Abstract
HIV viremia is associated with a wide range of immune dysfunctions that contribute to the immunocompromised state. HIV viremia has been shown to have a broad effect on several immune cell types and/or their interactions that are vital for mounting an effective immune response. In this study, we investigated the integrity of plasmacytoid dendritic cell (pDC)-NK cell interactions among HIV viremic, aviremic, and seronegative individuals. We describe a critical defect in the ability of pDCs from HIV-infected individuals to secrete IFN-α and TNF and subsequently activate NK cells. We also describe an inherent defect on NK cells from HIV-infected individuals to respond to pDC-secreted cytokines. Furthermore, we were able to demonstrate a direct effect of HIV trimeric gp120 on NK cells in vitro similar to that described ex vivo. Finally, we were able to establish that the HIV gp120-mediated suppressive effect on NK cells was a result of its binding to the integrin α4β7 expressed on NK cells. These findings suggest a novel mechanism by which HIV is capable of suppressing an innate immune function in infected individuals.
Introduction
Natural killer (NK) cells are an integral component of the innate immune system primarily responsible for killing virus-infected cells and tumor cells.1 These cells were originally identified by their ability to lyse certain tumor cells without prior activation; however, they do not express clonally distributed antigen-specific receptors.2 Furthermore, it has been established that HLA-class I molecules inhibit cytolytic activity of NK cells by their interaction with a series of cytotoxicity inhibitory receptors (iNKR).3,4 NK cells also express activating receptors (NCRs) on their surface, which bind to ligands on target cells.5 The activation of NK cells and their subsequent cytotoxic activity result from a balance between the stimulation of iNKRs and the cytotoxicity receptors.4,6 Activated NK cells also secrete cytokines and chemokines that induce inflammatory responses, regulate cell proliferation and function of monocytes and granulocytes, and influence the type of adaptive immune responses generated.7
Several previous studies have demonstrated the effects of HIV viremia on the immune system.8 HIV viremia is associated with global suppressive effects on all immune-competent cells including CD4+ T cells, CD8+ T cells, NK cells, plasmacytoid dendritic cells (pDCs), and myeloid dendritic cells.8 Recently, it has been demonstrated that HIV viremia is associated with several functional and phenotypic defects including increased expression of iNKRs, decreased expression of NCRs, and decreased secretion of CC-chemokines and cytokines.9 Furthermore, recent studies have shown that exposure to gp120 decreases cellular proliferation and increases the propensity of NK cells to undergo apoptosis.10 These studies also demonstrate that exposure to the HIV envelope inhibits NK-mediated lysis of target cells, thereby allowing the virus to replicate. Similar effects of HIV viremia and HIV gp120 on pDCs have also been demonstrated.11 Ashkar and Rosenthal have shown that stimulation of NK cells using TLR-9 agonists can activate NK cells and provide protective immunity against herpes virus infection.12 A recent study has illustrated that this effect of TLR-9 agonists on NK cells is mediated by pDCs, probably through the secretion of interferon (IFN)-α.13 Given the profound suppressive effects of HIV viremia and HIV envelope proteins on both NK cells and pDCs, we investigated the integrity of pDC-NK cell cross-talk in the context of TLR-9-mediated activation in HIV viremic individuals in this study.
Materials and Methods
Study subjects
Peripheral blood mononuclear cells (PBMCs) were obtained from HIV-negative (N = 20) and HIV-infected (N = 40) donors. Each donor underwent leukapheresis after signing informed consents approved by the Institutional Review Board of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. HIV-infected viremic (N = 20) patients were either off antiretroviral therapy (ART) for over 3 months or naive to ART at the time of the study (HIV VL mean 33,453 ± 2345 copies/ml, CD4+ T cell count 432 ± 98 cells/mm3). HIV-infected aviremic (N = 20) individuals were receiving ART that consisted of at least two nonnucleoside reverse transcriptase inhibitors and a protease inhibitor or nonnucleoside reverse transcriptase inhibitor at the time of the study (HIV VL <50 copies/ml and CD4+ T cells 542 ± 101 cells/mm3). The median duration of HIV viral load suppression was 7.2 months.
PBMC isolation and cell culture conditions
PBMCs were isolated by Ficoll-Paque PLUS (GE Healthcare) density gradient centrifugation and cultured in RPMI 1640 media at 37°C in the presence or absence of ODN-C, M362, 5′-tcgtcgtcgttc:gaacgacgttgat-3′ (25-mer with phosphorothiorate bases) (Invivogen) (1 μg/ml), and trimeric R5-gp120 (92Ug037,1 μg/ml, 2 μg/ml) and/or 5 μg/ml each of anti-IFN-αR antibody (Calbiochem), anti-TNF and anti-interleukin (IL)-12 antibodies (R & D Systems, Inc., Minneapolis, MN). Culture supernatants were collected after 24 h and stored at –80°C for later analysis. Control ODN without CpG motifs, ODN1585 (Invivogen) 5′-ggGGTCAAGCTTGAgggggg-3′, was used as negative control (1 μg/ml). Experiments utilizing the transwell format were set up in which pDCs and NK cells cultured with the transwell tissue culture plates (0.4 μm membrane with 6.5 mm insert, Costar), where the pDCs were cultured in the upper chamber and the NK cells in the lower chamber without any cell-to-cell contact between them for a period of 24 h. Purified NK cells were also cultured in complete RPMI and IL-2 (20 IU/ml) with each of the following cytokines [rIFN-α2b 100 IU/ml, tumor necrosis factor (TNF) 10 ng/ml and IL-12 10 ng/ml, R & D Systems Inc, Minneapolis, MN] for a period of 24 h before staining for levels of activation markers and cytokine secretion. The α4 integrin monoclonal antibody (HP2/1) was purchased from Santa Cruz Biotechnology Inc., Santa Cruz, CA.
