Abstract
The prevalence of cardiovascular disease is lower in middle-aged and older women than men. Increased endothelin-1-mediated vasoconstriction has been linked to the etiology of a number of cardiovascular diseases, including atherosclerosis, heart failure, and hypertension. It is unknown whether a sex difference in endothelin-1-mediated vasoconstrictor tone exists in middle-aged and older adults. Therefore, we tested the hypothesis that middle-aged and older men would demonstrate greater ET-1-mediated vasoconstrictor tone than age-matched women. Forearm blood flow in response to intra-arterial infusions of endothelin (ET)-1, BQ-123 (a selective ETA receptor antagonist), and BQ-788 (a selective ETB receptor antagonist) was assessed by venous occlusion plethysmography in 21 women (age: 58 ± 1 yr; body mass index: 26.0 ± 1.0 kg/m2) and 25 men (age: 57 ± 2 yr; body mass index: 26.8 ± 0.7 kg/m2). In response to BQ-123, the increase in forearm blood flow from baseline was significantly higher in the men than the women (24 ± 5% vs. 9 ± 5%; P < 0.05). In contrast, the increase in forearm blood flow in response to BQ-123 coinfused with BQ-788 was greater in the women than the men, such that the maximum vasodilation to dual endothelin receptor blockade was similar between men and women (∼25%). There was no difference in the vasoconstrictor response to ET-1 between the sexes. These results indicate that middle-aged and older men are under greater ETA receptor-mediated vasoconstrictor tone than age-matched women. Since the ETA receptor is the predominant receptor subtype in the coronary vasculature, this sex difference in vasoconstrictor tone may be a mechanism contributing to the sex difference in the prevalence of coronary heart disease in middle-aged and older adults.
Keywords: endothelin-1, endothelin receptor antagonist, endothelium, vascular function, sex differences
endothelin-1 (et-1) is the most abundant and important vasoconstrictor molecule released from the vasculature. Produced by the endothelium, ET-1 is predominantly released abluminally to activate ETA and ETB receptors located on the vascular smooth muscle causing smooth muscle contraction, cell proliferation, and hypertrophy (35). ETB receptors on the vascular endothelium produce vasodilation via a nitric oxide mechanism and are also a prominent clearance mechanism for circulating ET-1. ETA receptors are the predominant subtype [10 times greater number than ETB (37)] in the arterial system and the coronary arteries, in particular (30, 31, 34). Because of this disparity in receptor number, the contribution of the ETB receptor to coronary vasoregulation is limited (25). Importantly, ET-1 expression is elevated in atherosclerotic vessels (18, 32), and ET-1-mediated vasoconstriction has been implicated in the pathogenesis of atherosclerotic vascular disease, including coronary heart disease (9, 20, 26, 40).
A significant sex difference in the incidence and prevalence of atherosclerotic vascular disease is found in middle-aged and older adults. Between the ages of 45 and 65 years, men have an annual incidence of cardiovascular disease (CVD), which is over twice that in women (24) and myocardial infarction, which is almost three times that of women (250,000 vs. 88,000) (1). The mechanism behind this sex difference in CVD remains unidentified. We have previously demonstrated that ET-1-mediated vasoconstrictor tone increases with age in men, suggesting that ET-1 production and vasoconstrictor tone may contribute to the increased coronary heart disease risk in middle-aged men (41). Currently, it is unknown whether a sex difference in ET-1-mediated vasoconstrictor tone exists. If so, this may contribute to the sex difference in coronary heart disease in middle-aged and older adults. Therefore, we tested the hypothesis that middle-aged and older men would demonstrate greater ET-1-mediated vasoconstrictor tone than age-matched women.
MATERIALS AND METHODS
Subjects.
Forty-six healthy, sedentary middle-aged and older adults participated in the study: 21 women (age range: 50 to 71 years) and 25 men (age range 45 to 70). All the subjects were nonobese (body mass index ≤30 kg/m2), normotensive (blood pressure ≤140/90 mmHg), nonsmokers, nonmedicated, and free of overt cardiovascular, metabolic, and hematologic disease assessed by medical history, resting and exercise electrocardiograms, and fasting blood chemistries. The women were at least 1 yr postmenopausal (range 1 to 20 years) and had never taken or had discontinued use of hormone therapy at least 1 yr before the start of the study. Before participation the subjects provided written informed consent according to the guidelines of the University of Colorado at Boulder. All experimental protocols adhere to the principles of the Declaration of Helsinki and were approved by the Human Research Committee of the University of Colorado at Boulder.
