Abstract
Receptors are at the heart of how a molecule transmits a signal to a cell. Two receptor classes for endothelin (ET) are recognized, the ETA and ETB receptors. Intriguing questions have arisen in the field of ET receptor pharmacology, physiology, and function. For example, a host of pharmacological studies support the interaction of the ETA and ETB receptor in tissues (veins, arteries, bronchus, arterioles, esophagus), but yet few have been able to demonstrate direct ETA/ETB receptor interaction. Have we modeled this interaction wrong? Do we have a truly selective ETA receptor agonist such that we could selectively stimulate this important receptor? What can we learn from the recent phylogenic studies of the ET receptor family? Have we adequately addressed the number of biological molecules with which ET can interact to exert a biological effect? Recent mass spectrometry studies in our laboratory suggest that ET-1 interacts with other hereto unrecognized proteins. Biased ligands (ligands at the same receptor that elicit distinct signaling responses) have been discovered for other receptors. Do these exist for ET receptors and can we take advantage of this possibility in drug design? These and other questions will be posed in this minireview on topics on ET receptors.
Keywords: heterodimerization, functional selectivity
in 1988, the endothelial cell-derived peptide endothelin (ET) was discovered (121). In the 21 years since its discovery, ET-1 has been implicated in a wide number of physiological systems. Implication here means that synthetic machinery for ET peptides, ET peptides themselves, and ET receptors have been localized to tissues in these systems, as well as demonstration that ET-1 causes a physiological function within that system. An incomplete list includes the cardiovascular (11, 12, 16, 98), renal (35, 58, 87), central nervous (103), gastrointestinal (45), pituitary (61), adrenal (24, 43, 95, 96), nasal (83), peripheral nervous (20, 33, 82), immune (76), hepatic (70), genitourinary (15), and endocrine systems (75).
Given ET's enormously diverse presence in physiological systems, it comes as no surprise that ET-1 has been targeted as a pathophysiological culprit in multiple diseases and/or conditions. An incomplete list of these diseases includes hypertension [pregnancy, pulmonary, portopulmonary, and essential systemic (11, 31, 65, 77, 86, 113)], congestive heart failure (81), cancer (8, 37, 38, 85), diabetes (50, 55, 60), glaucoma (17), pain (41, 56), sexual dysfunction (112), fibrosis (108), renal failure (4, 63, 93), inflammation (4), and cerebral vasospasm (6).
If the involvement of ET-1 in disease is as pervasive as the many diseases listed above suggests, then development of compounds that block the effects of ET is imperative. This requires knowledge as to how ET exerts its biological activity. The classical view is that ET peptides stimulate their biological and pathophysiological effects through plasma membrane-bound ET receptors, the ETA and ETB receptors. This review is focused on a few current ideas/issues regarding ET receptor pharmacology and functioning in mammalian systems. Development of compounds for individual receptors as it pertains to therapy will not be discussed. There are several brilliant reviews that discuss, in far more detail than will be done here, ET peptides, classic knowledge of ET receptors (pharmacology, physiology and function), and models for study of ET in disease, and the reader is directed to some of them here (9, 22, 23, 26, 73, 88, 90, 91, 100, 104, 109, 110, 116).
CURRENTLY CLASSIFIED ET RECEPTORS AND THEIR PHARMACOLOGY
The International Union of Pharmacology (IUPHAR) officially defines receptor classification. This was done for ET receptors in a first report in 1994 (74) and updated in 2002 (21). At this time, only two classes are recognized as ET receptors: the ETA receptor and the ETB receptor (http://www.iuphar.org/).
ETA Receptor
Originally cloned in 1990 (5), this protein is a 7α helical transmembrane domain molecule that is a Class A member of G protein-coupled receptors, possessing an extracellular amino terminus and intracellular carboxy terminus. Antagonists selective for this subtype of ET receptor include ZD4054, atrasentan, darusentan, macitentan, ambrisentan, and sitaxsentan. ETA receptors are recognized by their relative insensitivity to stimulation by the agonist ET-3, whereas ET-2 and ET-1 have similar affinities at the ETA receptor. Selective agonists of this receptor have been poorly defined, although the ET peptide ET-1[1–31] has been used as an ETA receptor agonist (95). While it has some utility, this peptide falls short of the ideal agonist because ET-1[1–31] can be subjected to proteolytic degradation, thereby modifying the molecule and its pharmacological properties (30). It is unclear what contributions the different enzymatic products of ET-1[1–31] metabolism make to ETA receptor stimulation, such that this peptide is limited in its usefulness. The ETA receptor has been localized to virtually every physiological system, including cardiovascular (heart, blood, blood vessels), respiratory (lung, trachea), central nervous, sensory nervous, immune (neutrophils, macrophages), genitourinary (kidney, prostate, ovary), gastrointestinal (intestines, stomach, liver), and endocrine systems (pancreas).
ETB Receptor
The ETB receptor was cloned shortly after the ETA receptor. Similarly to the ETA receptor, it belongs to class A of G protein-coupled heptahelical receptors with an external amino terminus and internal carboxy terminus and binding sites intrinsic to the heptahelical portions of the receptor (99). Similar to the ETA receptor, the endogenous agonist ET-1 has a high affinity for the ETB receptor. The pharmacology of the ETB receptor is somewhat richer than that of the ETA receptor in that multiple agonists of the ETB receptor are recognized, including sarafotoxin 6c (S6c) and IRL1620. Selective antagonists of the ETB receptor include BQ788, A192621, RES7011, and IRL2500.
However, a number of real differences between the ETA and ETB receptor exist. First, unlike the ETA receptor, all three endogenous ET peptides (ET-1, ET-2, and ET-3) have similar affinity for the ETB receptor. Second, variants in the ETB receptor gene exist in the human population to a seemingly greater extent than for the ETA receptor (80, 107). In a similar vein, it has been suggested that ETB1 and ETB2 receptors exist, here discriminated by their geographical location (B1 = endothelial cell, B2 = smooth muscle in a blood vessel). The IUPHAR and ET receptor committee have not formally adopted this nomenclature in the absence of data that support the existence of two different ETB receptor genes or proteins that are distinct pharmacological entities. This is thus a nomenclature that would seem wise to not use. Third, the ETB receptor is not expressed as extensively as the ETA receptor, or at least the data supporting ETB receptor expression are not as readily available. The ETB receptor has been localized to cardiovascular tissues, the pulmonary system, neurons, bone, pancreas, and kidney.
