Abstract
Many important physiological, behavioral, and psychoemotional effects of intravenous (IV) cocaine (COC) are too fast and transient compared with pharmacokinetic predictions, suggesting a possible involvement of peripheral neural mechanisms in their triggering. In the present study, we examined changes in cortical electroencephalogram (EEG) and neck electromyogram (EMG) induced in freely moving rats by IV COC administration at low, reinforcing doses (0.25–1.0 mg/kg) and compared them with those induced by an auditory stimulus and IV COC methiodide, which cannot cross the blood-brain barrier. We found that COC induces rapid, strong, and prolonged EEG desynchronization, associated with decrease in alpha and increase in beta and gamma activities, and EMG activation and that both begin within 2–6 s following the start of a 10-s injection; immediate components of this effect were dose independent. The rapid COC-induced changes in EEG and EMG resembled those induced by an auditory stimulus; the latter effects had shorter onset latencies and durations and were fully blocked during urethane anesthesia. Although urethane anesthesia completely blocked COC-induced EMG activation and rapid components of EEG response, COC still induced EEG desynchronization that was much weaker, greatly delayed (∼60 s), and associated with tonic decreases in delta and increases in alpha, beta, and gamma activities. Surprisingly, IV saline delivered during slow-wave sleep (but not quite wakefulness) also induced a transient EEG desynchronization but without changes in EMG activity; these effects were also fully blocked during anesthesia. Peripherally acting COC methiodide fully mimicked rapid EEG and EMG effects of regular COC, but the effects at an equimolar dose were less prolonged than those with regular COC. These data suggest that in awake animals IV COC, like somato-sensory stimuli, induces cortical activation and a subsequent motor response via its action on peripheral neural elements and involving rapid neural transmission. By providing a rapid neural signal and triggering transient neural activation, such an action might play a crucial role in the sensory effects of COC, thus contributing to the learning and development of drug-taking behavior.
Keywords: electroencephalography, electromyography, arousal, sensory effects, ionic channels, reinforcement
many important effects of cocaine (COC), such as euphoria (14, 63), increase in arterial blood pressure (41), and peripheral vasoconstriction (6), occur either during or within seconds following an intravenous (IV) injection. Although it seems logical to assume that these centrally mediated effects result from the drug's direct interaction with brain substrates, the unusually fast onset latencies of these responses are difficult to reconcile with the timing necessary for peripherally administered COC to reach brain vessels, cross the blood-brain barrier, diffuse in brain tissue, and interact with brain receptor sites. In contrast to pharmacodynamic predictions (55), all of these effects are relatively transient, peaking well within the first postinjection minute, and they are mostly or entirely dose independent. Moreover, COC-induced euphoria, vasoconstriction, and a blood pressure increase remain generally intact during a dopamine (DA) receptor blockade (16, 23, 41, 49), suggesting the importance of non-DA mechanisms.
Although COC has a high affinity to monoamine transporters (42, 44, 46) and these substrates are usually viewed as primary targets of its action in the brain (20, 60), COC also interacts with various ionic channels (Na+, K+, Ca2+, possibly transient receptor potential (TRP); 9, 42, 43, 62), which are abundantly expressed not only on central neurons, but on the terminals of sensory nerves (see Ref. 29 for review). These sensory nerves densely innervate blood vessels (35) and other primary locations of COC self-administration and its absorption (i.e., lung alveoli, intranasal space). Therefore, a direct interaction of COC with these ionic channels or other peripherally located neural substrates could produce an excitatory afferent signal that is rapidly transmitted to the central nervous system (CNS) via pathways engaged in transmission of visceral sensory information. Therefore, in addition to the direct action on brain substrates, COC might induce central effects indirectly via its action on sensory nerve terminals, involving neural transmission.
The similarity in several physiological effects induced by regular COC and its methiodide derivative that cannot cross the blood-brain barrier (50) supports this peripheral mechanism. With IV administration at equimolar doses, COC methiodide mimics acute cardiovascular and peripheral vasoconstrictive effects of COC (6, 13), similarly increases brain and muscle temperatures (6), and induces comparable phasic excitations of most striatal neurons in awake rats (24; see also Ref. 7). Although procaine, a structurally similar local anesthetic with negligible effects on monoamine uptake (140- to 2,000-fold lower affinity to monoamine transporters vs. COC; 44) is not an addictive drug, like COC; with rapid IV administration it induces strong sensory and subjective effects in drug-naive individuals (48) and mimics acute COC-induced euphoria in drug-experienced individuals (2). Similar to COC, IV procaine induces transient sympathoexcitation (40), peripheral vasoconstriction, and elevations in brain and body temperatures (6).
In this work we employed two traditional electrophysiological techniques, electroencephalography (EEG) and electromyography (EMG), to examine rapid effects of IV COC in freely moving rats and clarify their possible mechanisms. In contrast to previous studies in which EEG was used to characterize relatively slow COC-induced changes in CNS activity over relatively long time intervals (10, 18, 30–32, 33), our analysis was focused on the immediate effects of COC and data were quantified with a high temporal resolution (4-s bins). COC was delivered at low doses (0.25, 0.5, and 1.0 mg/kg), covering the range of detection and most self-administration studies. The effects of COC were compared with those induced by an auditory stimulus, which is known to induce cortical EEG desynchronization and phasic motor response. Since DA mechanisms play an important role in mediating the reinforcing effects of COC (44, 60) and could affect drug-induced responses, we also examined COC-induced changes in EEG and EMG during a full DA receptor blockade induced by a mixture of selective D1 and D2 DA antagonists (SCH23390 and eticlopride). We then examined how EEG and EMG effects of IV COC and an auditory stimulus are altered during general anesthesia, which is known to greatly attenuate or fully block neural and physiological responses to sensory stimuli. Finally, to directly verify the role of peripheral mechanisms in mediating the acute central effects of COC, we examined changes in EEG and EMG induced by IV COC methiodide, a peripherally acting COC derivative.
MATERIALS AND METHODS
Animals and surgery.
Data were obtained from 14 male Long-Evans rats (400 ± 50 g) supplied by Charles River Laboratories (Greensboro, NC). All animals were housed individually under standard laboratory conditions (12-h light cycle beginning at 07:00) with free access to food and water. Protocols were performed in compliance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication 865-23) and were approved by the National Institute on Drug Abuse, Intramural Research Program Animal Care and Use Committee.
Each rat was surgically prepared for chronic EEG and EMG recording and implanted with a jugular IV catheter. Under general anesthesia (0.33 ml/100 g Equithesin; dose of 32.5 mg/kg pentobarbital sodium and 145 mg/kg chloral hydrate), each rat was implanted with three stainless steel screws threaded into the skull and two stainless steel electrodes implanted bilaterally in deep neck muscle. Screws with extension wires and gold pin connectors were purchased from Pinnacle Technology (Lawrence, KS), and each EMG electrode was prepared in-house from four insulated 50-μm wires, which were twisted together. All of these wires were soldered to a gold pin connector, similar to that for the EEG electrode. After implantation, all connectors were inserted into a plastic socket and fixed with dental acrylic as a head assembly. During the same surgical session, each rat was implanted with a chronic jugular catheter, which was run subcutaneously to the head mount and secured with dental acrylic in the same head assembly. After a 3–4 day period of recovery and habituation to the experimental chamber, recording sessions were held once daily over the next 5–8 days. The top of the plastic socket was protected between sessions by a plastic cap.
EEG/EMG recording.
EEG signals were recorded differentially, using two active screws threaded into the skull on the left side (A-L: 2–2 mm; P-L: 4–1.5 mm; according to Ref. 38) and a reference screw implanted on the right side (A-L: 5–1.0 mm). The latter screw was also used as a reference electrode for differential EMG recording, with two active EMG electrodes implanted bilaterally in deep neck muscle. Electrical activity from EEG and EMG electrodes passed trough the preamplifier (Pinnacle Technology) incorporated inside of an extension cord and electrical swivel to the main amplifiers (models P15D and P55; Grass Electronics), which were used for additional signal amplification and filtering. EEG signals were filtered from 1 to 30 Hz, and EMG signals were filtered between 10 and 1,000 Hz. The filtered signals then entered a Micro 1401 MK2 interface (Cambridge Electronic Design, Cambridge, UK), allowing its acquisition, recording, and analysis using a Spike2 interface (Cambridge Electronic Design).
Experimental protocol.
