Abstract
The maternal physiological changes that occur in normal pregnancy induce complex endocrine and immune responses. During a normal pregnancy, thyroid gland volume may enlarge, and thyroid hormone production increases. Hence, the interpretation of thyroid function during gestation needs to be adjusted according to pregnancy-specific ranges. The elevated prevalence of gestation-related thyroid disorders (10%–15%) and the important repercussions for both mother and fetus reported in multiple studies throughout the world denote, in our opinion, the necessity for routine thyroid function screening both before and during pregnancy. Once thyroid dysfunction is suspected or confirmed, management of the thyroid disorder necessitates regular monitoring in order to ensure a successful outcome. The aim of treating hyperthyroidism in pregnancy with antithyroid drugs is to maintain serum thyroxine (T4) in the upper normal range of the assay used with the lowest possible dose of drug, whereas in hypothyroidism, the goal is to return serum thyroid-stimulating hormone (TSH) to the range between 0.5 and 2.5 mU/L.
Introduction
In recent years, a number of important studies have been published addressing the special circumstances of thyroid physiology and pathophysiology in the gravid woman.1,2 Our increased knowledge has improved the management of pregnant women with a variety of thyroid disorders. This has also resulted in important expanded guidelines for the management of thyroid disease in pregnancy by international thyroid associations.3
Maternal thyroid during pregnancy
During pregnancy, several important physiological changes occur, with substantial repercussions for women's thyroid gland. At the same time, maternal thyroid hormones (TH) play a vital role in the development and function of both the fetus and the placenta. Thyroid gland volume usually enlarges during pregnancy, and TH synthesis increases about 50% above the preconception level. These changes are in response to several factors.
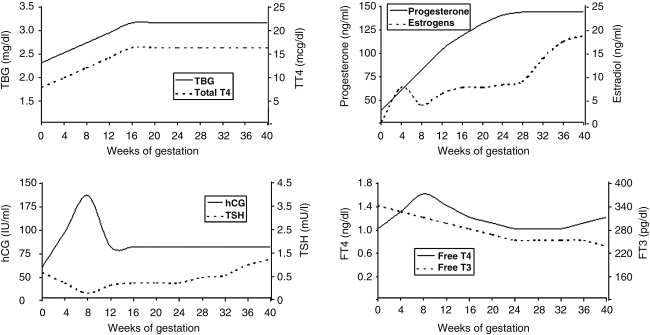
The normal pattern of human chorionic gonadotropin (hCG) secretion during pregnancy demonstrates a major increase during the first trimester and a plateau during midgestation, where it persists until shortly after delivery.4 hCG has a much researched thyroid-stimulating hormone (TSH)-like activity secondary to specificity crossover at the TSH receptor (TSHR). As a result, serum thyroxine (T4) and triiodothyronine (T3) levels are elevated, whereas serum TSH levels are reduced. Pregnancy-related hyperestrogenism induces a 100% rise in serum thyroxine-binding globulin (TBG) as a result of changes in TBG half-life secondary to altered glycosylation. As a consequence, by week 10 of gestation, total T4 and T3 serum concentrations are increased and plateau at this level until delivery.4,5 Other physiological adjustments also increase TH synthesis, such as elevation in the maternal glomerular filtration rate (GFR) and transplacental passage of T4.6 These changes mean that adjusted normal reference ranges for thyroid function tests, unique to pregnancy, must be consulted (Figs. 1 and 2).
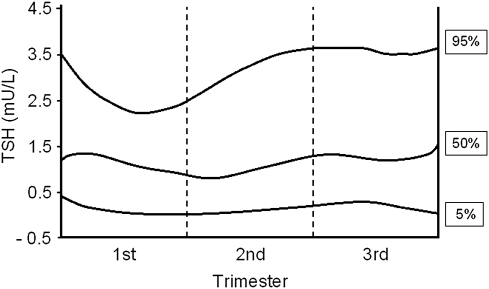
FIG. 1.
Thyroid-stimulating hormone (TSH) serum levels expressed in percentiles according to gestational age. (Adapted from Panesar et al.,7 with permission.)
FIG. 2.
Variation in serum levels of thyroid function test and pregnancy-related hormones according to course of gestation. TBG, thyroxine-binding globulin; T4, thyroxine; T3, triiodothyronine; hCG, human chorionic gonadotropin; TSH, thyroid-stimulating hormone. (Adapted from Glinoer8 and Brent,9 with permission.)
Immune modifications
For a successful pregnancy outcome, the maternal immune system must tolerate the fetus. Placental trophoblast cells, therefore, not only provide a physical barrier but also secrete a variety of cytokines and hormones, including several immunomodulatory molecules. These secretions induce detectable immune changes, including enhanced regulatory T cell function10,11 (Table 1), which have great importance for many autoimmune diseases. The result is a general improvement in autoimmune intolerance during gestation.12 In addition, taking advantage of this immunosuppressive condition, fetal cells cross the placental barrier and survive maternal intolerance, allowing them to take up residence in maternal tissues while maternal cells settle in the fetus. There is abundant evidence indicating that the thyroid gland is one of the important organs where fetal microchimeric cells may become established.13 The consequences of fetal immune cells within the maternal thyroid gland remain an attractive explanation for the postpartum exacerbation of autoimmune thyroid diseases (AITD), but this influence is far from confirmed.
Table 1.
Immune Adaptation to Pregnancya
| Place event | Adaptation | Result |
|---|---|---|
| Local | Trophoblast cells express several immune-modulating molecules (Fas-L,b HLA-G and indoleamine 2,3-dioxigenase) as well as secreting a variety of cytokines | Fas-L induces apoptosis on fetal antigen-reactive maternal lymphocytes |
| HLA-G inhibits both NK cell function and maturation of dendritic cells | ||
| Indoleamine 2,3-dioxigenase catalyzes triptophane in lymphocytes, which is critical in the maintenance of allogenic pregnancy | ||
| Systemic | T cell | Decreased CD4+, increased CD8+ T cells and increasing activity of T regulatory cells |
| The immune response is turned from Th1 (cellular) to Th2 (humoral), with an increase in Th2 cytokine production | ||
| B cell | Despite the shift to Th2, the relative B cell production and activity are downregulated, leading to a reduction in antibody production | |
| Hormonal changes | Increase in plasma levels of estrogen, progesterone, and corticosteroids | |
| Estrogen produces negative regulation of B cell activity | ||
| Progesterone generates variation in cytokine profiles | ||
| Corticosteroids induce immune cell apoptosis and immunosuppression | ||
| Postpartum | Recovery of prepregnancy immune function | Increased titers of serum antibodies, reversed ratio CD4+/CD8+ T cells, and change in cytokine profiles favor Th1 responses |
During pregnancy there are important adaptations of the maternal immune system. A physiological immunosuppression response creates an immune-privileged state protecting the fetus from rejection. Natural immunity and both arms of the adaptative immune responses (cell-mediated and humoral) are attenuated. This placental immune suppression may contribute to establish fetal microchimerism.