Thus we know, at the present, that two bona fide ET receptor subtypes exist. What are some of the outstanding questions regarding these two receptors and their stimulation by ET-1?
OUTSTANDING QUESTIONS IN THE FIELD
In this section, topic areas in which progress could provide our field with better tools, or issues with which the field of ET (and, in particular, the author's laboratory) is wrestling will be addressed. It should be noted that these topics are the opinion of the author of the present review, and thus are arguable.
Identification of an ETA Receptor Agonist
For pharmacologists, it is well established that receptors are best characterized when a full armamentarium of tools is available to define that receptor. This includes positive agonists (substances that bind to and stimulate a biological response through the receptor) and antagonists (substances that bind to and prevent a biological response through the receptor). Collectively, these pharmacological tools can be used to provide a “fingerprint” for a receptor. The ETB receptor has S6c as an agonist, but the ETA receptor has no known parallel tool. The limitation of ET-1[1–31] as an ETA receptor agonist was described above, and this is not an ideal situation. Without an ETA receptor selective agonist, one lacks the ability to activate the ETA receptor in the absence of other ET receptors. Such a tool would enable the study of receptor trafficking, desensitization, resensitization, and signal transduction elements directly utilized by the ETA receptor. To be sure, ET-1 itself can do this, but there is always the caveat that ET-1 will stimulate any ETB receptors present. In artificial systems, one can test the proof-of-concept studies of how ET stimulation modifies ETA receptor movement in the absence of an ETB receptor, but this only approaches an endogenous physiological situation. For example, most blood vessels (arteries and veins) possess both smooth muscular ETA and ETB receptors. It would be ideal to compare and contrast how the ETA receptor moves and signals independently in these two different blood vessel types that serve significantly different purposes in the body. Using ET-1 is not good enough. An agonist (ideally a small molecule) that activated the ETA receptor selectivity (>50-fold difference from ETB) would be a beneficial tool. Recently, several studies have dissected structural determinants for selective recognition of ETA and ETB receptor ligands and developed imaging proteins for the ETA receptor (7, 44, 64). With such information, we can hope to develop better tools for studying the ETA receptor.
Biased Ligands for ET Receptors?
Receptor signal transduction has classically been considered in a sequential fashion. The agonist (ET-1 in our case) activates a heptahelical receptor, which activates a G protein, which activates an effector that produces or modifies production of a second messenger such that a biological message is sent. This scenario suggests that a message is black/white (it is there or not), and once the receptor is stimulated, the message is sent. In the late 1990s, the idea of differential signaling was introduced (18); some of the original work was described in the serotonin receptor field. This idea stems from findings that different agonists could elicit different signal transduction events through the same receptor (for review see Refs. 53, 54, 114, 115). The mechanism by which this occurs has best been illustrated by the ANG II (AT1) receptor. This receptor couples to both G proteins (G protein dependent) and β-arrestin (G protein independent; a src/Erk MAPK pathway) to elicit biological effects. When ANG II is an agonist, both pathways (G protein-dependent and β-arrestin-dependent) are stimulated. However, the substituted ANG II peptide Sar1, Ile4, Ile8-ANG II (SII) activates only the β-arrestin pathways (115). SII has thus been called a biased agonist, in that its activation of the AT1 receptor is biased towards one particular signal transduction pathway.
Endothelin activates src and the Erk MAPK pathways in multiple cell types; and more recently, the ETA receptor has been linked to β-arrestin in signaling to β-catenin in ovarian cancer cells (94). Thus, it is probable that, like the AT1 receptor, ET receptors can function in a biased manner. Which ligands that we use possess this capability? Would the design of ligands that are biased be beneficial experimental or therapeutic tools? Does the context of the receptor modify whether an agonist is biased? Makita et al. (68) demonstrated that ET-3 showed significant affinity for the ETA receptor in superior cervical ganglion. Is this an issue of truly different ET receptor subtypes or ET receptor behaving with different signaling/pharmacology bias? This field is open for discovery.
ETA/ETB Receptor Heterodimerization
Heptahelical receptors like the ET receptors have classically been described as functional monomers which, when stimulated, activate G proteins and subsequent effectors. Evidence accrued over the last decade suggests that heptahelical receptors function as physical homodimers (e.g., 2 GABA receptors together) or heterodimers (e.g., angiotensin AT1 and bradykinin BK2) (14, 25, 34, 39, 72, 79, 119). Heptahelical receptors can dimerize through venus fly trap elements of the NH2 terminus, the heptahelical domains, covalent cysteine bonds in the NH2 termini, and twinning motifs in the COOH terminus (39, 66). The pharmacology and function of a multisubunit receptor complex (e.g., heterodimer, homodimer) can be different from that of the individual monomers, with changes in agonist potency, affinity, and antagonist-sensitivity reported. One member of the dimer pair may serve as a chaperone or modify the function of the other partner just as the receptor activity modifying proteins do for the B family of G protein-coupled receptors (105). Physical interaction of receptors occurs not only in the plasma membrane but also in the endoplasmic reticulum. If heterodimerization occurs physiologically, it could have a profound impact on pharmacological therapy of disease (117, 123). Dimerization is, however, controversial for class A/rhodopsin-like heptahelical receptors, which include the ET receptors, because they irregularly include classical motifs for interaction. Sequences of the rat and human ETA and ETB receptor contain NH2 terminus cysteine residues that could form covalent bonds and incomplete twinning motifs in the COOH terminus. Additionally, exactly what constitutes functional dimerization is arguable (39). The partners in heterodimers that can be coimmunoprecipited with one another typically have significant physical interaction, such as covalent bonds through cysteine residues in the NH2 terminus. This suggests these receptors live and operate together as a functional unit for extended periods of time. Do two partners that come together for a brief and noncovalent protein-protein interaction (a “kiss-and-run” interaction) also constitute a heterodimer? This should be so if that interaction, however brief, modifies the pharmacology and/or function of one of the partners. The question can then be asked: is this type of kiss-and-run association simply par for the course in signal transduction, a process clearly dependent on noncovalent and brief interactions?