Experiments were conducted in an electrically insulated cage (38 × 47 × 47 cm) placed inside a sound- and light-attenuated plastic box (60 × 56 × 70 cm) under continuous weak light insulation (20 W) and in view of a small video camera mounted above the cage. After placement in the cage, the rat was connected to the recording cable and a plastic catheter extension. This catheter extension was connected to a liquid swivel and an additional catheter extension, allowing stress-free drug delivery from outside of the cage by minimizing detection of the IV injection procedure by the animal. Each rat was intensively habituated to the recording environment both before (2–3 daily sessions ∼5–7 h each) and after (2–3 sessions) surgery. The first recording session was always an additional habituation session, during which the rat was observed and only sound stimuli were presented.
Each rat was exposed to several sound stimulations (clap) and IV saline injections (0.2 ml over 15 s) during the next two recording sessions. During the following two recording sessions, we examined the effects of COC in three doses (0.25, 0.50, and 1.0 mg/kg). While the latter two doses are traditionally used in self-administration experiments, COC at a 0.25 mg/kg dose is also detected by rats, self-injected, and induces significant locomotor activation (57, 58). In each of these 2 days, only two doses were tested, and the rat received 1–3 injections at each dose. The intervals between injections were at least 60 min for low and moderate doses and at least 2 h for a high dose. As shown in our previous studies, physiological and behavioral effects of COC remain relatively stable following several repeated injections at these low doses and extended between-injection time intervals. To avoid sensory responses associated with catheter refilling or its change, the catheter was filled on each testing day with different COC solutions, and the dose increase was achieved by increasing the volume and duration of the injection. On the first testing day, COC was dissolved to deliver a 0.25 mg/kg dose in 0.15 ml (over 10 s) and a 0.50 mg/kg in 0.30 ml (over 20 s). On the next day, COC was dissolved to deliver a 0.50 mg/kg dose in 0.15 ml (over 10 s) and a 1.0 mg/kg dose in 0.30 ml (over 20 s). Therefore, 0.25, 0.5, and 1.0 mg/kg doses were delivered over 10, 15 (an average of 10 and 20 s), and 20 s, respectively.
We also examined how EEG and EMG changes induced by COC at a moderate dose (0.5 mg/kg over 15 s) are affected by a DA receptor blockade or general anesthesia. Some rats received both treatments with one free day in between, and other rats received only one treatment. DA receptor blockade was induced by a mixture of D1- and D2-selective DA antagonists (SCH23390 and eticlopride, respectively), which have a high affinity to all types (D1-D5) of DA receptors (36). These drugs were freshly dissolved in saline and delivered subcutaneously. At doses used (0.2 mg/kg for each drug or 0.7 and 0.6 μM), combination of these two drugs provides effective blockade of DA transmission for at least 2 h as tested previously by the antagonism of striatal neuronal responses to iontophoretic DA (25). At these doses, a SCH23390 and eticlopride combination fully blocks locomotor activation induced by IV COC (23). In these tests, rats received at least one COC injection before and two injections after (+40 and +100 min) treatment. General anesthesia was induced by urethane (1.25 g/kg sc) and similarly, the effects of IV COC (0.5 mg/kg) were tested before and twice (+60 and +120 min) during urethane anesthesia. Control data for these two treatments were combined in one group, which was used for statistical comparisons. Two additional urethane-anesthetized rats were used to examine EMG and EEG effects of sensory stimuli and saline injections. The session with urethane was always the last recording session and, after the completion of testing, rats were euthanized.
In four separate rats, we examined changes in EEG and EMG induced by COC methiodide, a peripherally acting COC derivative. This drug was obtained from the National Institute on Drug Abuse, Intramural Research Program and used at a 0.67 mg/kg dose, equimolar to the moderate dose of COC-HCl used in primary experiments.
Both sensory stimuli and injections were made when the rat was in quiet wakefulness or drowsiness/sleep state (see results) with no movements during the 60 s preceding injection. Cases in which we observed movements or sudden changes in EEG during the prestimulus period were removed from analysis. Such cases were more often encountered with COC, which at moderate and high doses slightly increased locomotion and increased the activity state for prolonged periods.
Data analysis.
EEG and EMG signals were analyzed based on 6-min recording durations, with 1 min preceding and 5 min following drug or stimulus presentation. The following parameters were analyzed for each response: total power of EEG, total power of EMG, and change in the power of delta (2–4 Hz), theta (4–8 Hz), alpha (8–15 Hz), beta (15–29 Hz), and gamma (29–58 Hz) frequencies. Since EEG and EMG signals in individual rats differ in their magnitude, absolute values of total power were transferred into relative changes, taking a basal value (mean for 60-s preinjection) as 100%. Therefore, our data represent fluctuations in EEG and EMG total powers assessed with 4-s averaging bin. Changes in each individual EEG band were analyzed in percents with respect to the preevent baseline. While EEG and EMG total powers are integral indices of electrical activity, dependent upon both signal amplitude and frequency, the powers of individual EEG waves (each shown in %) reflect their respective proportions of EEG total power calculated for specified signal frequencies; the sum of all five EEG wave powers equals total EEG power (100%). Since EMG signals showed robust and highly variable increases following COC administration, its total power was analyzed statistically as natural logarithmic derivatives.
One-way ANOVA with repeated measures (followed by Fisher post hoc test) was used as a primary tool for evaluating statistical significance of EEG and EMG changes. Student's t-test was used for between-group comparisons. The use of the words “increase,” “decrease,” and “significant” means the presence of a statistically significant change in the parameter or differences between the compared groups or conditions (with at least P < 0.05) revealed by either ANOVA or Student’s t-test. To simplify the text, most quantitative results of statistical comparisons are presented in figure captions.
RESULTS
Data described in this study were obtained in 14 rats during 54 recording sessions; 174 EEG and EMG responses were included in data analyses. These data will be presented in logical order that differs from the chronology of individual tests. First, we describe the EEG and EMG effects of COC and their changes during DA receptor blockade and general anesthesia. Second, we describe the effects of a simple sensory stimulus and their changes during general anesthesia. Third, we describe the effects of COC methiodide. Finally, we describe the effects of IV saline injections in freely moving, drug-free conditions and during urethane anesthesia.
Rats habituated to the experimental environment were typically generally inactive during daytime recording (10:00–16:00), showing transient periods of comfort behaviors (slight locomotion, chewing, yawning, grooming) within more prolonged periods of full inactivity verified by video monitoring. These periods of motor inactivity maintained for > 60 s were used as baseline for presentation of a sensory stimulus and IV injections. The EEG signal during these periods typically showed high-amplitude, low-frequency fluctuations (slow-wave sleep) periodically interrupted by brief desynchronized activity of lower amplitude and higher frequency.
EEG and EMG responses to IV COC administration in freely moving rats.
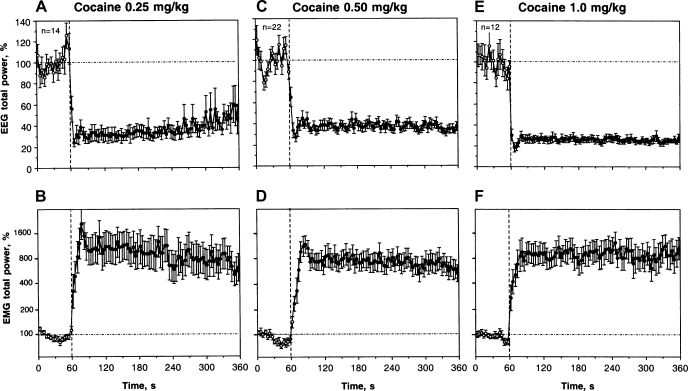
IV COC administration induced rapid, powerful, and prolonged changes in EEG and EMG at each dose tested (Fig. 1). EEG total power decreased to its minimum within the injection interval and remained at these levels for at least the next 5 min. Averaged EMG had a similar but inverted pattern, rapidly increasing during the injection and remaining increased for the entire analysis interval. Time to the peak of the EMG signal was longer than that for the EEG, and the maximal increase (10–20 fold vs. baseline) was seen at 20–32 s after the start of injection. The pattern of EEG and EMG responses was similar for each dose tested, and the EEG changes were maximal for the largest, 1.0 mg/kg dose. The EMG response was strong and quantitatively similar at each dose (∼8- to 10-fold increase). As shown in Fig. 2 (S78-D-1), EEG and EMG changes induced by IV COC at a moderate dose had short onset and both signals remained affected for a long time, exceeding the 5-min postinjection analysis interval.
Fig. 1.