Body composition and metabolic measurements.
Body mass was measured to the nearest 0.1 kg with a medical beam balance. The percentage of body fat was determined by dual energy X-ray absorptiometry (Lunar). Body mass index (BMI) was calculated as weight (kg) divided by height (m) squared. Minimal waist circumference was measured as previously described (41). Fasting plasma glucose, insulin, lipid, and lipoprotein concentrations were determined with standard techniques.
Intra-arterial infusion protocol.
All experimental infusion protocols were performed in a temperature-controlled room between 7 AM and 10 AM after a 12-h overnight fast. Under strict aseptic conditions and following local anesthesia (1% lidocaine), a 5-cm, 3 French catheter was inserted into the brachial artery of the nondominant arm. Arterial blood pressure and heart rate were continuously measured throughout the infusion protocol. Forearm blood flow (FBF) was measured with strain-gauge venous occlusion plethysmography (D. E. Hokanson, Bellevue, WA) at rest and in response to each pharmacological agent as previously described (10). Baseline FBF was measured for 5 min and for 5 min prior to each drug infusion. To exclude the possibility of nonspecific differences in the response to vasoconstrictor agents between sexes, the vascular response to norepinephrine was determined. Norepinephrine was infused at a rate of 260 pmol/min for 5 min with the FBF response measured during the final 3 min of the infusion.
Following a 20-min recovery period to allow FBF to return to baseline levels, ET-1 (Clinalfa, AG) was infused at a rate of 5 pmol/min for 20 min with the FBF response measured during the final 3 min of the infusion. Following a 30-min recovery period to allow FBF to return to baseline levels, BQ-123 (Clinalfa, AG), a selective ETA receptor antagonist, was infused at a rate of 100 nmol/min for 60 min with the FBF response measured every 10 min during the infusion. The dose of BQ-123 has been demonstrated to completely inhibit the vasoconstrictor effect of ET-1 in the human forearm of healthy adults (5, 8). After completing 60 min of ETA receptor blockade, FBF in response to nonselective ET-1 receptor blockade was evaluated with coadministration of BQ-123 and BQ-788 (Clinalfa, AG), a selective ETB receptor antagonist, for an additional 50 min. BQ-788 was infused at a rate of 50 nmol/min, a dose demonstrated to effectively inhibit ETB receptors (6). Because of difficulties with drug availability, studies demonstrating the effect of BQ-788 alone on FBF were unable to be performed. Identical sequential infusion protocols (BQ-123 followed by BQ-123 coadministered with BQ-788) have previously been reported by us and others to reliably determine vasomotor responses with aging (41), hypertension (8), diabetes (5), and hypercholesterolemia (7).
Statistical analysis.
Differences in subject baseline characteristics and the FBF response to norepinephrine and ET-1 were determined by between-group ANOVA. Group differences in FBF responses to BQ-123 and BQ-123 combined with BQ-788 were determined by repeated-measures ANOVA. Relations between variables of interest were assessed by linear regression analysis. All data are expressed as means ± SE. Statistical significance was set a priori at P < 0.05.
RESULTS
Baseline characteristics.
Selected subject characteristics are presented in Table 1. Although none of the subjects were hypertensive or dyslipidemic, the resting systolic blood pressure was higher, and the HDL was lower in the men (P < 0.05). While BMI was similar between groups, the men demonstrated greater body mass and waist circumference and the women had higher percent body fat.
Table 1.