Fas-L, Fas-Ligand; HLA-G, Human leukocyte antigen-G; NK, natural killer; Th1, T helper cell 1; Th2, T helper cell 2.
Consequences of thyroid autoantibodies
The first reports of an association between an increase in early pregnancy loss and the presence of thyroid autoimmunity came from our studies more than 15 years ago.14 Since then, the observation has been confirmed in multiple independent studies. This association, however, exists in the absence of a significant relationship with TSH,15 although women with thyroid autoantibodies (TAbs) to thyroid peroxidase (TPO) and thyoglobulin may have higher plasma TSH levels, within the normal range, reflective of subtle thyroid dysfunction. In contrast, TAbs may be merely reflective of a more general immune diathesis in the women. Although early studies found that levothyroxine (LT4) treatment did not prevent miscarriage in women with TAbs after in vitro fertilization,16 the same investigators recently reported successful prevention of pregnancy loss in euthyroid women using this approach.17 If confirmed, these data suggest that it may be appropriate to start treatment with LT4 at the time of pregnancy in order to reduce the risk of miscarriage and prematurity in euthyroid women with TAbs and suggest that a thyroid diathesis is responsible.16
Fetal thyroid development
The fetus starts to produce THs by the end of the first trimester. Before this time, the fetus is dependent on a supply of maternal THs which are metabolically controlled by the placental deiodinase enzymes and the TH cell transporters.18–23 Normal maternal thyroid function is, therefore, critical in order to provide adequate THs for early fetal development and, in particular, early central nervous system (CNS) development.
Assessment of thyroid function in pregnancy
Gestation is a prime time for diagnosis of thyroid diseases, inasmuch as women wishing to become pregnant and already pregnant woman usually seek regular medical care.24 Although thyroid disorders are an important barrier to pregnancy, significant thyroid dysfunction is still found in 1%–2% of gravid women, and subclinical forms of thyroid disease are even more prevalent.25,26
To screen or not to screen
The need for systematic thyroid function and TAb evaluation in early pregnancy may seem fairly obvious, but this is far from being a unanimous opinion.3,27–31 It has been 8 years since we called for thyroid function screening in pregnancy32 and 5 years since the American Association of Clinical Endocrinologists (AACE) recommended thyroid function screening in all women during the first trimester of pregnancy.33 The most recent joint guideline published by the Endocrine Society recommends an aggressive case-finding approach during early pregnancy in high-risk populations rather than routine screening.3 According to these most recent guidelines,3 additional evidence is required prior to the recommendation of routine thyroid function screening before and during pregnancy. The issues to be answered include the thyroid test(s) to be used, timing of the determination, thresholds to characterize as an abnormality, appropriate intervention mechanisms, and methods of monitoring.
Although these issues appear to have clear answers and because the association of thyroid abnormalities and adverse outcomes during pregnancy and the postpartum is impossible to ignore, we continue to advocate for routine screening in all women in early, and preferably before, pregnancy.30 Nevertheless, the lack of critical outcome trials of T4 replacement in mild hypothyroid pregnant women remains a major deficiency. Trials in progress should resolve this question in the next few years. It is also important to note that the current Endocrine Society guidelines enumerate many situations that, according to their criteria, determine enough risk to justify case finding for thyroid function testing.3 This list includes women with (1) a past history of thyroid disease or thyroid lobectomy, (2) a family history of thyroid disease, (3) goiter, (4) TAbs (when known), (5) symptoms or clinical signs suggestive of thyroid underfunction or overfunction, including anemia, elevated cholesterol, and hyponatremia, (6) type 1 diabetes, (7) other autoimmune disorders, (8) infertility, (9) previous therapeutic head and neck irradiation, and (10) history of miscarriage or preterm delivery.
This long list of situations where case finding is recommended includes many women because the symptoms of thyroid deficiency and excess are so nonspecific. The advice against a universal screening program to detect thyroid dysfunction in all fertile women is, therefore, superseded by the clinical situation. It is also difficult to reconcile the advice to treat pregnant women with increased TSH levels with the advice not to routinely measure their TSH.30 It should also be noted that very often blood parameters, such as renal or hepatic function, the practical utility of which may be less than that of thyroid function testing, are determined without hesitation. A recently published study of over 1560 consecutive pregnant women demonstrated that when TSH, free T4 (FT4), free T3 (FT3), and TPO-Ab were tested at the first antenatal visit, the prevalence of raised serum TSH (>4.2 mU/L) was 2.6%. The TSH was more often abnormal (6.8%) in high-risk patients (those with a personal history of thyroid dysfunction or other autoimmune disorder or family history of thyroid disease) vs. those women with low-risk conditions (1%). However, 30% of patients with high serum TSH were in the low-risk group. The authors concluded that targeting thyroid function testing of only high-risk patients would miss about one third of pregnant women with overt or subclinical hypothyroidism.34 These authors used an insensitive TAb assay, and their data may have been even more striking using more sensitive techniques. Such information and the fact that normal maternal thyroid function is most likely essential for normal intellectual development of the child make screening for thyroid dysfunction, in our opinion, an important part of a careful medical assessment.25,35–39 Hence, the recommendation for aggressive case finding in women with an increased risk for thyroid disease has been shown to have limited meaning in light of the high frequency of these disorders.27,40
Selecting screening tests
Selecting only one unique test for thyroid dysfunction screening in pregnancy has been claimed to be inadequate because serum TSH and T4 levels may not always be coincident. Preliminary data of The Controlled Antenatal Thyroid Screening (CATS) study showed that half of so-called hypothyroid pregnant women displayed low T4 serum levels without a high TSH, and this may or may not be a pathological state.41 An equal number of women have high TSH serum concentrations, with little change in serum T4 (so-called mild thyroid failure). In fact, very few women have both a low T4 and a high TSH.41 To avoid misinterpretation, we should bear in mind that low TSH serum levels can also be a physiological response during the first trimester of pregnancy. Whereas agreement exists on the usefulness of TSH measurements, different opinions persist on whether serum total T4 (TT4) or FT4 determination is the complementary test of choice (Table 2). We normally use a T4 index with an appropriate binding assessment, such as TBG or a T3 resin-binding assay, as such indices give results in pregnancy similar to those in normal women. In view of the association between anti-TPO and pregnancy loss and the usefulness of anti-TPO in predicting postpartum thyroiditis, it would also be appropriate to consider anti-TPO in any thyroid screening.42,43
Table 2.