Depletion of lymphocyte subsets
PBMCs were depleted of pDCs, B cells, CD4+ T cells, and CD8+ T cells using respective magnetic bead separation techniques following the manufacturer's instructions (Invitrogen Corporation, Carlsbad, CA; Miltenyi Biotech Inc., Auburn, CA). Depleted PBMCs were highly pure (>99%).
Isolation of natural killer cells
Highly purified NK cells were obtained from PBMCs using a magnetic bead separation technique as previously described (Stem Cell Technologies Inc., Vancouver, Canada).14 Isolated NK cells had a purity of >90%.
Isolation of plasmacytoid dendritic cells
Eighty million PBMCs were resuspended in cold MACS buffer (PBS, 2 mM EDTA, 0.5% BSA) at a concentration of 100 million cells/0.3 ml and incubated with 50 μl FcR blocking reagent (Miltenyi Biotech Inc., Auburn, CA) for 10 min at 4°C followed by a 15-min incubation with 50 μl anti-BDCA-4 beads (Milenyi Biotech Inc., Auburn, CA). Cells were then washed, resuspended in MACS buffer (200 million cells/ml), and run over an LS column as previously described.15 Eluted cells were counted and plated in a 24-transwell plate at a concentration of 2 million cells/ml.
Cytokine detection
The cytokines IFN-γ, TNF, RANTES, MIP-1α, and MIP-1β were detected on the BD FACSArray bioanalyzer utilizing the BD Cytometric Bead Array (CBA) Flex Sets. Acquisition and analysis were performed according to the manufacturer's protocol (BD Biosciences, San Jose, CA). IFN-α, TNF, and IL-12 levels from the culture supernatants were detected using ELISA performed according to the manufacturer's protocol (R & D Systems, Minneapolis, MN).
Flow cytometry
CD69 expression on NK cells defined by CD56 APC and CD16 PE-Cy7 staining was measured by flow cytometry utilizing PE-conjugated anti-CD69 antibodies after overnight incubation with cytokines, respective antibodies, and CpG using a BD Biosciences FACSArray bioanalyzer as previously described.14 Analysis was performed using FlowJo software (Treestar Inc., Ashland, OR) as previously described.14
bDNA multiplex assay
Expression of interferon-inducible genes (IFIG) in PBMCs was measured using a multiplex bDNA assay capable of detecting the expression of 35 genes. The RNA transcripts are released from cells in the presence of Lysis Mixture and hybridized to the Probe Sets. The RNA-Probe Set complexes are captured to their respective Capture Beads through the cooperative hybridization of multiple CEs with the Capture Probes on the Capture Beads during an overnight incubation. Signal amplification is performed by sequential hybridization of the bDNA Amplifier and biotinylated Label Probe. The streptavidin-conjugated R-phycoerythrin (SAPE) binds to the biotinylated Label Probe. The Capture Beads are analyzed using a Luminex instrument. The amount of each target RNA present in a sample is quantified by determining the amount of SAPE fluorescence signal and the identity of the beads. The levels of gene expression of B2M, GAPDH, APOBEC3A, APOBEC3B, EIFAK2, G1P3, IFI27, IFI44, IFIT1, IFIT3, IFITM3, IFNA2, IFNB, IFNG, IRF7, ISG15, ISG20, LY6E, MX1, MX2, OAS1, OAS2, PLSCR1, PPIA, SP110, and STAT1 were measured.
Statistical analysis
Analysis of variance with Tukey's multiple comparison test was used to compare means of the three independent groups. The paired t test with the Bonferroni adjustment for multiple testing was used to compare paired responses.
Results
Activation of NK cells by CpG-ODN-C (CpG) treatment of PBMCs is mediated by secretion of IFN-α and TNF by pDCs
Unfractionated PBMCs stimulated by CpG yielded a higher proportion of activated NK cells as indicated by increased expression of CD69. Further experiments using transwells that separate pDCs and NK cells indicate that CpG-mediated activation of NK cells was an indirect effect mediated by pDCs, mostly by the secretion of soluble factors. When we measured the levels of cytokine and chemokine secretion by NK cells, our results indicate that CD69 expression is a reliable marker of NK cell activation. Blocking of the major cytokines secreted by pDCs (IFN-α and TNF) clearly establishes that activation of NK cells is mediated by secretion of IFN-α and TNF. Both cytokines were capable of activating NK cells and supernatants of CpG-stimulated pDCs contained high levels of both TNF and IFN-α, which was consistent with the results observed using blocking antibodies. These results indicate that IFN-α and TNF are the major cytokines secreted by CpG-stimulated pDCs resulting in the activation of NK cells. Human pDCs do not secrete IL-12 in response to CpG stimulation and hence do not play a major role in our experimental conditions (data not shown).