Selected subject characteristics
| Men | Women | |
|---|---|---|
| Variable | (n = 25) | (n = 21) |
| Age, yr | 57 ± 2 | 58 ± 1 |
| Years postmenopausal, yr | —- | 8 ± 1 |
| Body mass, kg | 85.4 ± 2.4 | 69.9 ± 3.0* |
| Body fat, % | 27.0 ± 1.4 | 41.1 ± 1.6* |
| Body mass index, kg/m2 | 26.7 ± 0.7 | 26.0 ± 1.0 |
| Waist circumference, cm | 95 ± 2 | 83 ± 2* |
| SBP, mmHg | 126 ± 2 | 120 ± 2* |
| DBP, mmHg | 80 ± 2 | 77 ± 1 |
| Total cholesterol, mmol/l | 5.1 ± 0.2 | 5.3 ± 0.2 |
| HDL cholesterol, mmol/l | 1.2 ± 0.1 | 1.5 ± 0.1* |
| LDL cholesterol, mmol/l | 3.3 ± 0.1 | 3.2 ± 0.2 |
| Triglycerides, mmol/l | 1.4 ± 0.1 | 1.3 ± 0.1 |
| Glucose, mmol/l | 5.2 ± 0.1 | 5.0 ± 0.1 |
| Insulin, pmol/l | 35.0 ± 4.3 | 40.4 ± 5.4 |
Values are expressed as means ± SE. HDL, high-density lipoprotein; LDL, low-density lipoprotein; SBP, systolic blood pressure; DBP diastolic blood pressure.
P < 0.05 vs. men.
FBF responses to selective and nonselective ET-1 receptor blockade.
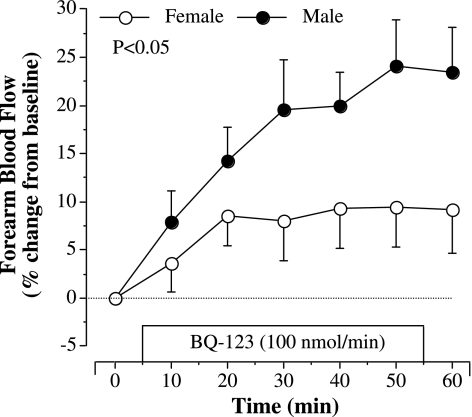
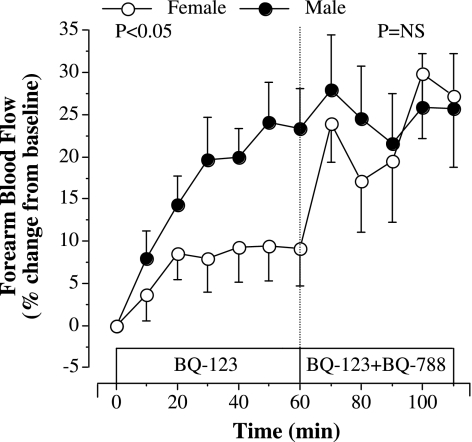
The FBF responses to selective ETA receptor blockade (BQ-123) were substantially different (P < 0.05) between groups (Fig. 1). In response to BQ-123, FBF increased ∼10% in women and ∼25% in men. The addition of BQ-788 to BQ-123 did not significantly affect the FBF response to BQ-123 in the men. However, in women, adding BQ-788 to BQ-123 resulted in an additional ∼20% increase in FBF over that demonstrated with BQ-123 alone (Fig. 2). As a result, the overall increase in FBF after the completed infusion protocol (BQ-123 for 60 min followed by BQ-788 for 50 min) was similar between the men and women. There were no correlations between FBF responses to BQ-123 or BQ-123 combined with BQ-788 with any anthropometric, hemodynamic, or metabolic parameters, or years after menopause. There were no changes in FBF in the noninfused control arm or in systemic blood pressure in response to the infusion protocol (data not shown).
Fig. 1.
Forearm blood flow (FBF) response to ETA receptor blockade with BQ-123 (100 nmol/min) in healthy middle-aged and older men and women. The P value refers to differences in the FBF response between the groups. Values are expressed as means ± SE.
Fig. 2.
Forearm blood flow responses to BQ-123 alone and BQ-123 combined with BQ-788 (50 nmol/min) in healthy middle-aged and older men and women. The P values refer to differences in the FBF response to selective ETA receptor antagonism (BQ-123) and dual ETA and ETB receptor blockade with BQ-123 combined with BQ-788. Values are expressed as means ± SE.
FBF responses to norepinephrine and ET-1.
There was no sex difference in the ET-1-mediated vasoconstrictor response (Women: 11 ± 3% and Men: 7 ± 3%, P = 0.32). The maximum vasoconstriction generated in response to norepinephrine was also not different between groups (Women: 3.0 ± 0.2 vs. Men: 3.3 ± 0.2 ml/100 ml tissue/min, P = 0.42).
