Abstract
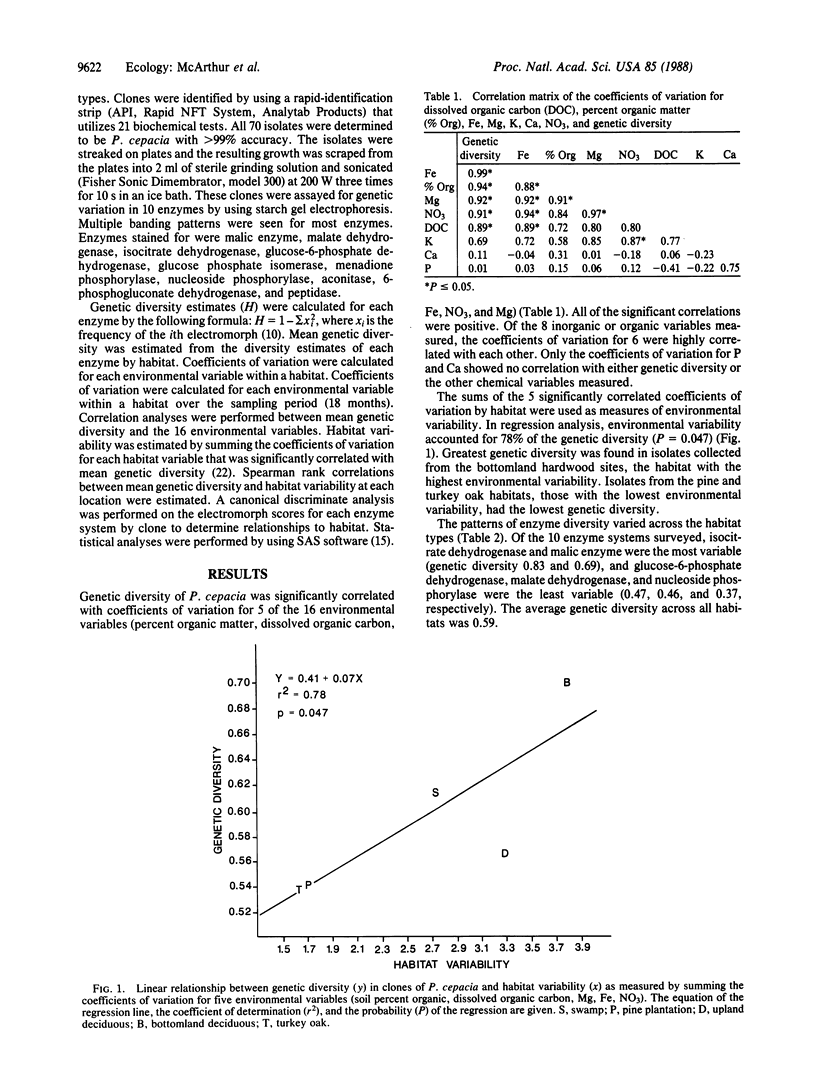
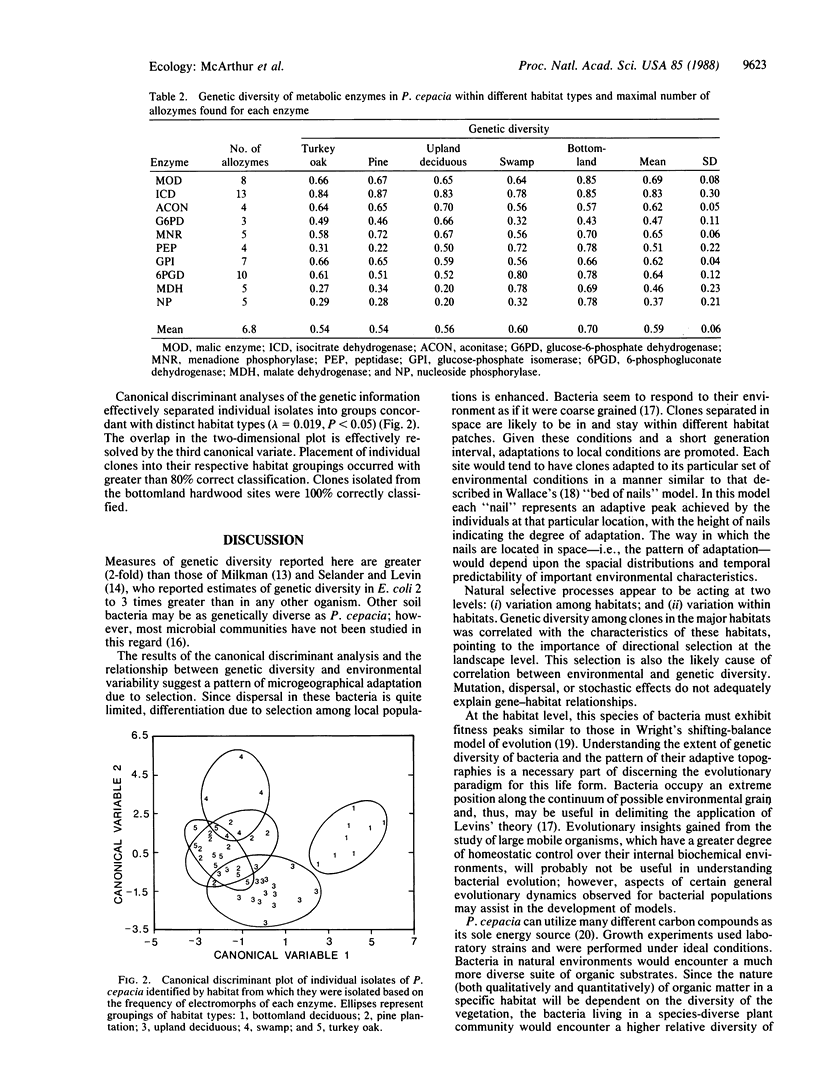
Genetic diversity in natural populations of the bacterium Pseudomonas cepacia was surveyed in 10 enzymes from 70 clones isolated along a landscape gradient. Estimates of genetic diversity, ranging from 0.54 to 0.70, were higher than any previously reported values of which we are aware and were positively correlated with habitat variability. Patterns of bacterial genetic diversity were correlated with habitat variability. Findings indicate that the source of strains used in genetic engineering will greatly affect the outcome of planned releases in variable environments. Selection of generalist strains may confer a large advantage to engineered populations, while selection of laboratory strains may result in quick elimination of the engineered strains.
Keywords: Pseudomonas cepacia, enzyme polymorphism, habitat variability, genetically engineered microorganisms
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hankinson T. R., Schmidt E. L. An acidophilic and a neutrophilic nitrobacter strain isolated from the numerically predominant nitrite-oxidizing population of an Acid forest soil. Appl Environ Microbiol. 1988 Jun;54(6):1536–1540. doi: 10.1128/aem.54.6.1536-1540.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulis L., Chase D., Guerrero R. Microbial communities. Bioscience. 1986 Mar;36(3):160–170. [PubMed] [Google Scholar]