Most studies investigating receptor heterodimerization use artificial systems in which receptors are transfected and are expressed at supraphysiological levels. Heterodimer pairs studied in such systems include the ANG II AT1 receptor with bradykinin B2 receptor (106), AT2 with B2 receptor (1), thromboxane α- and β-receptor (120), dopamine D1 and D2 receptors (27), α2C- and β2-adrenergic receptors (92), α1B- and α1D-adrenergic receptors (40), and vasopressin V1a and V2 receptors (111). This list is far from complete. One exception to transfected cell experiments is AT1/B2 receptor heterodimerization in cultured renal mesangial cells from the spontaneously hypertensive rat but not from normotensive controls, with heterodimerization contributing to the hyperresponsiveness of spontaneously hypertensive rat cells to ANG II (2).
We have revealed an interaction of the ETA and ETB receptor in venous but not arterial tissues. A similar interaction of ET receptors has been described in a number of tissues, being first described in the vasculature in 1995 (3, 32, 46, 49, 67, 69, 78, 89, 102). At the base of this interaction is the failure of ETA receptor antagonists to shift venous contraction to ET-1, unless ETB receptor function is ablated. S6c/ETB receptor desensitization renders veins sensitive to ETA receptor blockade. An explanation for the failure of the ETA receptor antagonist to shift ET-1-induced venous contraction may be that activated ETB receptors physically/pharmacologically uncouple the ETA receptor through heterodimerization. Exposure to high concentrations of S6c removes the actions of the ETB receptor. This allows ETA receptors to participate in contraction in a pharmacologically definable manner. Heterologously expressed human ETA and ETB receptors can form heterodimers (19, 28, 29, 36). More recently, ETA and ETB receptor heterodimerization has been suggested to occur in pulmonary arteries, but this is the only example in a physiological and pathological situation (101, 102). The question foremost in this field is under what conditions heterodimer formation contributes to physiological function. We have been unable to demonstrate coimmunoprecipitation of the ETA and ETB receptor in veins, having tried in at least a dozen different immunoprecipitation approaches (different kits, buffers, urea gels, etc.). Interaction of the ETA and ETB receptor is the most logical reason for our pharmacological results, but we’ve begun to look at other possible explanations given our lack of ability to explain the unusual pharmacology described above by ETA/ETB receptor heterodimerization. ET receptors may interact with other non-ET receptors to form a new pharmacophore. This was recently described for the ETB receptor, which heterodimerize with the dopamine D3 receptor in a cell culture of renal proximal tubule cells (122). This represents one possible explanation for the pharmacological results observed. Another question that deserves thought is whether ET-1 itself is a bivalent ligand, being capable of stimulating both the ETA and ETB receptor with different portions of the ET-1 molecule. This idea has been proposed by Hirada et al. (42). This group took a slightly different tactic, concluding that the ETB receptor could not independently recognize ET-1 without the ETA receptor and suggesting that ET-1 itself is a bivalent ligand. Another explanation includes alternative targets for ET itself, and these are next described.
Other Targets for ET-1?
Dual angiotensin ET receptor.
The dual angiotensin ET receptor (DEAR) was characterized in 1998 by Ruiz-Opazo et al. (97). Structural analysis predicted a receptor polypeptide that, unlike the native ET and AT receptors, possesses only one transmembrane region, an extracellular amino terminus and intracellular carboxy terminus. This receptor possesses both ANG II- and ET-1-specific binding sites, as well as the ability to mobilize calcium. Expression of this receptor mRNA was found in the brain and the heart, suggesting it participates in cardiovascular control. More recently, DEAR has been implicated in the female Dahl salt-sensitive model of hypertension (51). The interaction of ET-1 and ANG II is supported by the basic knowledge that ANG II can cause ET-1 release, and both modify the function of NADPH oxidase (62). The pharmacology and physiology of DEAR has not been developed. Could the DEAR receptor be a biological target of endogenous ET peptides?
The G protein-coupled peptide receptor 37 gene.
In 1997, GPR37, a gene located on chromosome 7 encoding a putative G protein-coupled peptide receptor, was cloned in human frontal brain (71). This has been called the ET receptor B-like protein receptor 1 because of its high degree of homology with the human ETB receptor. The most recent scientific research suggests this protein more likely functions as a substrate for Parkin, a polypeptide with similarities to ubiquitin protein ligase E3. This protein is now called the Parkin-associated ET receptor-like receptor (Pael-R; 48). Research of this protein as a bona fide ET receptor has not progressed.
ETC.
The ETC receptor was proposed early in ET receptor history, being cloned in Xenopus laevis (52, 74). A similar receptor from Xenopus was cloned by Kumar et al. (59). Recent phylogenetic reports suggest that the ETC receptor gene and protein was lost in placental and marsupial mammals and thus is not relevant to human physiology and pharmacology (13, 47).
Other targets of ET-1.
A tenet of pharmacology is that an agonist interacts with a receptor to exert a biological effect. This is certainly true, and pharmacology has taken advantage of this interaction to develop pharmaceutical therapies that have, without a doubt, benefited human health. However, it is becoming increasingly apparent that agonists (hormones, neurotransmitters, peptides) may exert their biological activity in decidedly different ways. For example, the small molecule serotonin (5-HT) can activate multiple plasma membrane receptors, but it can also be used to covalently modify proteins (serotonylation) and change protein function (118).
Could ET-1 Interact with Other Biological Molecules to Exert its Effects?