Usual Recommendations for Thyroid Evaluation in Normal Pregnancya
| Recommendation | Study | |
|---|---|---|
| Screening | Thyroid function | TSH and T4, interpretation should be trimester specific |
| Presence of AITD | TPO-Ab and Tg-Ab | |
| Ultrasound | Advisable when nodular disease is suggested by clinical examination | |
| Iodine supplement | 250 μg/day (range 200–300 μg/day) |
Once the diagnosis of pregnancy in confirmed, or even better, before pregnancy occurs, screening for thyroid disease and starting with iodide supplements are recommended.
Neck ultrasound
Although scintigraphy scans are contraindicated during pregnancy and rarely needed these days anyway, routine ultrasound may be considered when a goiter or nodular disease is suggested by clinical history and examination. This procedure is useful both to characterize thyroid size and degree of thyroiditis present and to delineate all nodules, allowing an evaluation of their growth characteristics on repeated measurements. In addition, sonography can help make the clinical diagnosis of Graves' disease (GD) (by excluding nodules) or Hashimoto's thyroiditis (by the typical heterogeneous patterning). Of course, the presence of a thyroid nodule does not exclude the diagnosis of GD and would normally initiate radioiodine uptake studies in a nonpregnant patient.
Diagnosis of suspected disease
Selecting diagnostic tests
There is a lack of consensus in choosing tests for the established pregnant patient under study. The most useful tests are determination of serum TSH, albeit normal values are pregnancy specific with a superior limit of <2.5 mU/L, and concurrent TT4 (which is expected to be 3–5 μg/dL [50–60 nmol/L]) above the normal range of nonpregnant women because of the increased TBG levels.44,45 A common practice in pregnancy has been determination of serum FT4 in order to bypass the changes in TBG serum levels.25,41,46 However, FT4 determined by commercial assays may be insensitive to the augmentation of serum transport proteins, as happens during pregnancy, leading to false readings in the presence of high TBG.44 Furthermore, there is no absolute value of FT4 that defines hypothyroxinemia. On the contrary, changes in TT4 during pregnancy are predictable, and the assays do not depend on the problem of high TBG concentrations. A rough superior reference range for TT4 during pregnancy can be estimated by multiplying the reference value of nonpregnant woman by 1.5.6 Again, as mentioned, the common practice in some centers is to use the relationship between TBG and TT4 to calculate the FT4 fraction or T4 index.
Reference ranges
Interpretation of thyroid function tests should be trimester-specific,28,47,48 and locally generated trimester-specific reference levels should be applied when available.47,49 Of note, it has been reported that the upper 95% Cl for plasma TSH in the first trimester is 2.5 mU/L.47 It is known that the TSH level descends 60%–80% by week 10 and recovers slowly thereafter, but it may not reach the preconception normal range until gestation ends.45
Hypothyroidism and pregnancy
Epidemiology and etiology
Ovulatory dysfunction is common in hypothyroidism; consequently, hypothyroid women have difficulty conceiving.16 Overall, the prevalence of hypothyroidism during pregnancy is ∼2.5%, the majority being subclinical cases.50 Transiently high serum TSH levels in the first trimester can also occur, however, probably related to iodine deficiency.50 The most common cause of hypothyroidism in women of reproductive age, in the absence of iodine deficiency, is AITD. A history of past total or subtotal thyroidectomy, radioiodine ablation, or transient thyroiditis accounts for most of the remaining cases of hypothyroidism in the pregnant population.51
Clinical features
The hypothyroid clinical features in pregnancy are similar to those in nonpregnant women, although only 20%–30% of patients with overt hypothyroidism develop clear clinical disease.25
Risks
In some studies, preterm delivery has been found to be 3-fold more common in hypothyroid pregnant women and has also been associated with an increase in spontaneous abortions,52–54 although no adverse outcomes were found in a very large study of mild (subclinical) disease.55 Another worrying danger associated with maternal hypothyroxinemia (especially when present during the first trimester) is the adverse consequences to child neuropsychointellectual development, as has been demonstrated by several studies6,16,25,56,57 (Table 3). Whether very mild hypothyroidism has the same associations remains uncertain.58
Table 3.
Hypothyroidism-Related Adverse Effects during Pregnancy
| Maternal | Fetus | |
|---|---|---|
| General health | Anemia, congestive heart failure, antepartum depression | |
| Course of gestation | Eclampsia, preeclamspia, gestational hypertension, placental abruption | Growth restriction, increased perinatal mortality |
| Delivery | Increased use of cesarean section, preterm delivery | Miscarriage |
| Long term | Postpartum depression, postpartum hypertension, lactation problems | Impaired neuropsychointellectual development |
Diagnosis
Documenting an elevated serum TSH level confirms the diagnosis of primary hypothyroidism.49 The presence of TAbs is a useful confirmatory finding. Serum TSH >2.5 mU/L in pregnant women should be used as a guide for thyroid dysfunction. It is clear that TSH >2.5 mU/L is too high for the first trimester,45 and when serum TSH is >4 mU/L irrespective of the presence (or absence) of TAbs, there is no doubt about the existence of thyroid underfunction.25 To help assess the severity, it is best to also measure TT445 or FT4.6,46,52 Because of the pregnancy-related changes in T4 in early gestation, any T4 level below the trimester-specific reference range suggests overt hypothyroidism, whereas in late gestation, an FT4 value decrease of around 20%–30% from prepregnancy levels may be physiological.25
Management
Prevention of hypothyroidism
Adequate iodine supplementation is crucial to prevent maternal hypothyroxinemia during pregnancy.56 Fertile women with normally functioning thyroid glands should have an average iodine intake of 150 μg/day. During pregnancy and breastfeeding, women should increase their daily iodine intake to 250 μg daily.46,49,59 LT4 therapy is needed if, despite the iodine supplementation, abnormal serum TSH levels are detected.
Levothyroxine supplements
Treatment of hypothyroidism depends on LT4 supplementation60 (Table 4). Hypothyroid pregnant women already on LT4 replacement therapy will require a dose increase from 25% to 50% on average to maintain desirable TSH concentrations because they have inadequate thyroid reserve.61 Most hypothyroid pregnant women need a dose increase during the first trimester. In the second trimester, there is generally a plateau in LT4 requirements, but 25%–40% of women may need a further dose increase during the third trimester.25,62 The increase is at least partly dependent on the thyroid reserve of the patient but also the size of the distribution space and the number of babies. Hence, those women with a history of a total thyroidectomy will be most dependent. Generally, patients with ongoing Hashimoto's thyroiditis require a 25% increase in LT4 dose, whereas the increase is more likely to be 50% in women on full replacement therapy.