Phasic and tonic changes in electroencephalogram (EEG; top) and electromyogram (EMG; bottom) total powers after intravenous cocaine (COC) administration at different doses. Mean ± SE values are shown in percents vs. preinjection baseline. Vertical hatched lines (0 s) show the start of injections and horizontal hatched lines show basal values (100%). Black symbols show values significantly different (Fisher F-test) from baseline (n = number of tests). ANOVA values for the effect of cocaine on EEG and EMG, respectively, are 0.25 mg/kg, F12.402 = 20.86, 9.41; 0.50 mg/kg, F19,610 = 19.48, 15.63; 1.0 mg/kg, F11,371 = 37.30, 11.10; each P < 0.001.
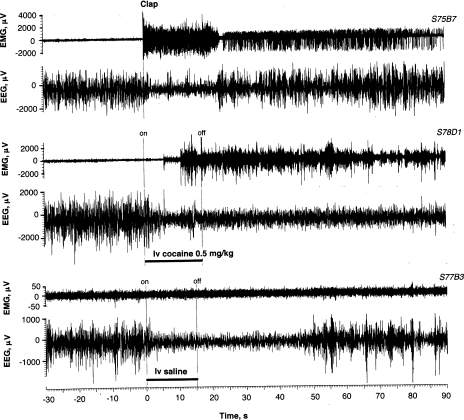
Fig. 2.
Original examples of EEG and EMG activity (μV at final amplification) recorded in freely moving rats following clap, intravenous COC, and saline injections. Onset (on) and offset (off) of the injection (15 s) is shown with vertical hatched lines. Data were obtained in 3 different rats (S75B7, S78D1, S77B3; first 3 symbols show rat number, the next symbol is session number, and the last symbol is the test number). While intravenous saline injection in many cases did not induce evident EEG changes, the example (S77B3) demonstrates a typical desynchronization when the injection was performed during a prolonged episode of slow-wave sleep.
As shown in the Table 1, COC at each dose had a highly significant effect (P < 0.001) on both EEG and EMG total powers calculated separately for the two time intervals following the injection (immediate change: 0–20 s and long-term change: 0–180 s). In each case, the effects were strong and dose-independent within the first three postadministration minutes.
Table 1.
Rapid and long-term changes in electroencephalogram (EEG) and electromyogram (EMG) total powers induced by intravenous administrations of cocaine (COC)-HCl, COC-methiodide, and saline as well as by clap in drug-free, freely moving conditions, during dopamine (DA) receptor blockade and urethane anesthesia
| Group | Immediate Change, 0–20 s | Long-Term Change, 1–180 s |
|---|---|---|
| EEG, % | ||
| COC 0.25 | 31.82 ± 2.78b | 28.82 ± 2.35b |
| COC 0.50 | 36.23 ± 3.25b | 34.53 ± 2.05b |
| COC 1.00 | 26.34 ± 3.21b | 27.50 ± 2.90b |
| COC 0.5* | 35.03 ± 3.52b | 31.65 ± 2.48b |
| COC 0.5+urethane | 101.32 ± 4.36e | 76.13 ± 3.38a,e |
| COC 0.5+DA antagonists | 51.91 ± 5.09b,d | 51.74 ± 6.32b,d |
| COC-methiodide 0.67 | 43.54 ± 4.80b | 40.33 ± 4.95b |
| Clap | 31.03 ± 1.99b | 63.99 ± 6.03b |
| Clap+urethane | 102.81 ± 4.62 | 110.61 ± 11.34 |
| Saline | 62.54 ± 5.57b | 99.04 ± 8.81 |
| Saline+urethane | 117.29 ± 10.18 | 103.56 ± 8.26 |
| EMG, ln, % | ||
| COC 0.25 | 6.67 ± 0.38b | 6.88 ± 0.39b |
| COC 0.50 | 5.87 ± 0.20b | 6.55 ± 0.22b |
| COC 1.00 | 6.22 ± 0.33b | 6.72 ± 0.27b |
| COC 0.5* | 6.42 ± 0.31b | 6.99 ± 0.29b |
| COC 0.5+urethane | 4.66 ± 0.07e | 4.61 ± 0.04e |
| COC 0.5+DA antagonists | 5.65 ± 0.21a,c | 6.03 ± 0.34b,c |
| COC-methiodide 0.67 | 6.25 ± 0.37b | 6.65 ± 0.15b |
| Clap | 6.90 ± 0.29b | 5.89 ± 0.29b |
| Clap+urethane | 4.64 ± 0.02 | 4.66 ± 0.02 |
| Saline | 4.67 ± 0.07 | 4.69 ± 0.06 |
| Saline+urethane | 4.66 ± 0.04 | 4.64 ± 0.04 |
Each value represents a mean ± SE of individual values of EEG total power in each group shown in % relative to baseline (mean of 15, 4-s values prestimulus or predrug). Each individual value for immediate change was a sum of 5, 4-s values and for long-term change, a sum of 45, 4-s values. The same procedure was used for quantification of EMG total power, but because of high and variable response magnitudes, natural logarithmic derivatives of %values were used (i.e., 4.61 = 100% and 6.67 = 788%). Symbols show statistical significance of changes:
P < 0.01 and
P < 0.001 vs. prestimulus or preinjection baseline
P < 0.05
P < 0.01; and
P < 0.001 vs. control group (COC in freely moving conditions).
Marks a separate control group (COC injections at 0.5 mg/kg in drug-free conditions preceding the injections of urethane and DA antagonists), which was used to evaluate the effects of anesthesia and DA receptor blockade on COC-induced changes in EEG and EMG (see captions to Figures for sample sizes). The values in this group were also used to evaluate the differences in effects of 2 COC derivatives delivered at equimolar doses.
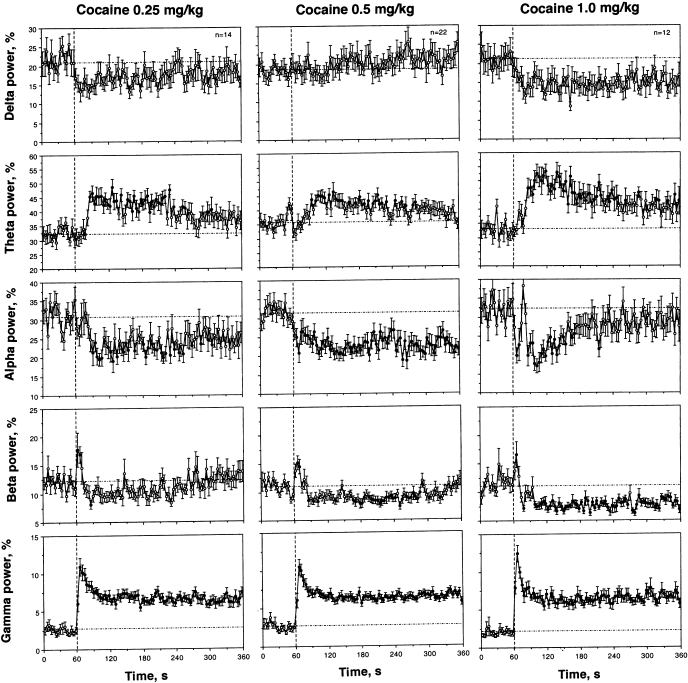
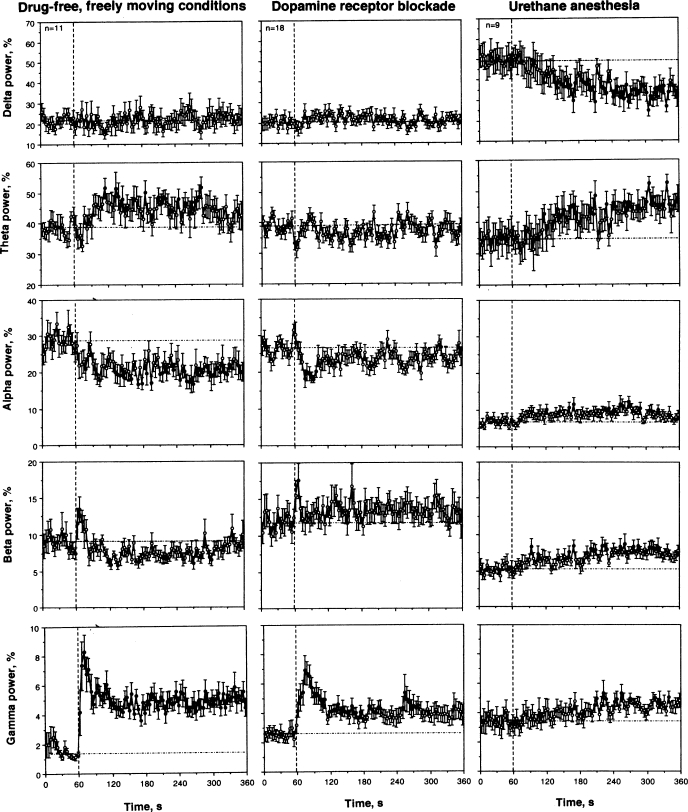
COC injection induced three principal changes in EEG wave activity (Fig. 3). First, beta activity rapidly, but transiently, increased during and immediately after the injection (0–12 s), but then slowly decreased for a prolonged time. While the phasic increase was dose independent, the tonic decrease became stronger and more prolonged with a dose increase. Second, COC induced a rapid and strong increase in gamma activity; the effect peaked during the injection but was maintained at significantly increased levels for the entire recording interval. While comparable in amplitude and duration at each dose tested, gamma activation appeared to be maximal at the highest dose. Third, alpha activity decreased after IV COC injection; in contrast to rapid changes in beta and gamma activities, this effect was less phasic, peaked at 1–2 min postinjection, and was more prolonged. COC also induced a tonic increase in theta and tonic decrease in delta activities, which became stronger as the dose was increased.
