatric Psychiatry. 2005;13(9):781–786. doi: 10.1176/appi.ajgp.13.9.781. [DOI] [PubMed] [Google Scholar]
- Hall TA, McGwin G, Owsley C. Effects of cataract surgery on cognitive function in older adults. Journal of the American Geriatrics Society. 2005;53(12):2140–2144. doi: 10.1111/j.1532-5415.2005.00499.x. [DOI] [PubMed] [Google Scholar]
- Hertzog C, Dixon RA, Hultsch DF, MacDonald SWS. Latent change models of adult cognition: Are changes in processing speed and working memory associated with changes in episodic memory. Psychology and Aging. 2003;18(4):755–769. doi: 10.1037/0882-7974.18.4.755. [DOI] [PubMed] [Google Scholar]
- Hertzog C, Nesselroade JR. Assessing psychological change in adulthhod: An overview of methodological issues. Psychology and Aging. 2003;28(4):639–657. doi: 10.1037/0882-7974.18.4.639. [DOI] [PubMed] [Google Scholar]
- Horn JL. The theory of fluid and crystallized intelligence in relation to concepts of cognitive psychology and aging in adulthood. In: Craik FIM, Trehub S, editors. Aging and cognitive processes. Vol. 8. Plenum; New York: 1982. pp. 237–278. [Google Scholar]
- Kane MJ, Hambrick DZ, Conway ARA. Working memory capacity and fluid intelligence are strongly related constructs: Comment on Ackerman, Beier, and Boyle. Psychological Bulletin. 2005;131(1):66–71. doi: 10.1037/0033-2909.131.1.66. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Baltes PB. Sensory functioning and intelligence in old age: A strong connection. Psychology and Aging. 1994;9(3):339–355. doi: 10.1037//0882-7974.9.3.339. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Baltes PB. Intellectual functioning in old and very old age: cross-sectional results from the Berlin Aging Study. Psychology and Aging. 1997;12(3):410–432. doi: 10.1037//0882-7974.12.3.410. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Scherer H, Baltes PB. The strong connection between sensory and cognitive performance in old age: Not due to sensory acuity reductions operating during cognitive assessment. Psychology and Aging. 2001;16(2):196–205. doi: 10.1037//0882-7974.16.2.196. [DOI] [PubMed] [Google Scholar]
- Lord SR, Lord SR. Visual risk factors for falls in older people. Age & Ageing. 2006;35(Suppl 2):ii42–ii45. doi: 10.1093/ageing/afl085. [DOI] [PubMed] [Google Scholar]
- Lovden M, Wahlin M. The sensory-cognition association in adulthood: Different magnitudes for processing speed, inhibition, episodic memory and false memory? Scandinavian Journal of Psychology. 2005;46:253–262. doi: 10.1111/j.1467-9450.2005.00455.x. [DOI] [PubMed] [Google Scholar]
- Luszcz MA, Bryan J. Toward understanding age-related memory loss in late adulthood. Gerontology. 1999;45(1):2–9. doi: 10.1159/000022048. [DOI] [PubMed] [Google Scholar]
- Mukunda KV, Hall VC. Does performance on memory for order correlate with performance on standardized measures of ability? A meta-analysis. Intelligence. 1992;16:81–97. [Google Scholar]
- Oberauer K, Schulze R, Wilhelm O, Süβ H-M. Working memory and intelligence-their correlation and their relation: Comment on Ackerman, Beier, and Boyle. Psychological Bulletin. 2005;131(1):61–65. doi: 10.1037/0033-2909.131.1.61. [DOI] [PubMed] [Google Scholar]
- Oster C. Sensory deprivation in geriatric patients. Journal of the American Geriatrics Society. 1976;24:461–464. doi: 10.1111/j.1532-5415.1976.tb03261.x. [DOI] [PubMed] [Google Scholar]
- Owsley C, Ball K, McGwin G, Sloane ME, Roenker DL, White MF, et al. Visual processing impairment and risk of motor vehicle crash among older adults. JAMA. 1998;279(14):1083–1088. doi: 10.1001/jama.279.14.1083. [DOI] [PubMed] [Google Scholar]