Table 4.
Management of Thyroid Dysfunction in Pregnancya
| Condition | Treatment | Indication | Recommendations | Comments |
|---|---|---|---|---|
| Hypothyroidism | LT4 | High TSHb | Dose | Maternal hormone target levels |
| Presence of TAbsc | New diagnosis: start with 1.8–2 μg/kg (overt disease) or 100 μg/day (mild cases, i.e., TSH <10 mU/L) | TT4: 12–18 μg/dL; FT4: 2–2.5 ng/dL TSH: 0.5–2.0 mU/L |
||
| Patients on LT4: increase dosage from 25% to 50% | ||||
| Monthly monitoring | Bear in mind possible drug interactions | |||
| Clinical: mother/Biochemical (TSH and T4): mother | Postpartum period require adjustments | |||
| Hyperthyroidism | Antithyroid drug | Drug of choice: PTU (50–300 mg/day) | Monthly monitoring | Maternal hormone target levels |
| Clinical: mother and fetus | TT4: 12–18 μg/dL; FT4: 2–2.5 ng/dL | |||
| Ultrasound: fetus | TSH: 0.1–0.4 mU/L (in late gestation) | |||
| Biochemical (TSH and T4d): mother | ||||
| Surgery | Uncontrolled maternal hyperthyroidism with high doses of ATD (>300 mg of PTU or 40 mg/day MMI) | Recommended during second trimester | ||
| Beta-adrenergic blockers | Preparation for surgery Severe hyperthyroidism | Should be used temporarily (no more than 4 weeks) until ATD becomes effective | Side effects: increased risk of miscarriage or small for dates | |
| Radioactive iodine therapy | Contraindicated | Possible teratogenic effects |
Management of any thyroid dysfunction during gestation demands frequent monitoring. Hypothyroidism treatment is with levothyroxine (LT4). It is important to avoid the adverse consequences of hypothyroidism in the fetus. Hyperthyroidism treatment is generally started with antithyroid drugs.
TSH, thyrotropin; ATD, antithyroid drugs; PTU, propylthiouracil; MMI, methimazole; TT4, total thyroxine; FT4, free thyroxine.
Some authors recommend starting LT4 in euthyroid pregnant women if elevated TAbs are found. However, this suggestion is not generally accepted and needs further study.
See section on Levothyroxine supplements on previous page.
When a high serum TSH is found for the first time during pregnancy in a previously euthyroid woman, the test should be repeated. Unless the TSH result will be available within 24 hours, treatment must be started before confirmation to avoid loss of time.6 Three factors have to be examined in selecting the appropriate dose at the moment the diagnosis is made: the trimester of gestation, the etiology, and the severity of the disease.25,63 From a practical point of view, the recommended starting LT4 daily dose should be 1.8–2 μg/kg for overt disease and 75–100 μg/day for mild cases (i.e., TSH <10 mU/L). The treatment end point is a maternal serum TSH concentration between 0.5 and 2.5 mU/L. As there is a large individual variability in dose requirements, dose adjustment requires thyroid function testing at regular intervals, such as every 4 weeks.61 Adjustments are made by increasing LT4 by 25–50 μg/day. Serum TSH and TT4 (FT4 according to some authors) should always be evaluated 3–4 weeks after every change of dosage.64 Although it is clear that excess thyroid hormone can lead to fetal loss,65 the fetal risk from maternal overtreatment in such patients is generally low.44
Possible LT4 interactions with coexisting diseases, such as gastritis,66 or medications, such as iron supplements, calcium, vitamins, or omeprazole, may reduce LT4 absorption.67 In those cases, it is best to advise a 4-hour delay between the medications and LT4.
Postpartum
After delivery, LT4 dosing generally can be returned to prepregnancy requirements. However, some patients may show a discordance in LT4 necessity before and after gestation, with a greater need developing in the postpartum period. This LT4 change happens mostly in women with AITD (70%) rather than athyreiotic women (20%) and may be secondary to long-term weight gain or ongoing thyroid destruction exacerbated in the postpartum.68
Hyperthyroidism and pregnancy
Epidemiology and etiology
Hyperthyroidism during pregnancy is relatively uncommon because of the low fertility state and increased pregnancy loss as well as gestation-related immunological changes suppressing autoimmune disease. The overall prevalence rate is around 0.1%–0.4% of pregnant women, with GD accounting for 85%–90% of all cases.69 Gestational transient thyrotoxicosis (GTT) occurs much more often (2%–3% of women), but it cannot be regarded as a disease.25
Clinical features
The features of thyroid overactivity during pregnancy may be masked by normal gestational manifestations, such as palpitations, excessive perspiration, dyspnea, and nervousness. Some signs or symptoms, such as deficient weight gain for gestational age, onycholysis, eye signs including lid lag, muscle weakness, and heart rate 100 beats/minute (which does not decrease with the Valsalva's maneuver) may help differentiate between thyrotoxicosis and the characteristic hypermetabolic state of pregnancy.70
Risks
Severe hyperthyroidism in pregnancy may be associated with serious complications. Maternal risks include congestive heart failure, thyroid storm, preterm labor, preeclampsia, and increased mortality,52 whereas the fetus may be stillborn or small for gestational age or may develop congenital malformations.44
Gestational transient thyrotoxicosis
GTT is defined as biochemical thyrotoxicosis in women with an otherwise normal pregnancy and is secondary to the thyrotrophic effects of hCG. This condition occurs in about 2%–3% of all pregnancies, especially where hCG levels are highest, such as in multiple pregnancies. In the more severe form, high serum concentrations of hCG occur along with hyperemesis gravidarum, defined by severe nausea and vomiting and leading to a 5% loss of body weight, dehydration, and ketosis. Typically, whether symptomatic or not, GTT usually resolves spontaneously by 20 weeks' gestation and does not require treatment.36 GTT must be distinguished from GD because the course, fetal risk, management, and follow-up are different.