DISCUSSION
The primary new findings of the current study are significant sex differences in ETA and ETB-receptor-mediated vasoconstrictor tone in healthy middle-aged and older adults. Men demonstrate greater ETA-mediated vasoconstrictor tone than women. In contrast, the contribution of the ETB receptor to ET-1-mediated vasoconstriction appears to be greater in women. Surprisingly, there was no difference in vasoconstriction to ET-1 between the sexes.
Elevated ET-1 mediated vasoconstrictor tone has been mechanistically linked to atherosclerotic vascular disease, as well as to several risk factors for vascular disease, such as hypertension. There are several possible mechanisms, whereby ET-1 promotes vascular disease. First, ET-1 is a powerful mitogen eliciting an inflammatory response in the vasculature (3, 39). Second, ET-1 immunoreactivity is enhanced in the walls of atherosclerotic human vessels (23, 33). Finally, ET-1 promotes fibrous tissue formation and inhibits endothelial NO synthesis (15, 22).
In humans, the vascular actions of ET-1 are mediated by two distinct ET receptor subtypes (ETA and ETB receptors). ETA receptors are located only on vascular smooth muscle cells, while ETB receptors are located on both vascular smooth muscle and endothelial cells. Activation of ETA or ETB receptors on smooth muscle causes contraction and subsequent vasoconstriction. In contrast, ETB receptor activation on the endothelial surface results in NO-mediated vasodilation. Thus, ETB receptors can lead to dual vasoregulatory effects. ET-1-mediated vasomotor effects are receptor dependent and therefore depend on receptor affinity, types of receptors activated, and number of receptors in a vascular bed.
In the present study, we have demonstrated for the first time that middle-aged and older men are under greater ETA-mediated vasoconstrictor tone and women have a greater vasoconstrictor response with the addition of ETB receptor blockade. Since, ETA receptors are the primary subtype in the coronary vasculature of both men and women (4, 13) and ET-1-mediated vasomotor effects are entirely receptor dependent, it is likely that coronary arteries in men are under greater ET-1-mediated vasoconstrictor tone than in women. The vasomotor response to the addition of BQ-788 to BQ-123 requires more evaluation to completely understand the mechanism underlying the sex difference.
Atypical endothelin receptor ligand binding properties have been demonstrated in a number of cell types coexpressing ETA and ETB receptors (11, 16, 21). Further investigation in cell culture using coimmunoprecipitation and fluorescence resonance energy transfer techniques have demonstrated that endothelin receptors form ETA/ETB heterodimers (12, 14). In human embryonic kidney 293 cells, functional differences between homodimers and heterodimers have been found (12). Considering that in the vasculature, only the smooth muscle layer demonstrates coexpression of both ET receptors (36), it is tempting to suggest that these functional differences may provide an additional layer of physiological regulation for smooth muscle contraction. However, while it remains unknown whether these heterodimers play an important role in the vasculature, a sex difference in heterodimer receptor formation (i.e., more ETA/ETB heterodimer receptors in women than men) could also explain our findings.
Sex steroids have been demonstrated to influence ET-1 production and ET-1-mediated vasoconstrictor tone. Estrogen suppresses ET-1 expression in a number of models, including humans (17, 19, 29, 43, 44). In addition, estrogen treatment of coronary artery rings reduces ET-1-mediated vasoconstriction (38). Because all of the women in the present study were postmenopausal and we found no significant relation (r = −0.05) between years after menopause and response to ETA receptor blockade, it is unlikely that residual estrogen effects are responsible for the sex differences in FBF to selective ETA antagonism. In contrast, testosterone increases ET-1 release from endothelial cells (29, 45) and potentiates ET-1-mediated coronary artery vasoconstriction (2, 38). It is possible that the augmented ETA-receptor-mediated vasoconstriction in the men in our study is due, at least in part, to the influence of testosterone. Unfortunately, we did not measure hormone levels in either sex; thus, further study of sex steroids and ET receptor subtype-mediated vasomotor regulation is needed to understand these potential interactions more completely.