NK cells from HIV-infected viremic individuals are not responsive to CpG-stimulated pDCs
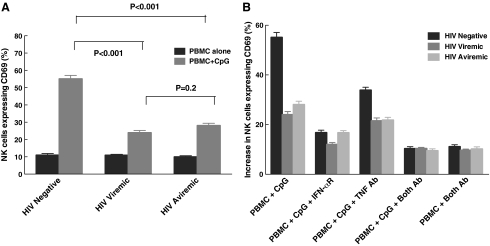
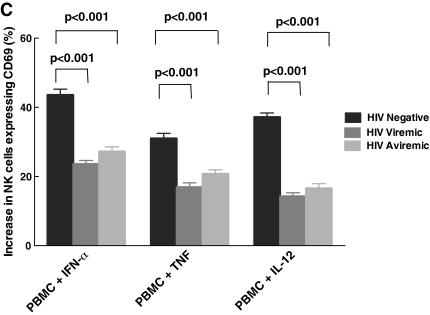
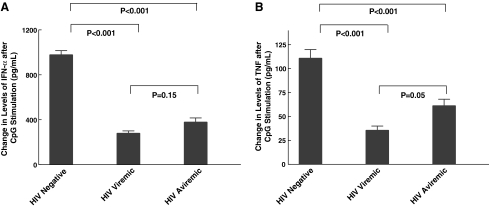
To understand the effect of HIV viremia on CpG-mediated activation of NK cells and to study the interactions between pDCs and NK cells carefully, we performed experiments measuring CD69 expression on PBMCs from HIV-negative, viremic, and aviremic individuals. As shown in Fig. 1A, NK cells from both HIV viremic and aviremic individuals had lower levels of CD69 expression after treatment with CpG when compared to those from normal HIV-negative individuals (24% ± 1, 28% ± 1, and 55% ± 2, respectively, p < 0.001). When we examined the underlying mechanism in activating NK cells among the three groups (Fig. 1B), we found that CD69 expression was mainly mediated by IFN-α and TNF. To distinguish between the effects of HIV viremia on pDCs versus NK cells, we performed experiments in which we treated PBMCs from HIV-negative, viremic, and aviremic individuals with IFN-α, TNF, and also IL-12, a cytokine, that activates NK cells. As seen in Fig. 1C, NK cells from HIV viremic and aviremic individuals had lower levels of CD69 expression upon stimulation with these cytokines when compared to HIV-negative individuals (for IFN-α, 24% ± 1, 27% ± 1, and 44% ± 2, respectively, p < 0.001; for TNF, 17% ± 1, 21% ± 1, and 31% ± 1, p < 0.001; and finally for IL-12, 14% ± 1.0, 17% ± 1, and 37% ± 1, respectively, p < 0.001). To determine whether the decrease in NK cell activation resulted from lower levels of IFN-α and TNF secreted by pDCs, we performed ELISA to measure the levels of each cytokine in the culture supernatants. The change in the levels of IFN-α (Fig. 2A) and TNF (Fig. 2B) was significantly lower in HIV-infected viremic and aviremic individuals than in HIV-seronegative controls, indicating that less cytokine is being secreted by pDCs in HIV-infected groups (for IFN-α, 278 pg/ml ± 23, 377 pg/ml ± 39, and 977 pg/ml ± 39, respectively, p < 0.001; for TNF, 35 pg/ml ± 5, 61 pg/ml ± 7, and 111 pg/ml ± 9, respectively, p < 0.001). These results indicate that the lower CD69 surface expression on NK cells from HIV-infected aviremic and viremic individuals is due to a nonresponsiveness of NK cells to stimulation by IFN-α and TNF secreted by pDCs as well as to a decline in the amount of IFN-α and TNF secreted by pDCs and/or the quantity of viable pDCs in the culture system.
FIG. 1.
NK cells from HIV-infected individuals failed to respond to CpG activation. (A) Levels of CD69 expression were measured in HIV-seronegative, HIV-viremic, and HIV-aviremic individuals. pDC-mediated stimulation of NK cells, as determined by CD69, was lower in both HIV-viremic and aviremic individuals compared to HIV seronegatives (p < 0.001). Levels in viremic and aviremic individuals were similar (p = 0.2). (B) PBMCs from all three groups of individuals were treated with blocking receptor for IFN-α and a blocking antibody for TNF. The results showed a significant decrease in CD69 expression with the addition of the antibodies, indicating that IFN-α and TNF are primarily responsible for pDC-mediated activation of NK cells. (C) To further understand the cause for this decrease in activation, PBMCs from each of the three groups of individuals were treated with or without IFN-α, TNF, and IL-12. Whereas NK cells from all three groups responded to the cytokines, NK cells from viremic and aviremic individuals responded less when compared to those from the HIV-seronegative group (p < 0.001 for each of the three cytokines). The level of CD69 on NK cells in PBMCs without any stimulation was subtracted from the levels obtained after stimulation with each cytokine.