- Owsley C, Sloane M, McGwin G, Jr., Ball K. Timed instrumental activities of daily living tasks: Relationship to cognitive function and everyday performance assessments in older adults. Gerontology. 2002;48:254–265. doi: 10.1159/000058360. [DOI] [PubMed] [Google Scholar]
- Owsley C, Sloane ME. Vision and aging. In: Boller F, Grafman J, editors. Handbook of Neuropsychology. Vol. 4. Elsevier; The Netherlands: 1990. pp. 229–249. [Google Scholar]
- Pelli DG, Robson JG, Wilkins AJ. The design of a new letter chart for measuring contrast sensitivity. Clinical Vision Sciences. 1988;2:187–199. [Google Scholar]
- Psychological Corporation . WASI (Wechsler Abbreviated Scales of Intelligence) Harcourt Brace and Company; San Antonio, TX: 1999. [Google Scholar]
- Roenker DL, Cissell GM, Ball KK, Wadley VG, Edwards JD. Speed-of-processing and driving simulator training result in improved driving performance. Human Factors. 2003;45(2):218–233. doi: 10.1518/hfes.45.2.218.27241. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological Review. 1996;103(3):403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Atkinson TM, Berish DE. Executive functioning as a potential mediator of age-related cognitive decline in normal adults. Journal of Experimental Psychology: General. 2003;132(4):566–594. doi: 10.1037/0096-3445.132.4.566. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Babcock RL. Decomposing adult age differences in working memory. Developmental Psychology. 1991;27(5):763–776. [Google Scholar]
- Salthouse TA, Czaja SJ. Structural constraints on process explanations in cognitive aging. Psychology and Aging. 2000;15(1):44–55. doi: 10.1037//0882-7974.15.1.44. [DOI] [PubMed] [Google Scholar]
- SAS . SAS Version 9. SAS Institute Publishing Co., Inc.; Cary, NC: 2002. [Google Scholar]
- Skeel RL, Schutte C, van Voorst W, Nagra A. The relationship between visual contrast sensitivity and neuropsychological performance in a healthy elderly sample. Journal of Clinical and Experimental Neuropsychology. 2006;28:696–705. doi: 10.1080/13803390590954173. [DOI] [PubMed] [Google Scholar]
- Tay T, Wang JJ, Kifley A, Lindley R, Newall P, Mitchell P. Sensory and cognitive association in older persons: Findings from an older Australian population. Gerontology. 2006;52:386–394. doi: 10.1159/000095129. [DOI] [PubMed] [Google Scholar]
- Valentijn SAM, van Boxtel MPJ, van Hooren SAH, Bosma H, Beckers HJM, Ponds RWHM, et al. Change in sensory functioning predicts change in cognitive functioning: Results from a 6-year follow-up in the Maastricht aging study. Journal of the American Geriatrics Society. 2005;53:374–380. doi: 10.1111/j.1532-5415.2005.53152.x. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Salthouse TA. Meta-analyses of age-cognition relations in adulthood: Estimates of linear and nonlinear age effects and structural models. Psychological Bulletin. 1997;122(3):231–249. doi: 10.1037/0033-2909.122.3.231. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WAIS-R Manual. The Psychological Corporation; New York: 1981. [Google Scholar]
- Wechsler D. WMS-III Administration and Scoring Manual. Psychological Corporation Harcourt & Brace Company; San Antonio: 1997. [Google Scholar]
- Whitebourne SK. Physical changes. In: Cavanaugh JC, Whitebourne SK, editors. Gerontology: An interdisciplinary perspective. Oxford University Press; New York: 1999. pp. 91–122. [Google Scholar]
- Wood KM, Edwards JD, Wadley VG, Clay OJ, Ball KK, Roenker D. Sensory and cognitive factors influencing functional ability in older adults. Gerontology. 2005;51:131–141. doi: 10.1159/000082199. [DOI] [PubMed] [Google Scholar]
- Zimprich D, Martin M. Can longitudinal changes in processing speed explain longitudinal age changes in fluid intelligence. Psychology and Aging. 2002;17(4):690–695. doi: 10.1037/0882-7974.17.4.690. [DOI] [PubMed] [Google Scholar]