There are important experimental considerations in the current study. Because of the cross-sectional study design, it is possible that lifestyle behaviors may have influenced our results. We attempted to minimize potential lifestyle influences by studying men and women of similar age who were sedentary, nonmedicated, nonsmokers without evidence of cardiometabolic abnormalities that are commonly associated with elevated ET-1 system activity, such as hypertension (8) or type 2 diabetes (5). The FBF response to BQ-123 alone was not evaluated between 60 and 120 min. Therefore, it is possible that the observed FBF response in women is simply a delayed response to ETA receptor blockade. However, the remarkably stable plateau in FBF from 20 to 60 min of the BQ-123 infusion and the subsequent change in FBF that is temporally related to the initiation of BQ-788 make a delayed ETA receptor response unlikely. It is possible that the differences identified may be due to differences in adipose tissue distribution between the men and women (42). A larger study would be necessary to determine the influence of regional adiposity on sex differences in FBF. Finally, from a clinical perspective, it is important to note that potential regional differences in ET receptor expression may limit our findings from the forearm to other vascular beds. However, there are significant parallels between vascular function in the forearm and in the coronaries (27, 28). Moreover, it is well documented that ETA is the predominant receptor subtype in the heart and coronary arteries of both men and women (4, 13, 30, 31, 34). Thus, regardless of the receptor density in the forearm, the greater degree of ETA-mediated vasoconstriction we have demonstrated in the men is likely to result in greater ET-1-mediated activity in the coronary vasculature in men.
Perspectives and Significance
The current investigation demonstrates for the first time a significant sex difference in endothelin receptor control of vasomotor function. Middle-aged and older men are under greater ETA-mediated vasoconstrictor tone than women. Since the ETA receptor predominates in the coronary circulation and endothelin-1-mediated vasoconstrictor tone has been linked to cardiovascular events, the observed sex-difference in vasoconstrictor tone may be responsible, in part, for the sex differences in coronary heart disease in middle-aged and older adults. Further investigation will be necessary to determine whether the sex difference in endothelin-mediated vasoconstrictor tone may provide a therapeutic option to improve cardiovascular event rates in adults.
GRANTS
This study was supported by National Institutes of Health Grants K08 HL080212, R01 HL077450, R01 HL076434, M01 RR00051, and UL1 RR025780.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank all of the subjects that participated in this study and Yoli Casas for her technical assistance.
REFERENCES
- 1.American Heart Association Heart Disease and Stroke Statistics: 2005 Update, http://www.americanheart.org, 2005
- 2.Ammar EM, Said SA, Hassan MS. Enhanced vasoconstriction and reduced vasorelaxation induced by testosterone and nandrolone in hypercholesterolemic rabbits. Pharmacol Res 50: 253–259, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Ammarguellat FZ, Gannon PO, Amiri F, Schiffrin EL. Fibrosis, matrix metalloproteinases, and inflammation in the heart of DOCA-salt hypertensive rats: role of ET(A) receptors. Hypertension 39: 679–684, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Bax WA, Bruinvels AT, van Suylen RJ, Saxena PR, Hoyer D. Endothelin receptors in the human coronary artery, ventricle and atrium. A quantitative autoradiographic analysis. Naunyn Schmiedebergs Arch Pharmacol 348: 403–410, 1993 [DOI] [PubMed] [Google Scholar]
- 5.Cardillo C, Campia U, Bryant MB, Panza JA. Increased activity of endogenous endothelin in patients with type II diabetes mellitus. Circulation 106: 1783–1787, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Cardillo C, Campia U, Kilcoyne CM, Bryant MB, Panza JA. Improved endothelium-dependent vasodilation after blockade of endothelin receptors in patients with essential hypertension. Circulation 105: 452–456, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Cardillo C, Kilcoyne CM, Cannon RO, 3rd, Panza JA. Increased activity of endogenous endothelin in patients with hypercholesterolemia. J Am Coll Cardiol 36: 1483–1488, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Cardillo C, Kilcoyne CM, Waclawiw M, Cannon RO, 3rd, Panza JA. Role of endothelin in the increased vascular tone of patients with essential hypertension. Hypertension 33: 753–758, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Dashwood MR, Tsui JC. Endothelin-1 and atherosclerosis: potential complications associated with endothelin-receptor blockade. Atherosclerosis 160: 297–304, 2002 [DOI] [PubMed] [Google Scholar]