FIG. 2.
CpG-induced stimulation of PBMCs from HIV-infected individuals results in lower levels of IFN-α and TNF secretion. IFN-α (A) and TNF (B) levels were measured from supernatants collected 24 h after treatment with CpG. The changes in IFN-α and TNF levels were significantly less in viremic and aviremic individuals compared to the changes in seronegative individuals (p < 0.001). Baseline levels of IFN-α present in the supernatants were not statistically different among HIV-negative, HIV-viremic, and aviremic subjects (mean 45.3 pg/ml, 38.4 pg/ml, and 33.95 pg/ml, respectively, p > 0.05). The change in the TNF levels was greater in aviremic than in viremic individuals (p = 0.05). The change in IFN-α was not (p = 0.15).
Refractoriness to exogenous IFN-α in HIV-infected individuals correlated to elevated IFIG expression in PBMCs
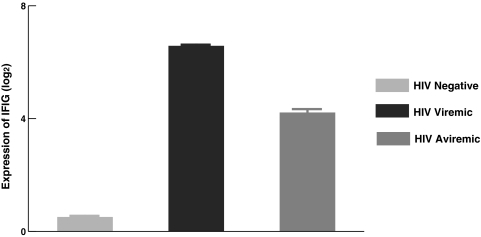
Previously we indicated that HIV-infected individuals with high levels of IFIG expression in PBMCs undergoing treatment for HCV do not respond to IFN-α in vivo.16 High baseline expression of IFIGs was also found to be associated with a poor in vivo antiviral effect of IFN-α in HIV mono-infected individuals (unpublished observations). Because the effects of endogenous IFN-α secretion on peripheral immune cells types have not been studied extensively, we explored the possibility of such an effect that was primarily responsible for the refractoriness of NK cells from HIV viremic individuals seen in this study. In this regard, we examined the levels of expression of IFIGs in PBMCs among all patients who participated in this study using a custom multiplex bDNA assay that can detect 20 IFIGs at the same time. The results indicate that there is increased expression of IFIGs in PBMCs of HIV-infected individuals, both viremic and aviremic, when compared to HIV-seronegative normal volunteers (Fig. 3, p < 0.01 and p < 0.02, respectively). NK cells from HIV aviremic individuals had lower expression of IFIGs when compared to those from HIV viremic individuals (p < 0.04); however, they were still higher than that seen in NK cells of normal volunteers (p < 0.02). These results suggest that high IFIG expression in PBMCs serves as a surrogate marker of refractoriness to IFN-α seen in HIV-infected patients. Our results show similar levels of CpG-induced activation of NK cells in both viremic and aviremic individuals, although at a much lower level when compared with normals. These findings suggest that although a variety of immune dysfunctions may be normalized by rendering a patient aviremic through the control of HIV replication by ART, some defects may be longer lasting than others.
FIG. 3.
Expression of interferon-inducible genes (IFIGs) in PBMCs from viremic, aviremic, and seronegative individuals was measured as described in Materials and Methods. PBMCs from HIV viremic and aviremic individuals expressed significantly higher levels of IFIGs when compared to those in PBMCs from HIV-negative individuals (p < 0.01 and 0.02, respectively). The Y axis is log2 expression of IFIG genes, which represents fold changes.
HIV gp120 induces refractoriness of NK cells to IFN-α by interaction with the integrin α4β7 receptor on NK cells
We performed experiments in vitro to successfully reproduce the defective pDC-NK cell interactions seen in HIV-infected individuals ex vivo by exposing PBMCs to HIV gp120 in vitro. When we stimulated PBMCs from HIV-seronegative individuals using CpG in the presence or absence of HIV gp120, we observed that the ability of CpG-stimulated pDCs to activate NK cells was completely abrogated by exposure to HIV gp120 (Fig. 4a). We further investigated whether this effect of HIV gp120 on pDC-NK interactions was predominantly mediated by the effect of HIV on pDCs or NK cells by repeating the same experiments by treating either pDCs alone, NK cells alone, or both with HIV gp120 before stimulation with CpG. The results indicate that HIV gp120 did exert profound suppressive effects on PDCs and NK cells, although maximal suppression was observed when both cell types were treated with HIV gp120 (Fig. 4b). Finally, we performed experiments to investigate the role of α4β7, the newly described HIV receptor on NK cells, CD4+, and CD8+ T cells,17 in interacting with HIV gp120, resulting in the suppression of NK cell activation (Fig. 4c). When we added CpG-stimulated pDCs to NK cells with or without gp120 treatment in the presence or absence of α4-integrin antibodies, pretreatment of NK cells with α4 integrin antibody completely reversed the effect of gp120 on NK cell activation (Fig. 4c). Treatment of α4 integrin antibodies did not have any effect on normal or CpG-stimulated NK and pDCs. The effect of gp120 on pDCs is complex as gp120 can bind to CD4 and DC-SIGN on the surface of pDCs other than the α4β7 integrin receptor.11 The effects of gp120 on pDCs have been recently described and our investigations were mainly focused on the effect of gp120 on NK cells, which seem to be mediated by binding to the α4β7 integrin receptor.