Fig. 3.
Mean changes in individual EEG frequencies [total power for delta, theta, alpha, beta, and gamma waves] following intravenous COC administration. Similar to Fig. 1, vertical and horizontal hatched lines in each graph show the starts of injections and basal values, respectively. ANOVA values for COC at 0.25 mg/kg [delta: F13,433 = 1.08, nonsignificant (NS); theta = 3.79, P < 0.001; alpha = 1.90, P < 0.01; beta = 2.85; P < 0.001; gamma = 8.59, P < 0.001]. ANOVA values for COC at 0.50 mg/kg (delta: F21,681 = 0.88, NS; theta = 3.40, P < 0.001; alpha = 1.93, P < 0.01; beta = 4.00; P < 0.001; gamma = 8.24, P < 0.001). ANOVA values for COC at 1.0 mg/kg (delta: F11.371 = 1.44, P = 0.06; theta = 4.61, P < 0.001; alpha = 3.32, P < 0.001; beta = 5.64; P < 0.001; gamma = 8.86, P < 0.001).
EEG and EMG responses to IV COC during DA receptor blockade and urethane anesthesia.
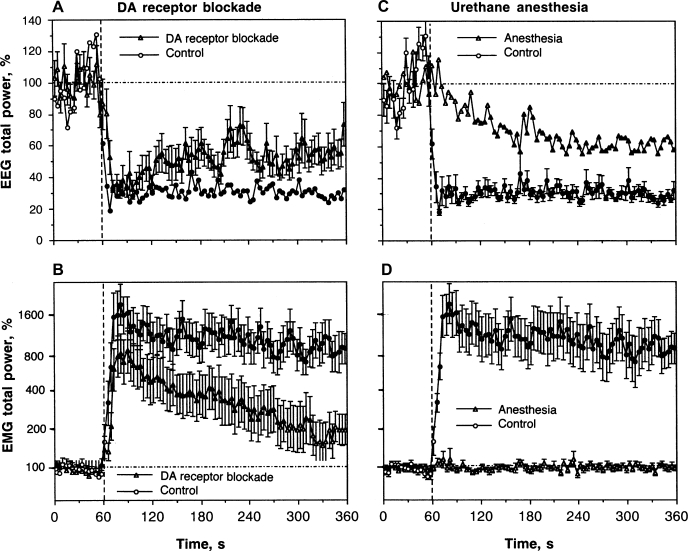
As shown in Fig. 4A, DA receptor blockade has no significant effect on rapid changes in the EEG signal induced by IV COC. Similar to control conditions, COC induced a rapid and strong decrease in EEG total power after the DA antagonist treatment. However, there were small between-group differences. The decrease in EEG total power in this group was slightly slower than that in controls and had a tendency to recover within the observation period. From ∼60 s after COC injection, the decrease became weaker than in control, and between-group differences became noticeable. The mean COC-decrease in EEG total power in the DA antagonist group was significantly weaker than in control from the second minute postinjection (51.02 ± 2.11 vs. 38.22 ± 1.46%), after which the difference became stronger. While similarly rapid, the EMG response to COC during DA receptor blockade (Fig. 4B) was also slightly weaker (at peak: 8-fold vs. 16-fold in control) and more transient, disappearing at ∼270 s postinjection, while the increase in the EMG signal in the control group was relatively stable during the entire observation interval. These between-group differences in the relative increase in EMG activity could be at least partially related to a higher basal EMG activity seen after the DA antagonist treatment (2.44 ± 0.15 vs. 2.31 ± 0.22). Since rats were generally adynamic during DA receptor blockage, an increased EMG total power results from tonic increases in EMG activity.
Fig. 4.
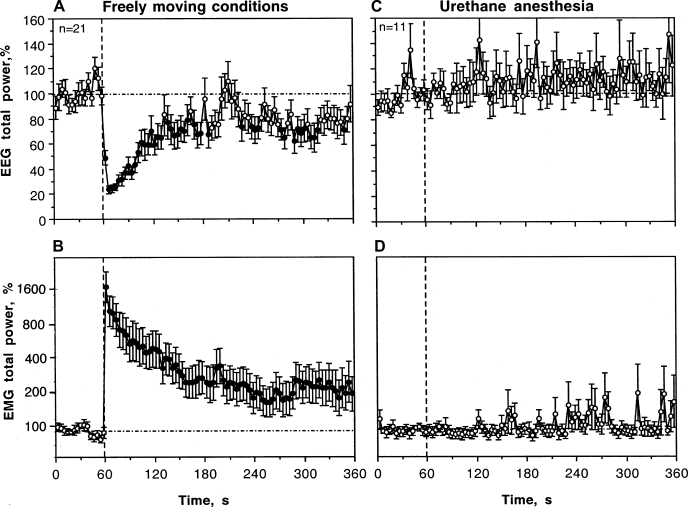
Mean changes in EEG (A and C) and EMG (B and D) total powers induced by intravenous COC (0.5 mg/kg) in drug-free, freely moving conditions (n = 11), during dopamine (DA) receptor blockade (SCH 23390 + eticlopride at 0.2 mg/kg sc, each; n = 18), and urethane anesthesia (1.25 g/kg ip, n = 11). Left: EEG and EMG effects of COC during DA receptor blockade and drug-free control. Right: effects of COC during urethane anesthesia and drug-free control. Black symbols show values significantly different from baseline. ANOVA values for EEG total power are: control, F10,240 = 7.81, P < 0.001; DA receptor blockade, F17,557 = 4.07, P < 0.01; anesthesia, F8,279 = 3.52, P < 0.01. ANOVA values for EMG total power are: control, F10,240 = 11.37, P < 0.001; DA receptor blockade, F17,557 = 7.11, P < 0.001; anesthesia, F8,279 = 0.78, NS.
Statistical analysis of mean changes (see Table 1) revealed that COC has a significant effect on both EEG and EMG total powers during a DA receptor blockade. However, this effect was significantly weaker than that for drug-free controls as both an immediate and long-term change.
Despite a clear locomotor hypoactivity evident following administration of DA antagonists, there were minimal changes in the basal EEG wave spectrum (compare baselines in Fig. 5, left and middle). However, statistical analysis revealed a significant decrease in alpha (26.42 ± 1.31% vs. 30.10 ± 1.35%, P < 0.05) and increase in beta and gamma activities (11.90 ± 1.23% and 2.60 ± 0.34% vs. 9.03 ± 0.64% and 1.66 ± 0.16%, respectively; both P < 0.05) in this condition compared with controls. Similarly weak between-group differences were found in changes of individual EEG waves (Fig. 5). All three major changes seen with COC in control (decrease in alpha and increase in beta and gamma activity) were evident when the drug was injected during DA receptor blockade. However, changes in alpha and gamma activity were weaker and much shorter, being evident for the first 40 and 120 s, respectively. Although beta activity transiently increased during the injection in both conditions, the subsequent decrease in beta waves seen in the control was absent during the DA receptor blockade. In contrast, beta activity has a tendency for tonic increases in this condition. Treatment with DA antagonists also fully blocked a tonic increase in theta activity induced by COC in control conditions.
Fig. 5.
Mean ± SE changes in individual EEG frequencies (delta, theta, alpha, beta, and gamma) following IV COC administration (0.5 mg/kg) in 3 conditions: drug-free control (left), DA receptor blockade (middle), and urethane anesthesia (right). Similar to Fig. 1, vertical and horizontal hatched lines in each graph show the start of injections and basal values, respectively. ANOVA values for the effect of COC in drug-free control (delta: F10,340 = 0.77, NS; theta = 1.94, P < 0.01; alpha = 1.62, P < 0.05; beta = 3.75; P < 0.001; gamma = 4.04, P < 0.001), following DA receptor blockade (delta: F17,557 = 1.00, P = 0.47; theta = 1.44, P = 0.06; alpha = 1.70, P < 0.02; beta = 1.10; NS; gamma = 4.57, P < 0.001), and during urethane anesthesia (delta: F8,278 = 2.84, P < 0.001; theta = 2.42, P < 0.001; alpha = 1.70, P < 0.02; beta = 1.39; NS; gamma = 1.20, NS).