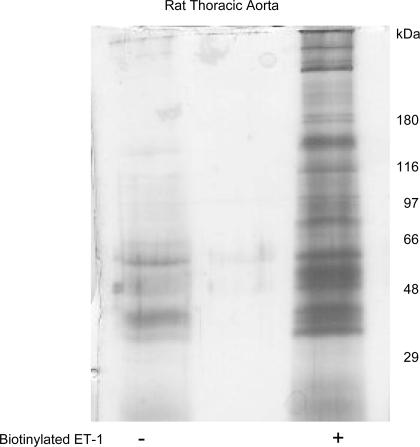
This is a long-term question that we are just beginning to address with experiments such as the following one. We used an ET-1 molecule biotinylated on the lysine 9 position (Genscript). This compound retains its biological activity as validated by its ability to cause arterial contraction (not shown). We incubated 100 μg of rat aortic homogenate (not whole tissue) with biotinylated ET-1 (∼1 μM final concentration) for 3 h at 4°C. The assumption is that ET-1 will find and, as it does with ET receptors, avidly bind to interacting proteins. Streptavidin-coated magnetic beads were added to the samples, and the samples tumbled overnight in a nonstringent buffer (PBS) at 4°C. Samples were washed three times and then the beads were collected, heated at 60°C for 10 min, and a sample (beads + eluted proteins) loaded onto a 10% polyacrylamide gel. The gel was run under standard conditions and taken through ProteoSilver staining. Figure 1 shows one example of such an experiment with no modifications. ET-1 clearly interacted with many proteins in the homogenate. These are in the process of being identified by tandem mass spectrometry. While these experiments must be performed in a whole tissue/whole cell, it suggests that we might need to take a broader look as to what ET interacts with in biological systems.
Fig. 1.
Gel of proteins brought down by streptavidin-coated magnetic beads from homogenates of normal Sprague-Dawley male rats incubated without (−) or with (+) biotinylated endothelin-1 (ET-1). Right, molecular weight markers.
PERSPECTIVES: WHAT DO WE NEED TO DO?
ET receptors have a rich, focused, but a still relatively new history compared with families like the adrenergic receptors. There is thus much we can learn and discover. I would hope that in the future we strive to
identify a truly selective ETA receptor agonist;
determine whether and how the concept of biased agonism applies to ET receptors;
determine mechanisms to show with confidence that ETA and ETB receptors heterodimerize in a physiological situation;
identify other biological targets for ET-1;
interrogate the current tools we have to study ET receptor pharmacology [Definitions of the tools we have used have been narrow (a compound is an agonist or antagonist), and one could speculate we have overlooked subtle differences in compounds such as bivalency, functional selectivity, and so on.];
answer the question of why ET receptors exist in the nucleus of the cell and modify calcium (10, 57, 84) (This fascinating finding is still at the fringes of ET pharmacology and deserves attention not only because of its relevance to ET-1 but because of the germane idea that classically known plasma membrane receptors may modify nuclear biochemistry.); and
reflect on recently published phylogenic studies (13, 47) (These studies described several gene losses in the evolution of the ET system, including that of the ETC receptor from placental and marsupial mammals. It has also been suggested that the ET core system is a vertebrate-specific innovation. These reports may be revealing as to other functions of ET-1.).
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant PO1-HL-70687.
ACKNOWLEDGMENTS
The work and ideas within this manuscript were generated through many discussions with colleagues and students that include Drs. Keshari Thakali, Theo Szasz, Gregory D. Fink, and James J. Galligan. My thanks to them for their remarkable brain power.
REFERENCES
- 1.Abadir PM, Periasamy A, Carey RM, Siragy H. Angiotensin II type 2 receptor bradykinin B2 receptor functional heterodimerization. Hypertension 48: 316–322, 2006 [DOI] [PubMed] [Google Scholar]
- 2.AbdAlla S, Abdel-Baset A, Lother H, el Massiery A, Quitterer U. Mesangial AT1/B2 receptor heterodimers contribute to angiotensin II hyperresponsiveness in experimental hypertension. J Mol Neurosci 26: 185–192, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Adner M, Shankley N, Edvinsson L. Evidence that ET-1, but not ET-3 and S6b, ETA receptor mediated contractions in isolated rat mesenteric arteries are modulated by co-activation of ETB receptors. Br J Pharmacol 133: 927–935, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angerio AD. Endothelin-1-mediated inflammation in acute renal failure. Crit Care Nurs Q 29: 152–156, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Arai H, Hori S, Aramori I, Ohkubo H, Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature 348: 730–732, 1990 [DOI] [PubMed] [Google Scholar]
- 6.Armstead WM. Endothelins and the role of endothelin antagonists in the management of posttraumatic vasospasm. Curr Pharm Des 10: 2185–2192, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Aubin J, Letourneau M, Francoeur E, Burgeon E, Fournier A. Identification of ETA and ETB binding domains using ET-derived photoprobes. Biochimie 90: 918–929, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Bagnato A, Rosano L. The endothelin axis in cancer. Int J Biochem Cell Biol 40: 1443–1451, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Barton M, Yanagisawa Endothelin M. 20 years from discovery to therapy. Can J Physiol Pharmacol 86: 485–499, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Bkaily G, Choufani S, Avedanian L, Ahmarani L, Nader M, Jacques D, D'Orleans-Juste P, Al Khoury J. Nonpeptidic antagonists of ETA and ETB receptors reverse the ET-1-induced sustained increase of cytosolic and nuclear calcium in human aortic vascular smooth muscle cells. Can J Physiol Pharmacol 86: 546–556, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Black SM, Kumar S, Wiseman D, Ravi K, Wedgwood S, Ryzhov V, Fineman JR. Pediatric pulmonary hypertension: roles of endothelin-1 and nitric oxide. Clin Hemorheol Microcirc 37: 111–120, 2007 [PubMed] [Google Scholar]