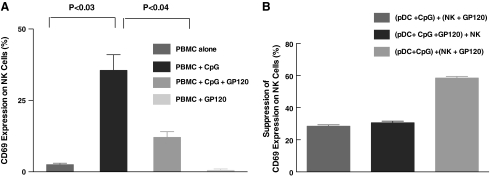
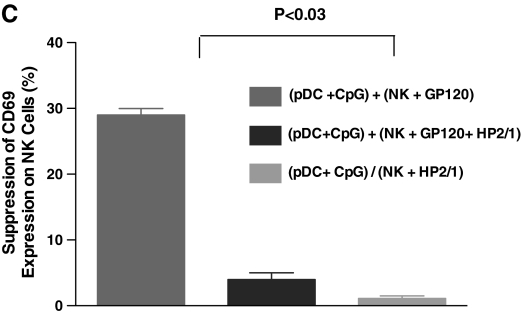
FIG. 4.
HIV gp120-mediated interference of pDC-NK cell cross-talk involves direct binding to the integrin α4β7 receptor on NK cells. We performed experiments of pDC-NK cell interactions in the presence or absence of trimeric HIV gp120 as described below. (A) PBMCs from healthy normal volunteers were treated with trimeric HIV gp120 (1 μg/ml) for 1 h at 37°C. The cells were washed and treated with CpG (1 μg/ml) overnight. CD69 expression on NK cells was determined by flow cytometry as described in Materials and Methods. Exposure to HIV gp120 markedly reduces the ability of NK cells to be activated, while gp120 by itself does not activate NK cells. (B) Purified pDCs and NK cells from healthy normal volunteers were isolated as described in Materials and Methods. Both pDCs and NK cells were exposed to trimeric HIV gp120 (1 μg/ml) for 60 min. Afterward the cells were washed and cocultured as follows. HIV untreated pDCs and NK cells with or without CpG (1 μg/ml), HIV gp120 treated or untreated pDCs with HIV gp120, and untreated or treated NK cells with or without CpG (1 μg/ml). The suppression of CD69 expression is calculated as a percentage of CD69 expression seen on NK cells with experimental conditions appropriate to that seen on NK cells cocultured with pDCs treated with CpG. Results indicate both cell types are affected by exposure to HIV gp120. (C) We performed experiments similar to those described in (B); when NK cells were pretreated with α4 integrin antibody that blocks HIV gp120 binding to NK cells, there was a complete reversal of the suppressive effects of HIV gp120.
Discussion
In this study, we report that CpG-induced activation of NK cells from HIV-infected individuals was significantly impaired when compared to that of HIV-seronegative individuals. Our results suggest that this dysfunction is due both to a nonresponsiveness of NK cells to the cytokines IFN-α and TNF and to a defect in secretion of IFN-α and TNF by pDCs from HIV-infected individuals. Both HIV viremic and aviremic individuals exhibited similar signs of dysfunction, indicating that this defect is probably a result of global activation by HIV infection and not entirely due to HIV viremia itself.
CpG binds to the intracellular TLR-9 receptor on immune-competent cells and activates them, thereby facilitating the development of a robust immune response.18 Hence, the use of CpG has been proposed as an adjuvant for vaccines.19–21 Although most immune-competent cells express TLR-9, CpG primarily exerts its effect on pDCs and B cells.22 Upon binding to TLR-9, pDCs and B cells are activated and express costimulatory molecules (CD80, CD86) and class II MHC molecules, and secrete cytokines that result in activation of other immune-competent cells.23 Although CpG has no direct effect on activation of NK cells when cultured with other pDCs, NK cells nonetheless become activated.13 CD69 expression on NK cells has been shown to correlate with NK cell cytotoxicity.24 Recently, it has been shown that CpG-adjuvant vaccination can result in prevention of herpes virus infection at the mucosal surface by recruiting NK cells in a murine model.25 Furthermore, the significance of the reciprocal interaction between pDCs and NK cells on innate and adaptive immune functions against microbes has been clearly described.26 Recent studies have shown that PBMCs27 or pDCs28 from HIV-infected individuals stimulated by TLR-9 agonists secrete less IFN-α. Moreover, some studies have also addressed the importance of IFN-α secretion for optimal function and activation of NK cells.29,30 In this study, we describe the specific defects in pDC-NK cross-talk in HIV-infected individuals. We are also able to reproduce the in vivo effects of HIV viremia to some degree utilizing direct interactions involving trimeric HIV gp120 and NK cells.