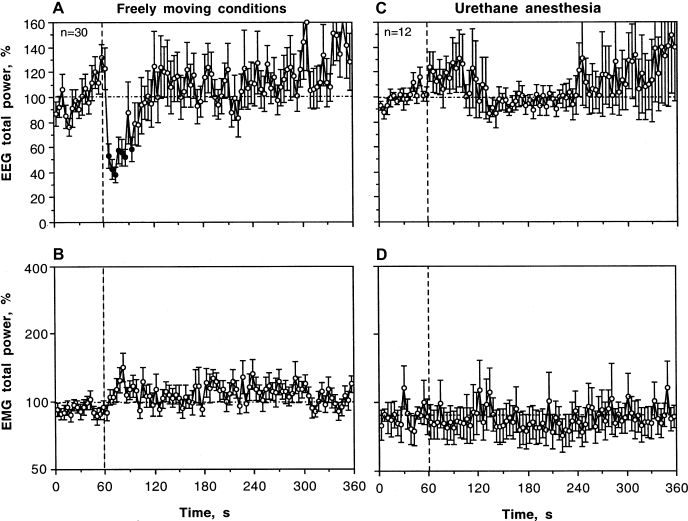
Urethane anesthesia dramatically affected EEG and EMG responses induced by IV COC (Fig. 4, C and D). Instead of a rapid decrease in EEG total power occurring during the injection, this integral parameter decreased slowly, being significantly different from the baseline from ∼ 50 s after the injection and minimal at the end of the analysis interval (5 min). Thus, between-group differences were most pronounced immediately after the injection and abated by the end of the observation period (see Table 1). COC induced no changes in EMG during anesthesia (Fig. 4D); between-group differences (anesthesia vs. control) were highly significant for both time intervals following drug administration (see Table 1).
As can be seen in Fig. 5 (baseline before drug injection), anesthesia dramatically changed the EEG power spectrum distribution with a robust increase in delta activity (49.54 ± 5.45% vs. 20.39 ± 1.45%, P < 0.001) and strong decreases in alpha and beta activity (6.91 ± 0.68% vs. 30.10 ± 1.35% and 5.24 ± 0.66% vs. 10.00 ± 0.65%; both P < 0.001). COC used during anesthesia (Fig. 5, right) did not induce any phasic changes in individual EEG waves after the injection, resulting in a full blockade of beta and gamma activation and alpha inhibition induced by COC in drug-free conditions. However, delta activity tonically decreased and theta activity tonically increased with ∼1-min onset latencies. All three waves that showed robust COC-induced changes in freely moving conditions (alpha, beta, and gamma) slightly increased starting from 1–2 min after the injection during anesthesia.
EEG and EMG responses to a sensory stimulus; effects of anesthesia.
Being presented during periods of synchronized EEG activity and motor inactivity, an auditory stimulus (clap) elicited rapid, strong, and relatively long-term changes in EEG and EMG (Fig. 6). EEG total power rapidly decreased after stimulus presentation, peaking at the first or second poststimulation values (2–6 s) and returning slowly to baseline for the next 2 min (Fig. 6A). EMG total power had a similar but inverted pattern, rapidly increasing immediately after clap (16-fold increase at peak) and slowly decreasing after (Fig. 6B). Statistical analysis (see Table 1) revealed that the immediate changes (0–20 s) induced by an auditory stimulus in EEG and EMG total powers are comparable in amplitude to those induced by IV COC. However, the effects of COC are much longer than those of a clap, resulting in significant differences in the long-term time scale (0–180 s). An original example of EEG and EMG responses induced by clap is shown in Fig. 2 (S75-B-7). In this case, EEG signal has a high-amplitude, slow-wave activity and low-magnitude EMG activity in baseline. These signals rapidly changed after clap and slowly restored from ∼20–30 s after sound stimulation.
Fig. 6.
Mean changes in EEG (A and C) and EMG (B and D) total powers (%) induced by clap in drug-free, freely moving conditions (A and B) and during urethane anesthesia (C and D). Vertical hatched lines (0 s) show the moments of sound presentation, and horizontal hatched lines show basal values (100% for both EEG and EMG). As assessed by ANOVA with repeated measures (for 120 s poststimulation with a basal value = mean for 60 s prestimulus), clap induced significant and strong effects on EEG and EMG in freely moving conditions (F20,650 = 6.98 and 11.43; both P < 0.001) but no effect on either parameter during general anesthesia (F10,340 = 0.94 and 0.84; P = 0.55 and 0.71, respectively). Black symbols show values significantly different (Fischer test) from baseline (mean for 1 min preceding stimulation). Data were compiled from 21 and 11 clap presentations.
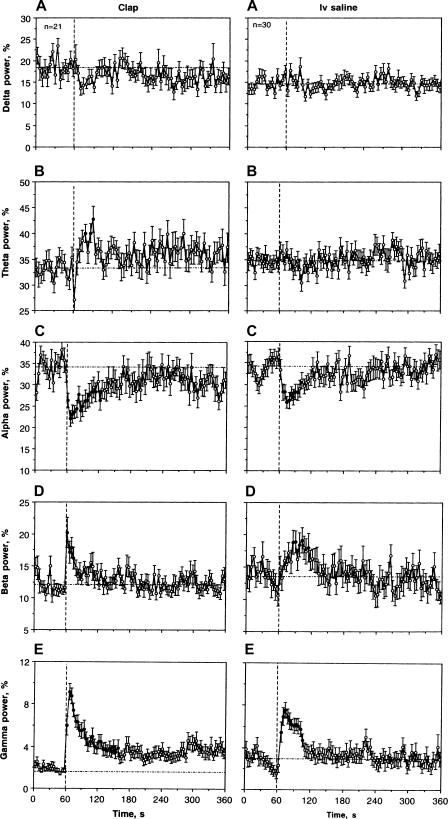
Three prominent changes induced by an auditory stimulus were found during quantitative analyses of EEG spectra (wave analysis; Fig. 7, left). While beta and gamma activities rapidly increased, alpha activity rapidly decreased. These effects were evident during the first postclap data point (0–4 s) and peaked at the second data point (4–8 s). Beta activation was most rapid and shortest in duration (2–16 s), while decreases in alpha and increases in gamma activities were more prolonged (2–80 s). These latter two waves showed inverted relationships with longer tails toward the prestimulus baseline. While sensory stimulus mimicked the pattern of changes in individual EEG waves seen with COC, these changes were shorter. For example, clap-induced gamma activation was comparable to that of COC in its amplitude, but it gradually decreased toward baseline. Increases in theta and decreases in alpha power were also more transient than those seen with COC.
Fig. 7.
Mean changes in individual EEG frequencies (delta, theta, alpha, beta, and gamma) following clap and IV saline administration in drug-free, freely moving conditions. Similar to Fig. 2, vertical and horizontal hatched lines in each graph show moments of stimulation and basal values, respectively. ANOVA values for clap are delta power: F20,650 = 1.26, NS; theta power = 1.98, P < 0.05; alpha power = 2.25, P < 0.01; beta power = 2.48, P < 0.01; gamma power = 10.54; P < 0.001. ANOVA values for IV saline are delta power: F29,929 = 1.60; theta power = 1.43, NS; delta power = 0.64, NS; alpha power = 2.86, P < 0.01; beta power = 0.97, NS; gamma power = 15.94; P < 0.001.
In sharp contrast to freely moving conditions, the clap did not induce any significant changes in EEG and EMG signals during urethane anesthesia (Fig. 6, right and Table 1). Total powers of both EEG and EMG remained very stable with a tendency of increased variability during the 5-min poststimulation interval. Although urethane anesthesia resulted in dramatic changes in individual EEG waves, they show no phasic changes after clap presentation in this condition.
Changes in EEG and EMG induced by IV administration of COC methiodide.