- 12.Bohm F, Pernow J. The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovasc Res 76: 8–18, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Braasch I, Volff JN, Schartl M. The endothelin system: evolution of vertebrate-specific ligand-receptor interactions by three rounds of genome duplication. Mol Biol Evol 26: 783–789, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Bulenger S, Marullo S, Bouvier M. Emerging role of homo- and heterodimerization in G protein coupled receptor biosynthesis and maturation. Trends Pharmacol Sci 26: 131–137, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Cameron IT, Bacon CR, Collett GP, Davenport AP. Endothelin expression in the uterus. J Steroid Biochem Mol Biol 53: 209–214, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Cernacek P, Stewart DJ, Monge JC, Rouleau JL. The endothelin system and its role in acute myocardial infarction. Can J Physiol Pharmacol 81: 598–606, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Chauhan BC. Endothelin and its potential role in glaucoma. Can J Ophthalmol 43: 356–360, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Clarke WP, Bond RA. The elusive nature of intrinsic efficacy. Trends Pharmacol Sci 19: 270–276, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Dai X, Galligan JJ. Differential trafficking, and desensitization of human ETA and ETB receptors expressed in HEK 293 cells. Exp Biol Med (Maywood) 231: 746–751, 2006 [PubMed] [Google Scholar]
- 20.Damon DH. Endothelin and post-ganglionic sympathetic neurons. Clin Exp Pharmacol Physiol 26: 1000–1003, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Davenport AP. International union of pharmacology. XXIX. Update on endothelin receptor nomenclature. Pharmacol Rev 54: 219–226, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Davenport AP, Maguire JJ. Endothelin. Handb Exp Pharmacol 176: 295–329, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Ducancel F. Endothelin-like peptides. Cell Mol Life Sci 62: 2828–2839, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delarue C, Conlon JM, Remy-Jouet I, Fournier A, Vaudry H. Endothelins as local activators of adrenocortical cells. J Mol Endocrinol 32: 1–7, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Devi LA. G-protein coupled receptor dimers in the lime light. Trends Pharmacol Sci 21: 324–329, 2000 [DOI] [PubMed] [Google Scholar]
- 26.D'Orleans-Just P, Labonte J, Bkaily G, Choufani S, Plante M, Honore JC. Function of the endothelin (B) receptor in cardiovascular physiology and pathophysiology. Pharmacol Ther 95: 221–238, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Dziedzicka-Wasylewska M, Faron-Gorecka A, Andrecka J, Polit A, Kusmider M, Wasylewski Z. Fluorescence studies reveal heterodimerization of dopamine D1 and D2 receptors in plasma membrane. Biochemistry 45: 8751–8759, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Evans NJ, Walker JW. Endothelin receptor dimers evaluated by FRET, ligand binding, and calcium mobilization. Biophys J 95: 483–492, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evans NJ, Walker JW. Sustained Ca2+ signaling and delayed internalization associated with endothelin receptor heterodimers linked through PDZ finger. Can J Physiol Pharmacol 86: 526–535, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Fecteau MH, Honore JC, Plante M, Labote J, Rae GA, D'Orleans-Juste Pedro. Endothelin-1 (1–31) is an intermediate in the production of endothelin-1 after big endothelin-1 administration in vivo. Hypertension 46: 87–92, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Feldstein C, Romero C. Role of endothelins in hypertension. Am J Ther 14: 147–153, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Fukuroda T, Ozaki S, Ihara M, Ishikawa K, Yano M, Miyauchi T, Ishikawa S, Onizuka M, Goto K, Nishikibe M. Necessity of dual blockade of endothelin ETA and ETB receptor subtypes for antagonism of endothelin-1-induced contraction in human bronchi. Br J Pharmacol 117: 959–999, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gershon MD. Endothelin and the development of the enteric nervous system. Clin Exp Pharmacol Physiol 26: 985–988, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Gouldson PR, Higgs C, Smith RE, Dean MK, Gkoutos GV, Reynolds CA. Dimerization and domain swapping in G-protein-coupled receptors: a computational study. Neuropsychopharmacol 23, Suppl 4: S60–S77, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Granger JP, Abram S, Stec D, Chandler D, LaMarca B. Endothelin, the kidney and hypertension. Curr Hypertens Rep 8: 298–303, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Gregan B, Jurgensen J, Papsdorf G, Furkert J, Schaefer M, Beyermann M, Rosenthal W, Oksche A. Ligand-dependent differences in the internalization of endothelin A and endothelin B receptor heterodimers. J Biol Chem 279: 27679–27687, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Grimshaw MJ. Endothelins and hypoxia inducible factor in cancer. Endocr Relat Cancer 14: 233–244, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Guise TA, Yin JJ, Mohammad KS. Role of endothelin-1 in osteoblastic bone metastases. Cancer Suppl 97: 779–784, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Gurevich VV, Gurevich EV. How and why do GPCRs dimerize? Trends Pharmacol Sci 29: 234–240, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hague C, Lee SE, Chen Z, Prinster SC, Hall RA, Minneman