Diagnosis
The diagnosis of hyperthyroidism is supported biochemically when concurrent low TSH plasma levels are found (<0.1 mU/L) in the presence of elevated T4 concentrations. Nevertheless, 10%–20% of pregnant women may have serum TSH levels that are low compared to the range of TSH found in nonpregnant women (either subnormal or fully suppressed) without coexisting thyrotoxic symptoms.25,71 When TSH is very low, a trend in the serum T4 and T3 serum levels may help in differentiating transient GTT from a truly thyrotoxic state. Therefore, more than just biochemistry is necessary for full confidence in the diagnosis. TSHR antibodies, eye disease, family history, goiter, weight loss, arrhythmias, and other factors contribute to the diagnosis.
Management
Significant hyperthyroidism during gestation, whatever it is caused by (GD or nodules), should be treated, but it is essential to consider that 2 patients are always involved.44 The management of GD with persisting TSHR antibodies able to cross the placenta requires special considerations, which are summarized in Table 5.
Table 5.
Specific Recommendations for Graves' Diseasea
| Situation | Management of mother | Management of fetus | Comments |
|---|---|---|---|
| Pregnancy occurs in active GD | Monitor TFTb monthly, and adjust ATD accordingly | Monitor fetal pulse, that should not be tachycardic (>160 bpm) | Mother should continue with ATD |
| Assessment of TSHR-Ab titers (3rd trimester) to evaluate if fetus is at risk | If maternal TSHR-Ab titers are elevated, it is recommended to investigate neonatal thyroid function after partum | Fetal tachycardia should respond to ATD | |
| Reassessment after partum | |||
| New diagnosis of GD during gestation | ATD therapy should be started as soon as diagnosis is made | ||
| GD relapse during 1st trimester | ATD therapy should be restarted | ||
| Gestation after previous ablative therapy (surgery or radioiodine) for GD | Maternal hyperthyroidism is not possible | If fetal tachycardia is noticed in fetus with maternal positive TSHR-Ab, it is advisable to initiate treatment with PTU 100–200 mg/8 h and also continue LT4 supplementation to mother to maintain maternal euthyroidism | |
| Reassess TSHR-Ab levels at beginning of pregnancy in order to evaluate possibility of fetal or postnatal hyperthyroidism |
Management of Graves' disease (GD) in pregnancy requires particular consideration, keeping in mind the consequences of treatment on both mother and fetus. Stimulatory TSHR-Abs may stimulate both thyroids.
TFT, thyroid function tests; ATD, antithyroid drugs; PTU, propylthiouracil.
Antithyroid drugs
Generally, the treatment of choice in pregnancy is an antithyroid drug (ATD). Many endocrinologists recommend the use of propylthiouracil (PTU) rather than methimazole (MMI) or carbimazole because of the widespread belief that PTU crosses the placenta to a lesser degree and causes fewer side effects in the fetus. This is doubtful, and if allergy or intolerance appears, it is still advisable that MMI be substituted.3 The starting dose depends on the severity of the disease. PTU is usually initiated at 100 mg/8 h, guided by maternal T4 levels.44 The therapeutic aim is to maintain the 2 patients in a euthyroid state, but fetal hypothyroidism must be avoided at all costs and is difficult to monitor. ATDs cross the placental barrier, and in order to avoid fetal hypothyroidism, it is important to use the lowest dose possible and to keep maternal T4 in the high normal range. With this double objective in mind, once ATDs are started, the 2 patients should be monitored every 4 weeks and the dose adjusted accordingly.25 Monitoring includes evaluation of maternal pulse, weight, thyroid volume, and determination of TT4 (or FT4) and TSH. The recommended therapeutic target is a TT4 of 12–18 μg/dL (or FT4 2–2.5 ng/dL).72 It is not desirable to normalize TSH in early pregnancy to the nonpregnant range, as the level in normal pregnancy often is low. Furthermore, if TSH is suppressed significantly by excess TH, it may continue to be suppressed for many weeks after regularization of peripheral THs. The recommended range for TSH in patients on ATD is 0.1–0.4 mU/L.25 The median time to normalization of maternal T4 is around 7–8 weeks. Monitoring by TSH becomes more useful in late gestation when the disease is controlled. The block and replacement method with ATDs (LT4 plus ATD) should be avoided in pregnancy because of the difficulty in monitoring fetal thyroid function and the increased risk of producing goiter and hypothyroidism.72
When hyperthyroidism is not well controlled by the middle trimester with reasonable doses of ATD (>300 mg/day of PTU or 40 mg/day of MMI), surgery may be recommended because of the difficulty in knowing the thyroid status of the fetus. However, this is an infrequent event, as the hyperthyroidism tends to ameliorate as pregnancy advances.
Managing the fetus
Surveillance of the fetus requires serial ultrasound evaluation for heart rate, thyroid size, growth, and hydropic variations. Thyroid echo-Doppler is also useful to evaluate gland function by the degree of vascularization. Goiters can be detected (when present) after week 32 but should be completely avoidable with appropriate treatment.73
Beta-adrenergic blocking drugs
Beta-blockers should be avoided in pregnancy or used temporarily for no more than 4 weeks in serious hyperthyroidism or as preparation for surgery.72
Surgery
Subtotal or total thyroidectomy is recommended only when ATDs fail to control the hyperthyroid disease.25 The appropriate time is the second trimester,72 and beta-blockade may be necessary through the surgical procedure.
Radioactive iodine therapy
During gestation, 131I is contraindicated44 because of the possible teratogenic effects of radiation. Nevertheless, the results of inadvertent administration of 5–10 mCi of 131I to pregnant women show that hypothyroidism occurred in only 3% of the fetuses.74
Discussion
Thyroid diseases, especially those of autoimmune origin, are common in women of childbearing age. These disorders are significantly influenced not only by a variety of changes in thyroid function that take place during normal gestation but also by the privileged immune state that occurs in pregnancy. Therefore, interpretation of thyroid function tests during gestation requires specific pregnancy ranges that are not widely available. In our opinion, adequate screening programs should be established in order to prevent the consequences of delay in or misdiagnosis of thyroid dysfunction during pregnancy, which can pose potentially significant adverse effects to both mother and child. The correct approach to the diagnosis and therapy of thyroid dysfunction in pregnancy requires monthly fetal and maternal monitoring and its continuation into the postpartum period when the onset of the postpartum thyroid syndromes should be expected.
Disclosure Statement
T.F.D. is a Member of the Board of Kronus Inc., which markets diagnostic kits, including those for thyroid autoantibodies, and is a lecturer for Abbott Corp, manufacturers of l-thyroxine. J.C.G. has received travel support from Genzyme Corp., manufacturers of recombinant TSH.