Our results clearly showed that NK cells from HIV-infected individuals had a poor response to CpG stimulation when compared to that of HIV-negative individuals. Such impairment could potentially be due to an inability of pDCs to secrete IFN-α and TNF cytokines, a lower number of pDCs secreting IFN-α and TNF, and/or a nonresponsiveness of NK cells to IFN-α and TNF stimulation. The results indicate that activation of NK cells from HIV-infected individuals was still mediated primarily by IFN-α and to a lesser degree by TNF. When we used recombinant cytokines to activate NK cells, we observed a reduced level of activation from all three cytokines tested suggesting that there is an inherent defect in NK cells from HIV-infected individuals to respond to these cytokines. Several studies have suggested that reconstitution of pDC numbers and function is not complete even after initiation of ART and complete suppression of HIV viral load in plasma.31–34 However, this does not rule out an alternate mechanism involving a reduction in the number of pDCs secreting IFN-α and TNF in HIV viremic patients. When we tested the supernatants of PBMCs activated by CpG for the levels of IFN-α and TNF from all three groups of patients, we found that HIV-infected individuals (both viremic and aviremic) had significantly lower levels of IFN-α and TNF. Because purified NK cells were unresponsive to recombinant IFN-α and TNF, the lack of secretion of IFN-α and TNF by pDCs is probably not a major mechanism in the dysfunction of NK cells activated by CpG in HIV-infected individuals. The failure of NK cells to respond to CpG-activated pDCs is due mainly in an inherent nonresponsiveness to the cytokines and not exclusively to an inability of pDCs to secrete these cytokines.
Most studies have shown that dysfunctions in immune-competent cells in HIV-infected individuals are associated with HIV viremia.9,35 Many of these defects are reversed by controlling ongoing HIV replication in vivo by ART.36,37 Our results show statistically similar levels of activation of NK cells in both viremic and aviremic individuals, however, at a much lower level when compared with normals. These findings suggest that although a variety of immune dysfunctions may be normalized by the control of HIV replication by ART, some defects are longer lasting than others. The clinical implications of these long-lasting effects are not clearly understood. It is also possible that these defects reflect a phenomenon associated with global immune activation, which can persist even after control of viremia.16 It is not yet clear why some individuals may have persistent immune defects even after control of HIV viremia and recovery of CD4+ T cells. However, our results suggest that HIV-induced immune dysfunction is much more pronounced in NK cells than in other cell types. Similar results of persistent IFN-α nonresponsive states have been previously described in vivo in HCV/HIV-coinfected individuals who fail to respond to pegylated interferon-α and ribavirin.16 The significance of this persistent immune activation and interferon nonresponsiveness is currently not understood. It is also plausible that recovery of complete immune function for pDCs and NK cells may need a period of viral control much more prolonged than the average time of viral suppression for the study subjects in this study, which was 11.3 months. Future studies will be focused on identifying the underlying mechanisms involved in the HIV-induced immune defects that render immune cells nonresponsive to cytokines.
Our study demonstrates a defective pDC-NK cell interaction in HIV-infected individuals; however, it should be noted that this does not explain all of the NK cell defects seen in HIV-infected subjects. To completely understand the mechanisms involved in HIV-mediated suppression of pDC-NK cross-talk seen in HIV-infected individuals, we attempted to reproduce ex vivo effects by treating PBMCs with HIV gp120 in vitro. When PBMCs were stimulated using CpG, in the presence of HIV gp120, we observed complete suppression of NK cell activation. It has previously been shown that HIV gp120 is capable of binding to pDCs and suppressing their ability to secrete IFN-α in response to CpG,11 which could partly explain the mechanism for this phenomenon. Moreover, HIV gp120 has been shown to directly suppress several NK cell functions in vitro.10 When we performed these experiments in which we either treated pDCs or NK cells or both with HIV gp120 in the presence of CpG, we were able to demonstrate that HIV gp120 had a significant suppressive effect on both cell types. These findings reiterate the deleterious effect of HIV on a variety of immune cell types that may result in a cumulative suppression of immune cell function. HIV gp120 can interact with pDCs by binding to and signaling through several receptors such as CD4, chemokine receptors, and/or DC-SIGN molecules.11 However, the receptor on NK cells responsible for their interactions with the HIV envelope in mediating this suppressive effect is not completely understood.
We have recently described a receptor for HIV gp120 on NK cells, CD8+ T cells, and CD4+ T cells.29 The receptor is integrin α4β7, which is the gut homing receptor on peripheral T cells.17 Our findings have suggested that this is the main receptor on NK cells that binds to HIV gp120, and that disruption of HIV gp120-α4β7 binding completely abrogated HIV gp120-mediated suppression of NK cell activation. Although there is a possibility that HP2/1 could be blocking α4β1, the only other α4 integrin expressed on NK cells, this likely represents a minor activity for two reasons. First, the interaction between HIV-1 gp120 and α4β1 is much weaker than gp120 interactions with α4β7. Second, the NK cells we employ express between 20-fold and 50-fold less β1 than β7. Therefore, the interactions with α4β1 are a minor component of the gp120 effect that is being described. These findings clearly establish that HIV gp120-mediated effects on NK cells is primarily, if not entirely, mediated by its binding to the α4β7 integrin receptor on NK cells.