KP. Heterodimers of α1B and α1D adrenergic receptor form a single functional entity. Mol Pharmacol 69: 45–55, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Hans G, Deseure K, Adriaensen H. Endothelin-1 induced pain and hyperalgesia: a review of pathophysiology, clinical manifestations and future therapeutic options. Neuropeptides 42: 119–132, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Hirada N, Himeno A, Shigematsu K, Sumikawa K, Niwa M. Endothelin-1 binding to endothelin receptors in the rat anterior pituitary gland: possible formation of an ETA-ETB receptor heterodimer. Cell Mol Neurobiol 22: 207–226, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hinojosa-Laborde C, Lange DL. Endothelin regulation of adrenal function. Clin Exp Pharmacol Physiol 26: 995–999, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Holtke C, Waldeck J, Kopka K, Heindel W, Schober O, Schaffers M, Bremer C. Biodistribution of a nonpeptidic fluorescent endothelin A receptor imaging probe. Mol Imaging 8: 27–34, 2009 [PubMed] [Google Scholar]
- 45.Huang SC. Endothelin receptor in gastrointestinal smooth muscle. Curr Protein Pept Sci 6: 547–557, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Huang SC, Chang BS. Endothelin causes contraction of human esophageal muscularis mucosae through interaction with both ETA and ETB receptors. Regul Pept 117: 179–186, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Hyndman KA, Miyamoto MM, Evans DH. Phylogeny, taxonomy, and evolution of the endothelin receptor gene family. Mol Phylogen Evol 52: 677–687, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Imai Y, Soda M, Inoue H, Hatori N, Mizuno Y, Takahashi R. An unfolded putative transmembrane polypeptide, which can lead to endoplasmic reticulum stress, is a substrate of Parkin. Cell 105: 891–902, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Inscho EW, Imig JD, Cook AK, Pollock DM. ETA and ETB receptors differentially modulate afferent and efferent arteriolar responses to endothelin. Br J Pharmacol 146: 1019–1026, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalani M. The importance of endothelin-1 for microvascular dysfunction in diabetes. Vas Health Risk Manag 4: 1061–1068, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaneko Y, Herrera VLM, Didishvilli T, Ruiz-Opazo N. Sex-specific effects of dual ET-1/ANG II receptor (DEAR) variants in Dahl salt-sensitive hypertension rat model. Physiol Genomics 20: 157–164, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Karne S, Jayawickreme CK, Lerner MR. Cloning and characterization of an endothelin-3 specific receptor (ETC receptor) from Xenopus laevis dermal melanophores. J Biol Chem 268: 19126–19133, 1933 [PubMed] [Google Scholar]
- 53.Kenakin T. Functional selectivity through protean and biased agonism: who steers the ship? Mol Pharmacol 72: 1393–1401, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Kenakin T. Collateral efficacy in drug discovery: taking advantage of the good (allosteric) nature of 7 TM receptors. Trends Pharmacol Sci 28: 407–415, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Khan ZA, Chakrabarti S. Endothelins in chronic diabetic complications. Can J Physiol Pharmacol 81: 622–634, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Khodorova A, Montmayeur JP, Strichartz G. Endothelin receptors and pain. J Pain 10: 4–28, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kockskamper J, Seidlmayer L, Walther S, Hellenkamp K, Maier LS, Pieske B. Endothelin-1 enhances nuclear Ca2+ transients in atrial myocytes through Ins(1,4,5)P3-dependent Ca2+ release from perinuclear Ca2+ stores. J Cell Sci 121: 186–195, 2008 [DOI] [PubMed] [Google Scholar]
- 58.Kohan DE. Endothelins: renal tubule synthesis and actions. Clin Exp Pharmacol Physiol 23: 337–344, 1996 [DOI] [PubMed] [Google Scholar]
- 59.Kumar CS, Nuthulaganti P, Pullen M, Nambi P. Novel endothelin receptors in the follicular membranes of Xenopus laevis oocytes mediate calcium responses by signal transduction through gap junctions. Mol Pharmacol 44: 153–157, 1993 [PubMed] [Google Scholar]
- 60.Lam HC, Lee JK, Lu CC, Chu CH, Chuang MJ, Wang MC. Role of endothelin in diabetic retinopathy. Curr Vasc Pharmacol 1: 243–250, 2003 [DOI] [PubMed] [Google Scholar]
- 61.Lange M, Pagotto U, Renner U, Arzberger T, Oeckler R, Stalla GK. The role of endothelins in the regulation of pituitary function. Exp Clin Endocrinol Diabetes 110: 103–112, 2002 [DOI] [PubMed] [Google Scholar]
- 62.Laplante MA, de Champlain J. The interrelation of the angiotensin and endothelin systems on the modulation of NAD(P)H oxidase. Can J Physiol Pharmacol 84: 21–28, 2006 [DOI] [PubMed] [Google Scholar]
- 63.Lariviere R, Lebel M. Endothelin-1 in chronic renal failure and hypertension. Can J Physiol Pharmacol 81: 607–621, 2003 [DOI] [PubMed] [Google Scholar]
- 64.Lattig J, Oksche A, Beyermann M, Rosenthal W, Krause G. Structural determinants for selective recognition of peptide ligands for endothelin receptor subtypes ETA and ETB. J Pept Sci 15: 479–491, 2009 [DOI] [PubMed] [Google Scholar]
- 65.LeMarca BD, Alexander BT, Gilbert JS, Ryan MJ, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension in response to placental ischemia during pregnancy: a central role for endothelin? Gend Med 5: S133–S138, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Levoye A, Dam J, Aoub MA, Guillame JL, Jockers R. Do orphan G protein coupled receptor have ligand-independent functions? New insights from receptor heterodimers. EMBO Rep 7: 1094–1098, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lodge NJ, Zhang R, Halaka NN, Moreland S. Functional role of endothelin ETA and ETB receptors in venous and arterial smooth muscle. Eur J Pharmacol 287: 279–285, 1995 [DOI] [PubMed] [Google Scholar]