References
- 1.Lazarus JH. Kokandi A. Thyroid disease in relation to pregnancy: A decade of change. Clin Endocrinol. 2000;53:265–278. doi: 10.1046/j.1365-2265.2000.01087.x. [DOI] [PubMed] [Google Scholar]
- 2.Smallridge RC. Glinoer D. Hollowell JG. Brent G. Thyroid function inside and outside of pregnancy: What do we know and what don't we know? Thyroid. 2005;15:54–59. doi: 10.1089/thy.2005.15.54. [DOI] [PubMed] [Google Scholar]
- 3.Abalovich M. Amino N. Barbour LA, et al. Management of thyroid dysfunction during pregnancy and postpartum: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2007;92:S1–47. doi: 10.1210/jc.2007-0141. [DOI] [PubMed] [Google Scholar]
- 4.Glinoer D. de Nayer P. Bourdoux P, et al. Regulation of maternal thyroid during pregnancy. J Clin Endocrinol Metab. 1990;71:276–287. doi: 10.1210/jcem-71-2-276. [DOI] [PubMed] [Google Scholar]
- 5.Ain KB. Mori Y. Refetoff S. Reduced clearance rate of thyroxine-binding globulin (TBG) with increased sialylation: A mechanism for estrogen-induced elevation of serum TBG concentration. J Clin Endocrinol Metab. 1987;65:689–696. doi: 10.1210/jcem-65-4-689. [DOI] [PubMed] [Google Scholar]
- 6.LeBeau SO. Mandel SJ. Thyroid disorders during pregnancy. Endocrinol Metab Clin North Am. 2006;35:117–136. doi: 10.1016/j.ecl.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Panesar NS. Li CY. Rogers MS. Reference intervals for thyroid hormones in pregnant Chinese women. Ann Clin Biochem. 2001;38:329–332. doi: 10.1258/0004563011900830. [DOI] [PubMed] [Google Scholar]
- 8.Glinoer D. The regulation of thyroid function in pregnancy: Pathways of endocrine adaptation from physiology to pathology. Endocr Rev. 1997;18:404–433. doi: 10.1210/edrv.18.3.0300. [DOI] [PubMed] [Google Scholar]
- 9.Brent GA. Maternal thyroid function: Interpretation of thyroid function tests in pregnancy. Clin Obstet Gynecol. 1997;40:3–15. doi: 10.1097/00003081-199703000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Somerset DA. Zheng Y. Kilby MD. Sansom DM. Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T cell subset. Immunology. 2004;112:38–43. doi: 10.1111/j.1365-2567.2004.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aluvihare VR. Kallikourdis M. Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 12.Ando T. Davies TF. Self-recognition and the role of fetal microchimerism. Best Pract Res Clin Endocrinol Metab. 2004;18:197–211. doi: 10.1016/j.beem.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Galofre JC. Davies TF. Microchimerism and thyroid disease, 2007. www.hotthyroidology.com www.hotthyroidology.com
- 14.Stagnaro-Green A. Roman SH. Cobin RH. el-Harazy E. Alvarez-Marfany M. Davies TF. Detection of at-risk pregnancy by means of highly sensitive assays for thyroid autoantibodies. JAMA. 1990;264:1422–1425. [PubMed] [Google Scholar]
- 15.Stagnaro-Green A. Chen X. Bogden JD. Davies TF. Scholl TO. The thyroid and pregnancy: A novel risk factor for very preterm delivery. Thyroid. 2005;15:351–357. doi: 10.1089/thy.2005.15.351. [DOI] [PubMed] [Google Scholar]
- 16.Negro R. Mangieri T. Coppola L, et al. Levothyroxine treatment in thyroid peroxidase antibody-positive women undergoing assisted reproduction technologies: A prospective study. Hum Reprod. 2005;20:1529–1533. doi: 10.1093/humrep/deh843. [DOI] [PubMed] [Google Scholar]
- 17.Negro R. Formoso G. Mangieri T. Pezzarossa A. Dazzi D. Hassan H. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: Effects on obstetrical complications. J Clin Endocrinol Metab. 2006;91:2587–2591. doi: 10.1210/jc.2005-1603. [DOI] [PubMed] [Google Scholar]
- 18.James SR. Franklyn JA. Kilby MD. Placental transport of thyroid hormone. Best Pract Res Clin Endocrinol Metab. 2007;21:253–264. doi: 10.1016/j.beem.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Bianco AC. Salvatore D. Gereben B. Berry MJ. Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002;23:38–89. doi: 10.1210/edrv.23.1.0455. [DOI] [PubMed] [Google Scholar]
- 20.Bianco AC. Kim BW. Deiodinases: Implications of the local control of thyroid hormone action. J Clin Invest. 2006;116:2571–2579. doi: 10.1172/JCI29812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hennemann G. Docter R. Friesema EC. de Jong M. Krenning EP. Visser TJ. Plasma membrane transport of thyroid hormones and its role in thyroid hormone metabolism and bioavailability. Endocr Rev. 2001;22:451–476. doi: 10.1210/edrv.22.4.0435. [DOI] [PubMed] [Google Scholar]
- 22.Tohyama K. Kusuhara H. Sugiyama Y. Involvement of multispecific organic anion transporter, Oatp14 (Slc21a14), in the transport of thyroxine across the blood-brain barrier. Endocrinology. 2004;145:4384–4391. doi: 10.1210/en.2004-0058. [DOI] [PubMed] [Google Scholar]
- 23.Heuer H. Maier MK. Iden S, et al. The monocarboxylate transporter 8 linked to human psychomotor retardation is highly expressed in thyroid hormone-sensitive neuron populations. Endocrinology. 2005;146:1701–1706. doi: 10.1210/en.2004-1179. [DOI] [PubMed] [Google Scholar]
- 24.Rosen IB. Korman M. Walfish PG. Thyroid nodular disease in pregnancy: Current diagnosis and management. Clin Obstet Gynecol. 1997;40:81–89. doi: 10.1097/00003081-199703000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Glinoer D. Management of hypo- and hyperthyroidism during pregnancy. Growth Horm IGF Res. 2003;13(Suppl A):S45–54. doi: 10.1016/s1096-6374(03)00055-8. [DOI] [PubMed] [Google Scholar]