Here we describe the profound effects of HIV viremia and HIV gp120 on yet another immune network involving pDCs and NK cells, thereby disrupting the ability of the host to mount effective immune responses. Furthermore, our findings indicate that the direct suppressive effects of HIV gp120 on NK cells is mediated by its binding to integrin α4β7 on NK cells. These findings could also have a significant impact on future clinical scenarios in which CpG-based vaccines utilized against various pathogens might be administered to HIV-infected individuals.
Acknowledgments
This research was supported in whole by the Intramural Research Program of the NIH [National Institute of Allergy and Infectious Diseases]. The content of this publication does not necessarily reflect the views or the policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. K.N. Reitano and S. Kottilil contributed equally to this article.
Disclosure Statement
No competing financial interests exist.
References
- 1.Trinchieri G. Biology of natural killer cells. Adv immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smyth MJ. Godfrey DI. Trapani JA. A fresh look at tumor immunosurveillance and immunotherapy. Nat immunol. 2001;2(4):293–299. doi: 10.1038/86297. [DOI] [PubMed] [Google Scholar]
- 3.Long EO. Regulation of immune responses through inhibitory receptors. Annu Rev Immunol. 1999;17:875–904. doi: 10.1146/annurev.immunol.17.1.875. [DOI] [PubMed] [Google Scholar]
- 4.Lanier LL. On guard—activating NK cell receptors. Nat Immunol. 2001;2(1):23–27. doi: 10.1038/83130. [DOI] [PubMed] [Google Scholar]
- 5.Moretta A. Bottino C. Vitale M, et al. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- 6.Mavilio D. Benjamin J. Daucher M, et al. Natural killer cells in HIV-1 infection: Dichotomous effects of viremia on inhibitory and activating receptors and their functional correlates. Proc Natl Acad Sci USA. 2003;100(25):15011–15016. doi: 10.1073/pnas.2336091100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper MA. Fehniger TA. Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22(11):633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 8.Fauci AS. Pantaleo G. Stanley S. Weissman D. Immunopathogenic mechanisms of HIV infection. Ann Intern Med. 1996;124(7):654–663. doi: 10.7326/0003-4819-124-7-199604010-00006. [DOI] [PubMed] [Google Scholar]
- 9.Fauci AS. Mavilio D. Kottilil S. NK cells in HIV infection: Paradigm for protection or targets for ambush. Nat Rev. 2005;5(11):835–843. doi: 10.1038/nri1711. [DOI] [PubMed] [Google Scholar]
- 10.Kottilil S. Shin K. Jackson JO, et al. Innate immune dysfunction in HIV infection: Effect of HIV envelope-NK cell interactions. J Immunol. 2006;176(2):1107–1114. doi: 10.4049/jimmunol.176.2.1107. [DOI] [PubMed] [Google Scholar]
- 11.Martinelli E. Cicala C. Van Ryk D, et al. HIV-1 gp120 inhibits TLR9-mediated activation and IFN-{alpha} secretion in plasmacytoid dendritic cells. Proc Natl Acad Sci USA. 2007;104(9):3396–3401. doi: 10.1073/pnas.0611353104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashkar AA. Rosenthal KL. Toll-like receptor 9, CpG DNA and innate immunity. Curr Mol Med. 2002;2(6):545–556. doi: 10.2174/1566524023362159. [DOI] [PubMed] [Google Scholar]
- 13.Marshall JD. Heeke DS. Abbate C. Yee P. Van Nest G. Induction of interferon-gamma from natural killer cells by immunostimulatory CpG DNA is mediated through plasmacytoid-dendritic-cell-produced interferon-alpha and tumour necrosis factor-alpha. Immunology. 2006;117(1):38–46. doi: 10.1111/j.1365-2567.2005.02261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kottilil S. Chun TW. Moir S, et al. Innate immunity in human immunodeficiency virus infection: Effect of viremia on natural killer cell function. J Infect Dis. 2003;187(7):1038–1045. doi: 10.1086/368222. [DOI] [PubMed] [Google Scholar]
- 15.Meyers JH. Justement JS. Hallahan CW, et al. Impact of HIV on cell survival and antiviral activity of plasmacytoid dendritic cells. PLoS ONE. 2007;2(5):e458. doi: 10.1371/journal.pone.0000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lempicki RA. Polis MA. Yang J, et al. Gene expression profiles in hepatitis C virus (HCV) and HIV coinfection: Class prediction analyses before treatment predict the outcome of anti-HCV therapy among HIV-coinfected persons. J Infect Dis. 2006;193(8):1172–1177. doi: 10.1086/501365. [DOI] [PubMed] [Google Scholar]
- 17.Arthos J. Cicala C. Martinelli E, et al. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat Immunol. 2008;9(3):301–309. doi: 10.1038/ni1566. [DOI] [PubMed] [Google Scholar]