- 10.DeSouza CA, Clevenger CM, Greiner JJ, Smith DT, Hoetzer GL, Shapiro LF, Stauffer BL. Evidence for agonist-specific endothelial vasodilator dysfunction with ageing in healthy humans. J Physiol 542: 255–262, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehrenreich H. The astrocytic endothelin system: toward solving a mystery focus on “distinct pharmacological properties of ET-1 and ET-3 on astroglial gap junctions and Ca2+ signaling”. Am J Physiol Cell Physiol 277: C614–C615, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Evans NJ, Walker JW. Endothelin receptor dimers evaluated by FRET, ligand binding, and calcium mobilization. Biophys J 95: 483–492, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Godfraind T. Evidence for heterogeneity of endothelin receptor distribution in human coronary artery. Br J Pharmacol 110: 1201–1205, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gregan B, Jurgensen J, Papsdorf G, Furkert J, Schaefer M, Beyermann M, Rosenthal W, Oksche A. Ligand-dependent differences in the internalization of endothelin A and endothelin B receptor heterodimers. J Biol Chem 279: 27679–27687, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Guarda E, Katwa LC, Myers PR, Tyagi SC, Weber KT. Effects of endothelins on collagen turnover in cardiac fibroblasts. Cardiovasc Res 27: 2130–2134, 1993 [DOI] [PubMed] [Google Scholar]
- 16.Harada N, Himeno A, Shigematsu K, Sumikawa K, Niwa M. Endothelin-1 binding to endothelin receptors in the rat anterior pituitary gland: possible formation of an ETA-ETB receptor heterodimer. Cell Mol Neurobiol 22: 207–226, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong HJ, Liu JC, Chan P, Juan SH, Loh SH, Lin JG, Cheng TH. 17β-estradiol downregulates angiotensin-II-induced endothelin-1 gene expression in rat aortic smooth muscle cells. J Biomed Sci 11: 27–36, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Ihling C, Szombathy T, Bohrmann B, Brockhaus M, Schaefer HE, Loeffler BM. Coexpression of endothelin-converting enzyme-1 and endothelin-1 in different stages of human atherosclerosis. Circulation 104: 864–869, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Juan SH, Chen JJ, Chen CH, Lin H, Cheng CF, Liu JC, Hsieh MH, Chen YL, Chao HH, Chen TH, Chan P, Cheng TH. 17β-estradiol inhibits cyclic strain-induced endothelin-1 gene expression within vascular endothelial cells. Am J Physiol Heart Circ Physiol 287: H1254–H1261, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Kelly JJ, Whitworth JA. Endothelin-1 as a mediator in cardiovascular disease. Clin Exp Pharmacol Physiol 26: 158–161, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Kitsukawa Y, Gu ZF, Hildebrand P, Jensen RT. Gastric smooth muscle cells possess two classes of endothelin receptors but only one alters contraction. Am J Physiol Gastrointest Liver Physiol 266: G713–G721, 1994 [DOI] [PubMed] [Google Scholar]
- 22.Lavallee M, Takamura M, Parent R, Thorin E. Crosstalk between endothelin and nitric oxide in the control of vascular tone. Heart Fail Rev 6: 265–276, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Lerman A, Edwards BS, Hallett JW, Heublein DM, Sandberg SM, Burnett JC., Jr Circulating and tissue endothelin immunoreactivity in advanced atherosclerosis. N Engl J Med 325: 997–1001, 1991 [DOI] [PubMed] [Google Scholar]
- 24.