- 26.García-Mayor RV. Cordido F. Valle-Inclán F. Lage M. Tomé MA. Casanueva FF. Prevalence of pregnancy and postpartum thyroid dysfunction in a homogeneous population of Spain. Gynecol Endocrinol. 1999;13:279–287. doi: 10.3109/09513599909167567. [DOI] [PubMed] [Google Scholar]
- 27.American College of Obstetrics and Gynecology. Thyroid disease in pregnancy. Int J Gynaecol Obstet. 2002;79:171–180. doi: 10.1016/s0020-7292(02)00327-2. [DOI] [PubMed] [Google Scholar]
- 28.Surks MI. Ortiz E. Daniels GH, et al. Subclinical thyroid disease: Scientific review and guidelines for diagnosis and management. JAMA. 2004;291:228–238. doi: 10.1001/jama.291.2.228. [DOI] [PubMed] [Google Scholar]
- 29.Gharib H. Tuttle RM. Baskin HJ. Fish LH. Singer PA. McDermott MT. Subclinical thyroid dysfunction: A joint statement on management from the American Association of Clinical Endocrinologists, the American Thyroid Association, and the Endocrine Society. J Clin Endocrinol Metab. 2005;90:581–585. doi: 10.1210/jc.2004-1231. [DOI] [PubMed] [Google Scholar]
- 30.Davies TF. Time for the American Thyroid Association to lead on thyroid screening in pregnancy. Thyroid. 2007;17:697–698. doi: 10.1089/thy.2007.1515. [DOI] [PubMed] [Google Scholar]
- 31.Spong CY. Subclinical hypothyroidism: Should all pregnant women be screened? Obstet Gynecol. 2005;105:235–236. doi: 10.1097/01.AOG.0000153143.89006.ac. [DOI] [PubMed] [Google Scholar]
- 32.Davies TF. The ATA, the Endocrine Society, and AACE confuse endocrinologists on thyroid disease in pregnancy. Thyroid. 2000;10:107. doi: 10.1089/thy.2000.10.107. [DOI] [PubMed] [Google Scholar]
- 33.American Association of Clinical Endocrinologists. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism. Endocr Pract. 2002;8:457–469. [PubMed] [Google Scholar]
- 34.Vaidya B. Anthony S. Bilous M, et al. Detection of thyroid dysfunction in early pregnancy: Universal screening or targeted high-risk case finding? J Clin Endocrinol Metab. 2007;92:203–207. doi: 10.1210/jc.2006-1748. [DOI] [PubMed] [Google Scholar]
- 35.Cooper DS. Subclinical thyroid disease: Consensus or conundrum? Clin Endocrinol. 2004;60:410–412. doi: 10.1111/j.1365-2265.2004.02031.x. [DOI] [PubMed] [Google Scholar]
- 36.Surks MI. Subclinical thyroid dysfunction: A joint statement on management from the American Association of Clinical Endocrinologists, the American Thyroid Association, and The Endocrine Society. J Clin Endocrinol Metab. 2005;90:586–587. doi: 10.1210/jc.2004-1231. [DOI] [PubMed] [Google Scholar]
- 37.Ringel MD. Mazzaferri EL. Subclinical thyroid dysfunction—Can there be a consensus about the consensus? J Clin Endocrinol Metab. 2005;90:588–590. doi: 10.1210/jc.2004-2173. [DOI] [PubMed] [Google Scholar]
- 38.Pinchera A. Subclinical thyroid disease: To treat or not to treat? Thyroid. 2005;15:1–2. doi: 10.1089/thy.2005.15.1. [DOI] [PubMed] [Google Scholar]
- 39.Ando T. Davies TF. Postpartum autoimmune thyroid disease: The potential role of fetal microchimerism. J Clin Endocrinol Metab. 2003;88:2965–2971. doi: 10.1210/jc.2002-021903. [DOI] [PubMed] [Google Scholar]
- 40.Brent GA. Diagnosing thyroid dysfunction in pregnant women: Is case finding enough? J Clin Endocrinol Metab. 2007;92:39–41. doi: 10.1210/jc.2006-2461. [DOI] [PubMed] [Google Scholar]
- 41.Lazarus JH. Premawardhana LD. Screening for thyroid disease in pregnancy. J Clin Pathol. 2005;58:449–452. doi: 10.1136/jcp.2004.021881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amino N. Mori H. Iwatani Y, et al. High prevalence of transient postpartum thyrotoxicosis and hypothyroidism. N Engl J Med. 1982;306:849–852. doi: 10.1056/NEJM198204083061405. [DOI] [PubMed] [Google Scholar]
- 43.Premawardhana LD. Parkes AB. John R. Harris B. Lazarus JH. Thyroid peroxidase antibodies in early pregnancy: Utility for prediction of postpartum thyroid dysfunction and implications for screening. Thyroid. 2004;14:610–615. doi: 10.1089/1050725041692828. [DOI] [PubMed] [Google Scholar]
- 44.Mandel SJ. Spencer CA. Hollowell JG. Are detection and treatment of thyroid insufficiency in pregnancy feasible? Thyroid. 2005;15:44–53. doi: 10.1089/thy.2005.15.44. [DOI] [PubMed] [Google Scholar]
- 45.Glinoer D. Lemone M. Bourdoux P, et al. Partial reversibility during late postpartum of thyroid abnormalities associated with pregnancy. J Clin Endocrinol Metab. 1992;74:453–457. doi: 10.1210/jcem.74.2.1730819. [DOI] [PubMed] [Google Scholar]
- 46.Morreale de Escobar G. Obregon MJ. Escobar del Rey F. Is neuropsychological development related to maternal hypothyroidism or to maternal hypothyroxinemia? J Clin Endocrinol Metab. 2000;85:3975–3987. doi: 10.1210/jcem.85.11.6961. [DOI] [PubMed] [Google Scholar]
- 47.Dashe JS. Casey BM. Wells CE, et al. Thyroid-stimulating hormone in singleton and twin pregnancy: Importance of gestational age-specific reference ranges. Obstet Gynecol. 2005;106:753–757. doi: 10.1097/01.AOG.0000175836.41390.73. [DOI] [PubMed] [Google Scholar]
- 48.Soldin OP. Tractenberg RE. Hollowell JG. Jonklaas J. Janicic N. Soldin SJ. Trimester-specific changes in maternal thyroid hormone, thyrotropin, and thyroglobulin concentrations during gestation: Trends and associations across trimesters in iodine sufficiency. Thyroid. 2004;14:1084–1090. doi: 10.1089/thy.2004.14.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Becker DV. Braverman LE. Delange F, et al. Iodine supplementation for pregnancy and lactation—United States and Canada: Recommendations of the American Thyroid Association. Thyroid. 2006;16:949–951. doi: 10.1089/thy.2006.16.949. [DOI] [PubMed] [Google Scholar]