- Milkman R. Electrophoretic variation in Escherichia coli from natural sources. Science. 1973 Dec 7;182(4116):1024–1026. doi: 10.1126/science.182.4116.1024. [DOI] [PubMed] [Google Scholar]
- Sadowsky M. J., Bohlool B. B., Keyser H. H. Serological Relatedness of Rhizobium fredii to Other Rhizobia and to the Bradyrhizobia. Appl Environ Microbiol. 1987 Aug;53(8):1785–1789. doi: 10.1128/aem.53.8.1785-1789.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt E. L., Zidwick M. J., Abebe H. M. Bradyrhizobium japonicum Serocluster 123 and Diversity among Member Isolates. Appl Environ Microbiol. 1986 Jun;51(6):1212–1215. doi: 10.1128/aem.51.6.1212-1215.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selander R. K., Levin B. R. Genetic diversity and structure in Escherichia coli populations. Science. 1980 Oct 31;210(4469):545–547. doi: 10.1126/science.6999623. [DOI] [PubMed] [Google Scholar]
- Trent J. D., Chastain R. A., Yayanos A. A. Possible artefactual basis for apparent bacterial growth at 250 degrees C. Nature. 1984 Feb 23;307(5953):737–740. doi: 10.1038/307737a0. [DOI] [PubMed] [Google Scholar]
- Woomer P., Singleton P. W., Bohlool B. B. Ecological indicators of native rhizobia in tropical soils. Appl Environ Microbiol. 1988 May;54(5):1112–1116. doi: 10.1128/aem.54.5.1112-1116.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. Evolution in Mendelian Populations. Genetics. 1931 Mar;16(2):97–159. doi: 10.1093/genetics/16.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]