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics–2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 119: e21–e181, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Maguire JJ, Davenport AP. ETA receptor-mediated constrictor responses to endothelin peptides in human blood vessels in vitro. Br J Pharmacol 115: 191–197, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyauchi T, Masaki T. Pathophysiology of endothelin in the cardiovascular system. Annu Rev Physiol 61: 391–415, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Newby DE, Wright RA, Labinjoh C, Ludlam CA, Fox KAA, Boon NA, Webb DJ. Endothelial dysfunction, impaired endogenous fibrinolysis and cigarette smoking: a mechanism for arterial thrombosis and myocardial infarction. Circulation 99: 1411–1415, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Newby DE, Wright RA, Labinjoh C, Ludlam CA, Fox KA, Boon NA, Webb DJ. Endothelial dysfunction, impaired endogenous fibrinolysis, and cigarette smoking: a mechanism for arterial thrombosis and myocardial infarction. Circulation 99: 1411–1415, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Pearson LJ, Yandle TG, Nicholls MG, Evans JJ. Regulation of endothelin-1 release from human endothelial cells by sex steroids and angiotensin-II. Peptides 29: 1057–1061, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Pierre LN, Davenport AP. Endothelin receptor subtypes and their functional relevance in human small coronary arteries. Br J Pharmacol 124: 499–506, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pierre LN, Davenport AP. Relative contribution of endothelin A and endothelin B receptors to vasoconstriction in small arteries from human heart and brain. J Cardiovasc Pharmacol 31 Suppl 1: S74–S76, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Ravalli S, Szabolcs M, Albala A, Michler RE, Cannon PJ. Increased immunoreactive endothelin-1 in human transplant coronary artery disease. Circulation 94: 2096–2102, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Rossi GP, Colonna S, Pavan E, Albertin G, Della Rocca F, Gerosa G, Casarotto D, Sartore S, Pauletto P, Pessina AC. Endothelin-1 and its mRNA in the wall layers of human arteries ex vivo. Circulation 99: 1147–1155, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Saetrum Opgaard O, Adner M, Gulbenkian S, Edvinsson L. Localization of endothelin immunoreactivity and demonstration of constrictory endothelin-A receptors in human coronary arteries and veins. J Cardiovasc Pharmacol 23: 576–583, 1994 [DOI] [PubMed] [Google Scholar]
- 35.Schiffrin EL. Role of endothelin-1 in hypertension and vascular disease. Am J Hypertens 14: 83S–89S, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Schiffrin EL. Vascular endothelin in hypertension. Vascul Pharmacol 43: 19–29, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Teerlink JR, Breu V, Sprecher U, Clozel M, Clozel JP. Potent vasoconstriction mediated by endothelin ETB receptors in canine coronary arteries. Circ Res 74: 105–114, 1994 [DOI] [PubMed] [Google Scholar]
- 38.Teoh H, Quan A, Leung SW, Man RY. Differential effects of 17β-estradiol and testosterone on the contractile responses of porcine coronary arteries. Br J Pharmacol 129: 1301–1308, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tostes RC, Touyz RM, He G, Ammarguellat F, Schiffrin EL. Endothelin A receptor blockade decreases expression of growth factors and collagen and improves matrix metalloproteinase-2 activity in kidneys from stroke-prone spontaneously hypertensive rats. J Cardiovasc Pharmacol 39: 892–900, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Touyz RM, Schiffrin EL. Role of endothelin in human hypertension. Can J Physiol Pharmacol 81: 533–541, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Van Guilder GP, Westby CM, Greiner JJ, Stauffer BL, DeSouza CA. Endothelin-1 vasoconstrictor tone increases with age in healthy men but can be reduced by regular aerobic exercise. Hypertension 50: 403–409, 2007 [DOI] [PubMed] [Google Scholar]
- 42.van Harmelen V, Eriksson A, Astrom G, Wahlen K, Naslund E, Karpe F, Frayn K, Olsson T, Andersson J, Ryden M, Arner P. Vascular peptide endothelin-1 links fat accumulation with alterations of visceral adipocyte lipolysis. Diabetes 57: 378–386, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Wang X, Barber DA, Lewis DA, McGregor CG, Sieck GC, Fitzpatrick LA, Miller VM. Gender and transcriptional regulation of NO synthase and ET-1 in porcine aortic endothelial cells. Am J Physiol Heart Circ Physiol 273: H1962–H1967, 1997 [DOI] [PubMed] [Google Scholar]
- 44.Webb CM, Ghatei MA, McNeill JG, Collins P. 17β-estradiol decreases endothelin-1 levels in the coronary circulation of postmenopausal women with coronary artery disease. Circulation 102: 1617–1622, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Wilbert-Lampen U, Seliger C, Trapp A, Straube F, Plasse A. Female sex hormones decrease constitutive endothelin-1 release via endothelial sigma-1/cocaine receptors: an action independent of the steroid hormone receptors. Endothelium 12: 185–191, 2005 [DOI] [PubMed] [Google Scholar]