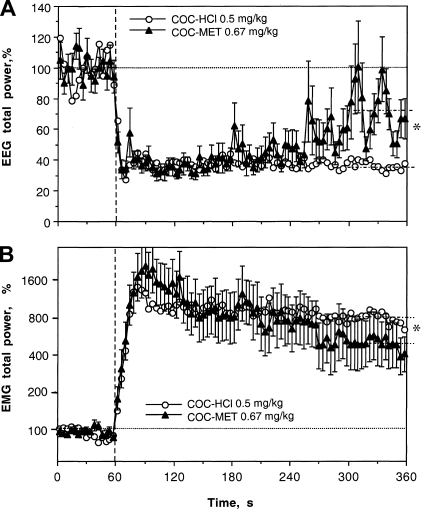
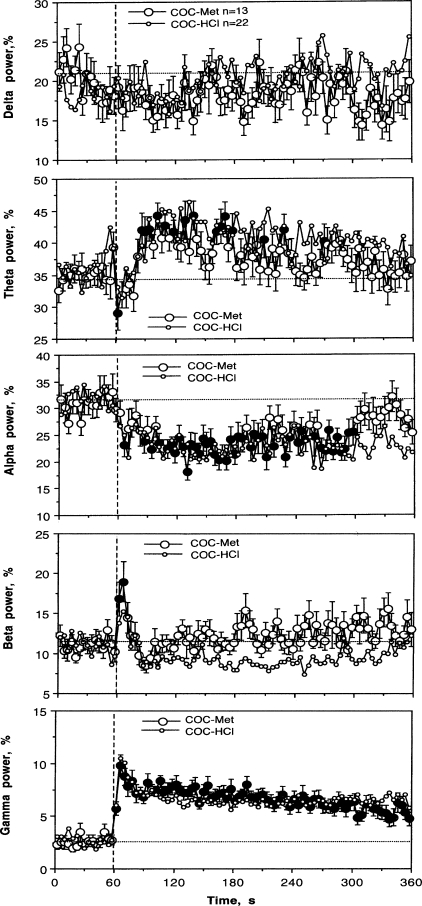
COC methiodide delivered IV at a moderate dose (0.67 mg/kg), equimolar to 0.5 mg/kg dose of COC-HCl, mimicked this drug in its ability to induce rapid EEG desynchronization and EMG activation (see Fig. 8 and Table 1). The EEG total power rapidly decreased from the first data point, remained low during the next 3 min, and slowly disappeared afterward. While there were no significant differences in this effect between the two COC derivatives for both the immediate and long-term scales (see Table 1), the effect of COC methiodide was clearly less prolonged, with a significantly weaker decrease vs. regular COC at the last minute of analysis (72.94 ± 4.47 vs. 36.47 ± 0.76%, P < 0.01).
Fig. 8.
Mean changes in EEG and EMG total powers induced by intravenous administration of COC methiodide (0.67 mg/kg or 1.4 μM) shown together with those induced by regular COC-HCl at equimolar dose (reproduced from Fig. 4 for comparison). For clarity, standard errors are shown only for COC methiodide. Vertical hatched lines (0 s) show the start of injections, and horizontal hatched lines show basal values. ANOVA values for the effect of cocaine methiodide on EEG and EMG are F18,588 = 6.32, 8.63; each P < 0.001. COC methiodide data represent the average of 13 responses obtained in 4 rats. Interrupted hatched lines at 300–360 s in both graphs show mean values of EEG and EMG total powers for 5th min postinjection. *Significant between-group difference.
As shown in Fig. 8 and Table 1, COC methiodide also mimicked regular COC-HCl in its ability to increase EMG activity rapidly and strongly. The averaged curves for both COC derivatives were virtually identical, and mean data were similar for both time scales. Similar to EEG, the effect of COC methiodide was somewhat shorter, with a weak but significant difference in mean EMG power vs. regular COC at the last minute of analysis (6.19 ± 0.05 vs. 6.68 ± 0.04; P < 0.01).
COC methiodide also mimicked regular COC-HCl by the pattern of changes in individual EEG frequencies (Fig. 9). All three basic desynchronization features, decrease in alpha and increases in beta and gamma activities, revealed with regular COC were also seen with COC methiodide. However, the beta response was monophasic without the subsequent decrease typical of COC-HCl. Similar to COC-HCl, COC methiodide tonically increased theta frequencies and had no effects on delta activity.
Fig. 9.
Mean changes in individual EEG frequencies (%total power for delta, theta, alpha, beta, and gamma waves) following intravenous administration of COC methiodide shown together with those induced by regular COC-HCl at equimolar dose (reproduced from Fig. 5 for comparison). Similar to Fig. 3, vertical and horizontal hatched lines in each graph show the start of injections and basal values, respectively. ANOVA values for the effect of COC methiodide are delta: F18,588 = 0.78, NS; theta = 3.25, P < 0.001; alpha = 2.08, P < 0.01; beta = 2.84; P < 0.001; gamma = 3.82, P < 0.001. Values significantly different from baseline are shown by black symbols.
Effects of IV saline administration in freely moving conditions and their changes during anesthesia.
In some tests, the EEG signal was clearly affected by the IV saline injection. The changes were typically evident when the injection was performed during high-amplitude, slow-wave activity (slow-wave sleep) and visually manifested as a transient decrease in signal amplitude with increase in its frequency. In contrast to clap, this transient EEG desynchonization was typically not associated with any changes in the EMG signal or observable changes in motor activity. When saline was injected at the EEG baseline with alternating episodes of slow-wave sleep and more desynchronized activity typical of quiet wakefulness, the EEG signal showed no evident changes. The example shown in Fig. 2 (S77-B-3) demonstrates rapid EEG desynchronization following IV saline injection delivered during a prolonged period of slow-wave sleep. Note that clear changes in the EEG signal were not accompanied by any change in the EMG signal.
Figure 10A shows mean changes in EEG total power following IV saline injection in a relatively large data sample (n = 30). Despite high signal variability, this integral parameter significantly decreased from the second to seventh data points (6–26 s) and then returned to baseline. The effect of saline injection was also evident during statistical analysis (see Table 1). Although the mean values of EEG total power significantly decreased during and immediately following the injection (0–20 s), the decrease was significantly lower than that for COC at each dose (62.54 ± 5.57% vs. 31.82 ± 2.78% for COC at 0.25 mg/kg, P < 0.001). While the effect of COC on the EEG signal persisted during the entire analysis interval, the effect of saline was absent on a longer time scale. In contrast, EMG total power did not change after IV saline injection. Similar to clap, EEG response elicited by IV saline administration was not observed during anesthesia. While not significant, EEG total power slightly increased after saline administration in anesthetized rats.
Fig. 10.
Mean changes in EEG (A and C) and EMG (B and D) total powers (%) induced by intravenous saline administration in drug-free, freely moving conditions (A and B) and during urethane anesthesia (C and D). Vertical hatched lines (0 s) show the moments of injection start, and horizontal hatched lines show basal values (100% for both EEG and EMG). As assessed by ANOVA with repeated measures (for 120 s poststimulation with a basal value = mean for 60 s prestimulus), intravenous saline administration induced a significant effect on EEG in freely moving conditions (F29,929 = 2.96) but no effect during anesthesia (F10,340 = 0.96, P = 0.59). The effect of intravenous saline on EMG was not significant in both conditions (F29,929 = 0.96, P = 0.54 for freely moving and F11,371 = 1.02, P = 0.44 for anesthetized conditions). Black symbols show values significantly different (Fischer test) from baseline (mean for 1 min preceding stimulation). Data were compiled from 30 and 12 saline injections.
The effects of saline injection were also evident in wave analysis (Fig. 7). Similar to clap, alpha activity decreased and gamma activity increased after the injection, but both effects were weaker. They were also much weaker than those seen with COC. Beta activity also showed a weak but significant increase, but its pattern was different that that seen with clap and COC. In contrast to the very rapid and strong increase induced by either clap or COC, this increase was seen at 26–46 s after the start of saline injection. Delta and theta activities did not show any changes following saline administration.
DISCUSSION
Cortical EEG and neck EMG were used in this study as two sensitive physiological parameters to characterize rapid central effects of IV COC and clarify their possible mechanisms. The neurophysiological mechanisms underlying EEG activity and its fluctuations have been the subject of discussion for years (see Refs. 8 and 19 for review), yet it is clear that EEG provides an objective measure of the variable CNS activity state, which is affected by various arousing challenges and drugs. Consistent with classic observations (34, 47), our present study revealed rapid and profound changes in cortical EEG following auditory stimulation. Being presented during high-amplitude EEG fluctuations typical of drowsiness and sleep, a clap caused an immediate decrease in signal amplitude and increase in its frequency (see Fig. 2 and 6). This effect, usually defined as an event-related desynchronization, manifested as a rapid decrease in EEG total power, associated with a fall in low-frequency alpha activity and a rise in high-frequency beta and gamma activities (see Fig. 7). These changes peaked within the first poststimulus data bin (0–4 s) and progressively dissipated within the subsequent 60 s. Stimulus-induced EEG desynchronization was tightly related to EMG activation, which followed the changes in brain activity. This shift was expected, obviously reflecting a time delay between neural activation and change in muscular activity triggered by this activation. Clap-induced EEG desynchronization and EMG activation were fully abolished during general anesthesia, which resulted in full motor inactivity and profound redistribution of basal EEG waves. Although urethane is a weaker central inhibitor than other general anesthetic drugs (i.e., barbiturates, chloral hydrate) and is unable to fully block EEG desynchronization induced by strong visceral and somatic “noxious” stimuli (12, 21, 34), disappearance of the EEG response to weak, auditory stimulation is consistent with its mechanism of action and previous observations (34).