- 50.Mandel SJ. Hypothyroidism and chronic autoimmune thyroiditis in the pregnant state: Maternal aspects. Best Pract Res Clin Endocrinol Metab. 2004;18:213–224. doi: 10.1016/j.beem.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 51.Kamijo K. Saito T. Sato M, et al. Transient subclinical hypothyroidism in early pregnancy. Endocrinol Jpn. 1990;37:397–403. doi: 10.1507/endocrj1954.37.397. [DOI] [PubMed] [Google Scholar]
- 52.Lao TT. Thyroid disorders in pregnancy. Curr Opin Obstet Gynecol. 2005;17:123–127. doi: 10.1097/01.gco.0000162179.15360.08. [DOI] [PubMed] [Google Scholar]
- 53.Abalovich M. Gutierrez S. Alcaraz G. Maccallini G. Garcia A. Levalle O. Overt and subclinical hypothyroidism complicating pregnancy. Thyroid. 2002;12:63–68. doi: 10.1089/105072502753451986. [DOI] [PubMed] [Google Scholar]
- 54.Casey BM. Dashe JS. Wells CE, et al. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol. 2005;105:239–245. doi: 10.1097/01.AOG.0000152345.99421.22. [DOI] [PubMed] [Google Scholar]
- 55.Cleary-Goldman J. Malone FD. Lambert-Messerlian G, et al. Maternal thyroid hypofunction and pregnancy outcome. Obstet Gynecol. 2008;112:85–92. doi: 10.1097/AOG.0b013e3181788dd7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lazarus JH. Epidemiology and prevention of thyroid disease in pregnancy. Thyroid. 2002;12:861–865. doi: 10.1089/105072502761016485. [DOI] [PubMed] [Google Scholar]
- 57.Auso E. Lavado-Autric R. Cuevas E. Del Rey FE. Morreale de Escobar G. Berbel P. A moderate and transient deficiency of maternal thyroid function at the beginning of fetal neocorticogenesis alters neuronal migration. Endocrinology. 2004;145:4037–4047. doi: 10.1210/en.2004-0274. [DOI] [PubMed] [Google Scholar]
- 58.Stagnaro-Green A. Glinoer D. Thyroid autoimmunity and the risk of miscarriage. Best Pract Res Clin Endocrinol Metab. 2004;18:167–181. doi: 10.1016/j.beem.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 59.Glinoer D. Iodine nutrition requirements during pregancy. Thyroid. 2006;16:947–948. doi: 10.1089/thy.2006.16.947. [DOI] [PubMed] [Google Scholar]
- 60.Mandel SJ. Larsen PR. Seely EW. Brent GA. Increased need for thyroxine during pregnancy in women with primary hypothyroidism. N Engl J Med. 1990;323:91–96. doi: 10.1056/NEJM199007123230204. [DOI] [PubMed] [Google Scholar]
- 61.Alexander EK. Marqusee E. Lawrence J. Jarolim P. Fischer GA. Larsen PR. Timing and magnitude of increases in levothyroxine requirements during pregnancy in women with hypothyroidism. N Engl J Med. 2004;351:241–249. doi: 10.1056/NEJMoa040079. [DOI] [PubMed] [Google Scholar]
- 62.Toft A. Increased levothyroxine requirements in pregnancy—Why, when, and how much? N Engl J Med. 2004;351:292–294. doi: 10.1056/NEJMe048110. [DOI] [PubMed] [Google Scholar]
- 63.Idris I. Srinivasan R. Simm A. Page RC. Maternal hypothyroidism in early and late gestation: Effects on neonatal and obstetric outcome. Clin Endocrinol. 2005;63:560–565. doi: 10.1111/j.1365-2265.2005.02382.x. [DOI] [PubMed] [Google Scholar]
- 64.Kaplan MM. Monitoring thyroxine treatment during pregnancy. Thyroid. 1992;2:147–152. doi: 10.1089/thy.1992.2.147. [DOI] [PubMed] [Google Scholar]
- 65.Anselmo J. Cao D. Karrison T. Weiss RE. Refetoff S. Fetal loss associated with excess thyroid hormone exposure. JAMA. 2004;292:691–695. doi: 10.1001/jama.292.6.691. [DOI] [PubMed] [Google Scholar]
- 66.Centanni M. Gargano L. Canettieri G, et al. Thyroxine in goiter, Helicobacter pylori infection, and chronic gastritis. N Engl J Med. 2006;354:1787–1795. doi: 10.1056/NEJMoa043903. [DOI] [PubMed] [Google Scholar]
- 67.Chopra IJ. Baber K. Treatment of primary hypothyroidism during pregnancy: Is there an increase in thyroxine dose requirement in pregnancy? Metabolism. 2003;52:122–128. doi: 10.1053/meta.2003.50019. [DOI] [PubMed] [Google Scholar]
- 68.Caixas A. Albareda M. Garcia-Patterson A. Rodriguez-Espinosa J. de Leiva A. Corcoy R. Postpartum thyroiditis in women with hypothyroidism antedating pregnancy? J Clin Endocrinol Metab. 1999;84:4000–4005. doi: 10.1210/jcem.84.11.6144. [DOI] [PubMed] [Google Scholar]
- 69.Glinoer D. Thyroid hyperfunction during pregnancy. Thyroid. 1998;8:859–864. doi: 10.1089/thy.1998.8.859. [DOI] [PubMed] [Google Scholar]
- 70.Burrow GN. The management of thyrotoxicosis in pregnancy. N Engl J Med. 1985;313:562–565. doi: 10.1056/NEJM198508293130907. [DOI] [PubMed] [Google Scholar]
- 71.Glinoer D. De Nayer P. Robyn C. Lejeune B. Kinthaert J. Meuris S. Serum levels of intact human chorionic gonadotropin (hCG) and its free alpha and beta subunits, in relation to maternal thyroid stimulation during normal pregnancy. J Endocrinol Invest. 1993;16:881–888. doi: 10.1007/BF03348950. [DOI] [PubMed] [Google Scholar]
- 72.Sherif IH. Oyan WT. Bosairi S. Carrascal SM. Treatment of hyperthyroidism in pregnancy. Acta Obstet Gynecol Scand. 1991;70:461–463. doi: 10.3109/00016349109007160. [DOI] [PubMed] [Google Scholar]
- 73.Luton D. Le Gac I. Vuillard E, et al. Management of Graves' disease during pregnancy: The key role of fetal thyroid gland monitoring. J Clin Endocrinol Metab. 2005;90:6093–6098. doi: 10.1210/jc.2004-2555. [DOI] [PubMed] [Google Scholar]
- 74.Stoffer SS. Hamburger JI. Inadvertent 131I therapy for hyperthyroidism in the first trimester of pregnancy. J Nucl Med. 1976;17:146–149. [PubMed] [Google Scholar]