- 68.Makita T, Sucov HM, Gariepy CE, Yanagisawa M, Ginty DD. Endothelins are vascular-derived axonal guidance cues for developing sympathetic neurons. Nature 452: 759–763, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Malik R, Vlasicova K, Sedo A. Functional cross talk of Ca2+-mobilizing endothelin receptors in C6 gloma cells. Physiol Res 51: 73–78, 2002 [PubMed] [Google Scholar]
- 70.Mallat A, Lotersztajn S. Multiple hepatic functions of endothelin-1: physiopathological relevance. J Hepatol 25: 405–413, 1996 [DOI] [PubMed] [Google Scholar]
- 71.Marazziti D, Golini E, Gallo A, Lombardi MS, Matteoni R, Tocchini-Valentini GP. Cloning of GPR37, a gene located on chromosome 7 encoding a putative G protein coupled peptide receptor, from a human frontal brain EST library. Genomics 45: 68–77, 1997 [DOI] [PubMed] [Google Scholar]
- 72.Marinissen JJ, Gutkind JS. G-protein-coupled receptors and signaling networks: emerging paradigms. Trends Pharmacol Sci 22: 368–376, 2001 [DOI] [PubMed] [Google Scholar]
- 73.Masaki T, Ninomiva H, Sakamoto A, Okamoto Y. Structural basis of the function of endothelin receptor. Med Cell Biochem 190: 153–156, 1999 [PubMed] [Google Scholar]
- 74.Masaki T, Vane JR, Vanhoutte PM. International Union of Pharmacology nomenclature of endothelin receptors. Pharmacol Rev 46: 137–142, 1994 [PubMed] [Google Scholar]
- 75.Meiden R, Levy N. The ovarian endothelin network: an evolving story. Trends Endocrinol Metab 18: 379–385, 2007 [DOI] [PubMed] [Google Scholar]
- 76.Mencarelli M, Pecorreli A, Carbotti P, Valacchi G, Grasso G, Muscetoola M. Endothelin receptor A expression in human inflammatory cells. Regul Pept 158: 1–5, 2009 [DOI] [PubMed] [Google Scholar]
- 77.Michel RP, Langleben D, Dupuis J. The endothelin system in pulmonary hypertension. Can J Physiol Pharmacol 81: 542–554, 2003 [DOI] [PubMed] [Google Scholar]
- 78.Mickley EJ, Gray GA, Webb DJ. Activation of endothelin ETA receptors masks the constrictor role of endothelin ETB receptors in rat isolated small mesenteric arteries. Br J Pharmacol 120: 1376–1382, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Minneman KP. Heterodimerization and surface localization of G protein coupled receptor. Biochem Pharmacol 73: 1043–1050, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mizuguchi T, Nishiyama M, Moroi K, Tanaka H, Saito T, Masuda Y, Masaki T, de Wit D, Yanagisawa M, Kimura S. Analysis of two pharmacologically predicted endothelin B receptor subtypes by using the endothelin B receptor gene knockout mouse. Br J Pharmacol 120: 1427–1430, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moe GW, Rouleau JL, Nguyen QT, Cernacek P, Stewart DJ. Role of endothelins in congestive heart failure. Can J Physiol Pharmacol 81: 588–597, 2003 [DOI] [PubMed] [Google Scholar]
- 82.Mortensen LH. Endothelin and the central and peripheral nervous systems: a decade of endothelin research. Clin Exp Pharmacol Physiol 26: 980–984, 1999 [DOI] [PubMed] [Google Scholar]
- 83.Mullol J, Picado C. Endothelin in nasal mucosa: role in nasal function and inflammation. Clin Exp Allergy 30: 172–177, 2000 [DOI] [PubMed] [Google Scholar]
- 84.Naik RD, McNeill JR, Wilson TW, Gopalakrishnan V. Nuclear Ca2+ signaling in endothelin-1 in rat aortic smooth muscle cells. J Cardiovasc Pharmacol 31: S199–S202, 1998 [DOI] [PubMed] [Google Scholar]
- 85.Nelson J, Bagnato A, Battistini B, Nisen P. The endothelin axis: emerging role in cancer. Nat Rev Cancer 3: 110–116, 2003 [DOI] [PubMed] [Google Scholar]
- 86.Neuhofer W, Gulberg V, Gerbes AL. Endothelin and endothelin receptor antagonism in portopulmonary hypertension. Eur J Clin Invest 36: 54–61, 2006 [DOI] [PubMed] [Google Scholar]
- 87.Neuhofer W, Pittrow D. Role of endothelin and endothelin receptor antagonists in renal disease. Eur J Clin Invest 36: 78–88, 2006 [DOI] [PubMed] [Google Scholar]
- 88.Ohlstein EH, Elliott JD, Feuerstein GZ, Ruffolo RR. Endothelin receptors: receptor classification, novel receptor antagonists, and potential therapeutic targets. Med Res Rev 16: 365–390, 1996 [DOI] [PubMed] [Google Scholar]
- 89.Pate MA, Chester AH, Brown TJ, Roach AG, Yacoub MH. Atypical antagonism observed with BQ-123 in human saphenous vein. J Cardiovasc Pharmacol 31, Suppl 1: S172–S174, 1998 [DOI] [PubMed] [Google Scholar]
- 90.Patocka J, Merka V, Hrdina V, Hrdina R. Pharmacological potential of endothelin receptors agonists and antagonists. Acta Medica 48: 67–73, 2005 [PubMed] [Google Scholar]
- 91.Pollock DM, Keith TL, Highsmith RF. Endothelin receptors and calcium signaling. FASEB J 9: 1196–1204, 1995 [DOI] [PubMed] [Google Scholar]
- 92.Prinster SC, Holmqvist TG, Hall RA. Alpha2c adrenergic receptors exhibit enhanced surface expression and signaling upon association with β2-adrenergic receptors. J Pharmacol Exp Ther 318: 974–981, 2006 [DOI] [PubMed] [Google Scholar]
- 93.Richter CM. The role of endothelin in chronic renal failure–developments in renal involvement. Rheumatology 45: iii36–iii38, 2006 [DOI] [PubMed] [Google Scholar]
- 94.Rosano L, Cianfrocca R, Masi S, Spinella F, Di Castro V, Biroccio A, Salvati E, Nicotra MR, Natali PG, Bagnato A. β-arrestin links endothelin A receptor to β-catenin signaling to induce ovarian cancer cell invastion and metastasis. Proc Natl Acad Sci USA 106: 2806–2811, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rossi GP, Andreis PG, Colonna S, Albertin G, Aragona F, Belloni AS, Nussdorfer GG. Endothelin-1[1–31]: a novel autocrine-paracrine regulator of human adrenal cortex secretion and growth. J Clin Endocrinol Metab 87: 322–328, 2002 [DOI] [PubMed] [Google Scholar]