Surprisingly, the cortical EEG signal was affected by IV saline injection. When the rat was fully inactive and showed large-amplitude EEG fluctuations (slow-wave sleep), saline injection typically induced a transient decrease in EEG total power, with a drop in alpha activity and a rise in beta and gamma activities, a pattern seen with an auditory stimulus. However, EMG did not show any changes in this case. In contrast, when the rat was in quiet wakefulness and basal EEG signal was more desynchronized, the EEG signal did not change at all following saline injection. Therefore, this finding suggests that the IV saline administration is detected by animals and results in transient EEG desynchronization, but only in freely moving conditions during slow-wave sleep. Since our protocol excluded the possibility of additional sensory stimuli associated with the procedure of IV injection, it appears that changes in pressure or/and temperature are likely factors that trigger this transient EEG response. In addition to its transient nature and differences in individual EEG waves, EEG desynchronization induced by IV saline drastically differed from a strong and prolonged EEG response induced by clap and COC; the latter response was always associated with robust phasic and tonic changes in EMG. While this finding was unexpected, it suggests that EEG is sensitive to detect both spontaneous fluctuations in the CNS activity state and transient desynchronization episodes induced by weak visceral sensory stimuli. The initial drowsiness/sleep state is obviously an important factor in revealing these weak responses.
The primary finding of this study is that IV COC induces very rapid, strong, and prolonged changes in EEG and EMG in freely moving rats. The rapid components of these changes basically mimicked those induced by sensory stimuli and were dose independent. They were fully blocked during urethane anesthesia but were generally unaffected by a DA receptor blockade. Although it is difficult to make a clear distinction, COC also induced more prolonged, tonic changes in EEG, which were affected by the DA receptor blockade, and remained (although in a changed pattern) during urethane anesthesia. Based on these data, it could be hypothesized that these two superimposed effects reflect different drug actions, with different underlying mechanisms. While the prolonged and COC-specific effects could reflect a direct drug action on brain substrates (“true” pharmacological effects), the nature and mechanisms underlying rapid, “sensory” effects of IV COC remain more obscure.
Cocaine-induced EEG desynchronization and its general mechanism.
While the cellular mechanisms underlying cortical EEG desynchronization are a matter of discussion (see 19, 53 for review), it is clear that this electrophysiological phenomenon reflects CNS activation, which could be triggered by various salient environmental stimuli, ranging from simple sensory to noxious somatic or visceral challenges. While the neural pathways conveying sensory afferent signals differ depending upon the stimulus' modality and strength, these signals converge to a number of subcortical structures, primarily the nuclei of the reticular formation and thalamus, finally resulting in generalized neural activation (52). Cortical EEG desynchronization could also occur “spontaneously” during transition from the slow-wave sleep to either active wakefulness or paradoxical sleep. Since IV COC injection mimics a sensory stimulus in its ability to induce rapid EEG desynchronization and EMG activation, it appears that it has a similar central excitatory action, inducing a generalized neural activation.
In support of this sensory mechanism, rapid EEG and EMG effects of both an auditory stimulus and IV COC were fully blocked during urethane anesthesia. Anesthesia also almost fully blocked excitatory responses of striatal and accumbal neurons induced by both somato-sensory stimuli (tail touch and tail pinch) and IV COC (24). Although DA antagonists at doses providing an effective blockade of all DA receptors completely blocked locomotor stimulation induced by IV COC (23), this treatment minimally affected rapid COC-induced EEG and EMG responses, suggesting their non-DA nature. This treatment also failed to block the initial pressor effects of IV COC (22, 41). While this effect is centrally mediated (27), arterial blood pressure peaks at ∼10 s following an IV COC injection, with a subsequent significant decrease toward baseline within the first postinjection minute (41). Despite different triggering mechanisms, the acute pressor effect of COC was similar to that elicited by arousing somato-sensory stimuli such as sound, light, and tactile stimulation (54). Similar to an apparent lack of dose dependency in the immediate effects of COC on EEG and EMG, acute increases of arterial blood pressure induced by IV COC were equally strong at 0.25 and 2.0 mg/kg doses (41), which cover the entire range of drug self-administration. In contrast, subsequent tonic blood pressure elevations induced by IV COC were dose dependent and attenuated by a DA receptor blockade. IV COC also induced surprisingly rapid drop in skin temperature (∼20 s from the injection start; 23), suggesting acute peripheral vasoconstriction (4), another centrally mediated effect of COC (17, 56) that is mimicked by various arousing somato-sensory stimuli (3, 4, 51). Rapid, transient skin hypothermia induced by IV COC (1 mg/kg) was both quantitatively and qualitatively similar to that induced by arousing stimuli (i.e., tail-pinch, social interaction), and it was unaffected by a DA receptor blockade (23).
While the ultrafast response dynamics, basic similarity in the response pattern to a sensory stimulus, and dramatic attenuation by anesthesia point toward the peripheral neural triggering of EEG desynchronization and EMG activation induced by IV COC, our tests with COC methiodide were critical to prove this basic mechanism directly. Because of chemical structure modification, this COC derivative fails to cross the blood-brain barrier, thus acting entirely in the periphery. Despite this principal difference, this drug used at low (0.67 mg/kg or ∼1.4 μM) doses, mimicked all basic fast-acting effects of regular COC-HCl, inducing equally rapid and strong EEG desynchronization (with quite similar changes in individual EEG bands) and EMG activation. Moreover, in contrast to regular COC, its methiodide derivative has much lower affinity to DA, norephinephrine, and serotonin transporters (30 to 60-fold; 1, 45), adding more evidence for the role of ionic channels on sensory nerve terminals as critical interaction substrates. In contrast to the CNS, where all three monoamine transporters are expressed abundantly, only norephinephrine transporters are present on peripheral neural elements and their involvements seems unlikely in light of very small COC doses (0.7–3.0 μM) and ultrafast response dynamics.
COC action on peripheral non-DA neural substrates as a possible trigger of its rapid central effects.
While this study provides abundant evidence for the peripheral neural triggering of ultrafast electrophysiological effects of IV COC, the nature of affected substrates remains less clear. It is known that peripheral vessels, especially the veins, are densely innervated by terminals of sensory nerves (35) that express various ionic channels (Na+, K+, Ca2+, TRP) (see Ref. 29 for review). Although COC binds with a high affinity to all these types of ionic channels (9), based on the high affinity (ED50 = 5–7 μM), ultrafast dynamics, and the pattern of action, K+ channels could be the most probable substrate that is able to produce ascending afferent signal (15, 42, 62, 64). Indirect data on other local anesthetics also suggest that TRP channels could be another possible substrate (5, 39). In contrast to COC-induced inhibition of Na+ channels that slows and eventually blocks neural activity (26) and nerve conductance (9), inhibition of K+ channels could increase neural activity (11), thus triggering excitatory neural drive from the periphery, which is rapidly transmitted to the CNS via pathways engaged in transmission of visceral somato-sensory information.
Although COC’s affinity to monoamine transporters (Ki between 0.15–1.60 μM for different transporters; 44, 45) clearly exceed that for ionic channels, IV administration results in very quick and strong increases in blood COC levels. If IV-administered COC is equally distributed in blood plasma (∼15 ml for a 400 g rat; see Ref 28), its levels could reach 54–218 μM with 0.25–1.0 mg/kg doses. Since the drug is injected as a high-concentration bolus, actual local levels inside blood vessels could transiently reach even higher concentrations. Such levels greatly exceed COC’s affinity to most types of ionic channels, which fluctuate within a 5–100 μM range (43, 59, 61). The issue of primary neural substrates that could be affected by COC in the periphery is a complex one and could be clarified in special experiments, using other techniques and experimental paradigms.
Relationships between rapid sensory and slow pharmacological effects of COC.