- 18.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev. 2006;5(6):471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 19.Lipford GB. Bauer M. Blank C. Reiter R. Wagner H. Heeg K. CpG-containing synthetic oligonucleotides promote B and cytotoxic T cell responses to protein antigen: A new class of vaccine adjuvants. Eur J Immunol. 1997;27(9):2340–2344. doi: 10.1002/eji.1830270931. [DOI] [PubMed] [Google Scholar]
- 20.Weiner GJ. Liu HM. Wooldridge JE. Dahle CE. Krieg AM. Immunostimulatory oligodeoxynucleotides containing the CpG motif are effective as immune adjuvants in tumor antigen immunization. Proc Natl Acad Sci USA. 1997;94(20):10833–10837. doi: 10.1073/pnas.94.20.10833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aderem A. Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406(6797):82–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 22.Bauer M. Heeg K. Wagner H. Lipford GB. DNA activates human immune cells through a CpG sequence-dependent manner. Immunology. 1999;97(4):699–705. doi: 10.1046/j.1365-2567.1999.00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartmann G. Weiner GJ. Krieg AM. CpG DNA: A potent signal for growth, activation, and maturation of human dendritic cells. Proc Natl Acad Sci USA. 1999;96(16):9305–9310. doi: 10.1073/pnas.96.16.9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sivori S. Carlomagno S. Moretta L. Moretta A. Comparison of different CpG oligodeoxynucleotide classes for their capability to stimulate human NK cells. Eur J Immunol. 2006;36(4):961–967. doi: 10.1002/eji.200535781. [DOI] [PubMed] [Google Scholar]
- 25.Harandi AM. Eriksson K. Holmgren J. A protective role of locally administered immunostimulatory CpG oligodeoxynucleotide in a mouse model of genital herpes infection. J Virol. 2003;77(2):953–962. doi: 10.1128/JVI.77.2.953-962.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerosa F. Gobbi A. Zorzi P, et al. The reciprocal interaction of NK cells with plasmacytoid or myeloid dendritic cells profoundly affects innate resistance functions. J Immunol. 2005;174(2):727–734. doi: 10.4049/jimmunol.174.2.727. [DOI] [PubMed] [Google Scholar]
- 27.Martinson JA. Roman-Gonzalez A. Tenorio AR, et al. Dendritic cells from HIV-1 infected individuals are less responsive to toll-like receptor (TLR) ligands. Cell Immunol. 2007;250(1–2):75–84. doi: 10.1016/j.cellimm.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tilton JC. Manion MM. Luskin MR, et al. Human immunodeficiency virus viremia induces plasmacytoid dendritic cell activation in vivo and diminished alpha interferon production in vitro. J Virol. 2008;82(8):3997–4006. doi: 10.1128/JVI.01545-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alter G. Suscovich TJ. Teigen N, et al. Single-stranded RNA derived from HIV-1 serves as a potent activator of NK cells. J Immunol. 2007;178(12):7658–7666. doi: 10.4049/jimmunol.178.12.7658. [DOI] [PubMed] [Google Scholar]
- 30.Tomescu C. Chehimi J. Maino VC. Montaner LJ. NK cell lysis of HIV-1-infected autologous CD4 primary T cells: Requirement for IFN-mediated NK activation by plasmacytoid dendritic cells. J Immunol. 2007;179(4):2097–2104. doi: 10.4049/jimmunol.179.4.2097. [DOI] [PubMed] [Google Scholar]
- 31.Azzoni L. Rutstein RM. Chehimi J. Farabaugh MA. Nowmos A. Montaner LJ. Dendritic and natural killer cell subsets associated with stable or declining CD4+ cell counts in treated HIV-1-infected children. J Infect Dis. 2005;191(9):1451–1459. doi: 10.1086/429300. [DOI] [PubMed] [Google Scholar]
- 32.Finke JS. Shodell M. Shah K. Siegal FP. Steinman RM. Dendritic cell numbers in the blood of HIV-1 infected patients before and after changes in antiretroviral therapy. J Clin Immunol. 2004;24(6):647–652. doi: 10.1007/s10875-004-6250-5. [DOI] [PubMed] [Google Scholar]
- 33.Killian MS. Fujimura SH. Hecht FM. Levy JA. Similar changes in plasmacytoid dendritic cell and CD4 T-cell counts during primary HIV-1 infection and treatment. AIDS. 2006;20(9):1247–1252. doi: 10.1097/01.aids.0000232231.34253.bd. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt B. Fujimura SH. Martin JN. Levy JA. Variations in plasmacytoid dendritic cell (PDC) and myeloid dendritic cell (MDC) levels in HIV-infected subjects on and off antiretroviral therapy. J Clin Immunol. 2006;26(1):55–64. doi: 10.1007/s10875-006-8401-3. [DOI] [PubMed] [Google Scholar]
- 35.Cohen OJ. Pantaleo G. Schwartzentruber DJ. Graziosi C. Vaccarezza M. Fauci AS. Pathogenic insights from studies of lymphoid tissue from HIV-infected individuals. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10(Suppl. 1):S6–14. [PubMed] [Google Scholar]
- 36.Simon V. Ho DD. HIV-1 dynamics in vivo: Implications for therapy. Nat Rev. 2003;1(3):181–190. doi: 10.1038/nrmicro772. [DOI] [PubMed] [Google Scholar]
- 37.Letvin NL. Walker BD. Immunopathogenesis and immunotherapy in AIDS virus infections. Nat Med. 2003;9(7):861–866. doi: 10.1038/nm0703-861. [DOI] [PubMed] [Google Scholar]