- 96.Rossi GP, Belloni AS, Nussdorfer GG, Pessina AC. Endothelin and the adrenal gland. J Cardiovasc Pharmacol 35: S17–S20, 2000 [DOI] [PubMed] [Google Scholar]
- 97.Ruiz-Opazo N, Hirayam K, Akimoto K, Herrera VLM. Molecular characterization of a dual endothelin-1/angiotensin II receptor. Mol Med 4: 96–108, 1998 [PMC free article] [PubMed] [Google Scholar]
- 98.Russell FD, Molenaar P. The human heart endothelin system: ET-1 synthesis, storage, release and effect. Trends Pharmacol Sci 21: 353–359, 2000 [DOI] [PubMed] [Google Scholar]
- 99.Sakurai T, Yanagisawa M, Takuwa Y, Miyazaki H, Kmura S, Goto K, Masaki T. Cloning of a cDNA encoding a non-isopeptide selective subtype of the endothelin receptor. Nature 348: 732–735, 1990 [DOI] [PubMed] [Google Scholar]
- 100.Sakurai T, Yanagisawa M, Masaki T. Molecular characterization of endothelin receptors. Trends Pharmacol Sci 13: 103–108, 1992 [DOI] [PubMed] [Google Scholar]
- 101.Sauvageau S, Thorin E, Caron A, Dupuis J. Evaluation of endothelin-1-induced pulmonary vasoconstriction following myocardial infarction. Exp Biol Med (Maywood) 231: 840–846, 2006 [PubMed] [Google Scholar]
- 102.Sauvageau S, Thorin E, Caron A, Dupuis J. Endothelin-1-induced pulmonary vasoreactivity is regulated by ETA and ETB receptor interactions. J Vasc Res 44: 375–381, 2007 [DOI] [PubMed] [Google Scholar]
- 103.Schinelli S. Pharmacology and physiopathology of the brain endothelin system: an overview. Curr Med Chem 13: 627–638, 2006 [DOI] [PubMed] [Google Scholar]
- 104.Schneider MP, Boesen EO, Pollock DM. Contrasting actions of endothelin ETA and ETB receptors in cardiovascular disease. Ann Rev Pharmacol Toxicol 47: 731–759, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sexton PM, Morfis M, Tilakaratne N, Hay DL, Udawela M, Christopoulos G, Christopoulos A. Complexing receptor pharmacology: modulation of family B G protein-coupled receptors by RAMPs. Ann NY Acad Sci 1070: 90–104, 2006 [DOI] [PubMed] [Google Scholar]
- 106.Shen B, Harrison-Bernard LM, Fuller AJ, Vanderpool V, Saifudeen Z, El-Dahr SS. The bradykinin B2 receptor gene is a target of angiotensin II type 1 receptor signaling. J Am Soc Nephrol 18: 1140–1149, 2007 [DOI] [PubMed] [Google Scholar]
- 107.Shyamala V, Moulthrop TH, Stratto-Thomas J, Takamp-Olson P. Two distinct human endothelin B receptors generated by alternative splicing from a single gene. Cell Mol Biol Res 40: 285–296, 1994 [PubMed] [Google Scholar]
- 108.Sticherling M. The role of endothelin in connective tissue diseases. Rheumatology 45: iii8–iiii10, 2006 [DOI] [PubMed] [Google Scholar]
- 109.Sokolvsky M. Endothelin receptor subtypes and their role in transmembrane signaling mechanisms. Pharmacol Ther 68: 435–471, 1995 [DOI] [PubMed] [Google Scholar]
- 110.Sokolovsky M. Endothelin receptor heterogeneity, G-proteins, and signaling via camp and cGMP cascades. Cell Mol Neurobiol 15: 561–570, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Terrillon S, Barberis C, Bouvier M. Heterodimerization of the V1a and V2 vasopressin receptors determines the interaction with beta-arrestin and their trafficking patterns. Proc Natl Acad Sci USA 101: 1548–1553, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tostes RC, Fortes ZB, Callera GE, Montezano AC, Touyz RM, Webb RC, Carvalho MH. Endothelin, sex and hypertension. Clin Sci 114: 85–97, 2008 [DOI] [PubMed] [Google Scholar]
- 113.Touyz RM, Schiffrin EL. Role of endothelin in human hypertension. Can J Physiol Pharmacol 81: 533–541, 2003 [DOI] [PubMed] [Google Scholar]
- 114.Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, Miller KJ, Spedding M, Mailman RB. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther 320: 1–13, 2007 [DOI] [PubMed] [Google Scholar]
- 115.Violin JD, Lefkowtiz RJ. β-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci 28: 416–422, 2007 [DOI] [PubMed] [Google Scholar]
- 116.Von Websky K, Heiden S, Pfab T, Hocher B. Pathophysiology of the endothelin system–lessons from genetically manipulated animal models. Eur J Med Res 28: 1–6, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Waldhoer M, Fong J, Jones RM, Lunzer MM, Sharma SK, Kostenis E, Portoghese PS, Whistler JL. A heterodimer-selective agonists shows in vivo relevance of G protein-coupled receptor dimers. Proc Natl Acad Sci USA 102: 9050–9055, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Watts SW, Priestley JRC, Thompson JM. Serotonylation of vascular proteins important to contraction. PLoS One 4: e5682, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wilkie TM. Treasures throughout the life-cycle of G protein coupled receptors. Trends Pharmacol Sci 22: 396–397, 2001 [Google Scholar]
- 120.Wilson SJ, Dowling JK, Zhao L, Carnish E, Smyth EM. Regulation of thromboxane receptor trafficking through the prostacyclin receptor in vascular smooth muscle cells: role of receptor heterodimerization. Arterioscler Thromb Vasc Biol 27: 290–296, 2007 [DOI] [PubMed] [Google Scholar]
- 121.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332: 411–415, 1988 [DOI] [PubMed] [Google Scholar]
- 122.Yu C, Yang Z, Ren H, Zhang Y, Han Y, He D, Lu Q, Wang X, Wang X, Yang C, Asico LD, Hopfer U, Eisner GM, Jose PA, Zeng C. D3 dopamine receptor regulation of ETB receptors in renal proximal tubule cells from WKY and SHRs. Am J Hypertens 22: 877–883, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang A, Liu Z, Kan Y. Receptor dimerization–rationale for the design of bivalent ligands. Curr Top Med Chem 7: 3343–345, 2007 [DOI] [PubMed] [Google Scholar]