In addition to rapid EEG desynchonization and an acute EMG response, IV COC also had certain unique effects that were absent with sensory stimuli and peripherally acting COC methiodide. However, it is difficult to distinguish these COC-specific effects from its nonspecific effects shared with other arousing stimuli. Although general anesthesia almost fully blocked phasic electrophysiological effects of both sensory stimuli and IV COC, the drug slowly decreased delta and increased theta, alpha, beta, and gamma activities during urethane anesthesia (see Fig. 5). These effects appeared with ∼1- to 2-min latencies and were relatively weak and prolonged. EEG total power also decreased by IV COC during anesthesia, but this steadily decreasing effect reached significance only ∼60 s after the injection (see Fig. 4). Although in agreement with a previous work (37), these changes could be viewed as a reflection of the activating effects of COC under anesthesia, pointing at central neural substrates in their mediation, urethane anesthesia resulted in dramatic redistribution of EEG waves not seen under natural conditions. Therefore, it is unclear to what extent these changes could reflect true pharmacological effects of COC occurring under behavioral conditions. It could be of interest to find a way of blocking the rapid sensory actions of COC and examine its physiological and behavioral effects, which are mediated via its action on monoamine transporters. However, because of multiple substrates simultaneously affected by COC, this task is quite difficult to accomplish. It is also unclear how the behavioral and physiological effects of this drug will be altered if its rapid, arousing effects will be somehow eliminated.
Perspectives and Significance
Our present data indicate that IV COC induces very rapid central excitatory effects and suggest that these effects could be triggered via drug interaction with peripheral, nonmonoamine neural substrates. Therefore, in addition to the direct action of COC on centrally located neural substrates (monoamine transporters, neuroreceptors, ionic channels), it could indirectly affect neural activity by interaction with peripheral neural elements at primary locations of drug administration (i.e., vessels, lung alveoli, intranasal space). Although COC's interaction with brain substrates, particularly monoamine transporters, is essential in mediating its unique psychoactive and reinforcing effects, these effects appear to be modulated and may critically depend on drug interaction with peripheral sensory systems that trigger an ascending neural drive and induce a generalized neural activation. By providing a rapid neural signal and triggering transient neural activation, such an action might play a crucial role in the sensory effects of COC, thus contributing to the learning and development of drug-taking behavior.
GRANTS
This research was supported by the Intramural Research Program of the National Institutes of Health National Institute on Drug Abuse.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thanks Dr. Barry Hoffer for valuable comments on this manuscript.
REFERENCES
- 1.Abraham P, Pitner JB, Lewin AH, Boja JW, Kuhar MJ, Carroll FI. N-modified analogues of cocaine: synthesis and inhibition of binding to the cocaine receptor. J Med Chem 35: 141– 144, 1992 [DOI] [PubMed] [Google Scholar]
- 2.Adinoff B, Brady K, Sonne S, Mirabella RF, Kellner CH. Cocaine-like effects of intravenous procaine in cocaine addicts. Addiction Biol 3: 189– 196, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Altschule MD. Emotion and circulation. Circulation 3: 444– 454, 1951 [DOI] [PubMed] [Google Scholar]
- 4.Baker M, Cronin M, Mountjoy D. Variability of skin temperature in the waking monkey. Am J Physiol 230: 449– 455, 1976 [DOI] [PubMed] [Google Scholar]
- 5.Briersley SM, Page AJ, Highes PA, Aden B, Liebregts T, Cooper NJ, Hoffman G, Liedtke W, Blackshaw LA. Selective role of TRP4 ion channels in visceral sensory pathways. Gastroenterology 134: 2059– 2069, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown PL, Kiyatkin EA. The role of peripheral Na+ channels in triggering the central excitatory effects of intravenous cocaine. Eur J Neurosci 24: 1182– 1192, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Brown PL, Kiyatkin EA. Sensory effects of intravenous cocaine on dopamine and non-dopamine ventral tegmental area neurons. Brain Res 1218: 230– 249, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buzsaki G. Rhythms of the Brain Oxford, UK: Oxford University Press, 2006 [Google Scholar]
- 9.Catterall W, Mackie K. Local anesthetics. In: Goodman and Gilman's the Pharmacological Basis of Therapeutics (11th ed.), edited by Brunton LL, Laso JS, Parker KL. New York: McGraw-Hill, 2006, p. 369– 386 [Google Scholar]
- 10.Chang AY, Kuo TB, Chan SH. Power spectral analysis of electroencephalographic desynchronization induced by cocaine in the rat. Neurosci Lett 170: 175– 178, 1994 [DOI] [PubMed] [Google Scholar]
- 11.Chen YH, Lin CH, Lin PL, Tsai MC. Cocaine elicits action potential burst in a central snail neuron: the role of delayed rectifying K+ channels. Neuroscience 138: 257– 280, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Conte B, Cutrufo C, Manzini S. Electrocorticographic desynchronization after application of visceral and somatic noxious stimuli in urethane-anesthetized rats: effect of intrathecal administration of tachikinin (NK1 or NK 2) receptor antagonists. J Pharmacol Exp Ther 276: 212– 218, 1996 [PubMed] [Google Scholar]
- 13.Dickerson LW, Rodak DJ, Kuhn FE, Wahlstrom SK, Tessel RE, Visner MS, Schaer GL, Gillis RA. Cocaine-induced cardiovascular effects: lack of evidence for a central nervous system site of action based on hemodynamic studies with cocaine methiodide. J Cardiovasc Pharmacol 33: 36– 42, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Fischman MW, Schuster CR. A comparison of the subjective and cardiovascular effects of cocaine and procaine in humans. Pharmacol Biochem Behav 18: 711– 716, 1983 [DOI] [PubMed] [Google Scholar]
- 15.Ferreira S, Crumb WJ, Carlton CG, Clarkson CW. Effects of cocaine and its major metabolites on the HERG-encoded potassium channel. J Pharmacol Exp Ther 299: 220– 226, 2001 [PubMed] [Google Scholar]
- 16.Gawin F. Neuroleptic reduction of cocaine-induced paranoia but not euphoria? Psychopharmacology 90: 142– 143, 1986 [DOI] [PubMed] [Google Scholar]
- 17.Gillis RA, Hernandez YM, Erzouki HK, Raczkowski VF, Mandal AK, Kuhn FE, Dretchen KL. Sympathetic nervous system mediated cardiovascular effects of cocaine are primary due to a peripheral site of action of the drug. Drug Alcohol Depend 37: 217– 230, 1995 [DOI] [PubMed] [Google Scholar]
- 18.Herning RI, Jones RT, Hooker WD, Mendelson J, Blackwell L. Cocaine increases EEG beta: a replication and extension of Hans Berger's historic experiments. EEG Clin Neurophysiol 60: 470– 477, 1985 [DOI] [PubMed] [Google Scholar]
- 19.Hobson JA. Sleep and dreaming. In: Fundamental Neuroscience, edited by Zigmond MJ, Bloom FE, Landis SC, Roberts JL, Squire LR. San Diego, CA: Academic, 1999, p. 1207– 1227 [Google Scholar]
- 20.Iversen L. Neurotransmitter transporters and their impact on the development of psychopharmacology. Br J Pharmacol 147, Suppl 1: S82– S88, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanosue K, Nakayama T, Andrew PD, Shen Z, Sato M. Neuronal activities in ventrobasal complex of thalamus and in trigeminal main sensory nucleus during EEG desynchronization in anesthetized rats. Brain Res 379: 90– 97, 1986 [DOI] [PubMed] [Google Scholar]
- 22.Kiritsy-Roy JA, Halter JB, Gordon SM, Smith MJ, Terry LC. Role of the central nervous system in hemodynamic and sympathoadrenal responses to cocaine in rats. J Pharmacol Exp Ther 256: 154– 160, 1990 [PubMed] [Google Scholar]
- 23.Kiyatkin EA, Brown PL. Dopamine-dependent and dopamine-independent actions of cocaine as revealed by brain thermorecording in freely moving rats. Eur J Neurosci 22: 930– 938, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Kiyatkin EA, Brown PL. I.v. cocaine induces rapid, transient excitation of striatal neurons via its action on peripheral neural elements: single-cell, iontophoretic study in awake and anesthetized rats. Neuroscience 148: 978– 995, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiyatkin EA, Rebec GV. Striatal neuronal activity and responsiveness to dopamine and glutamate after selective blockade of D1 and D2 dopamine receptors in freely moving rats. J Neurosci 19: 3594– 3609, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiyatkin EA, Rebec GV. Dopamine-independent action of cocaine on striatal and accumbal neurons. Eur J Neurosci 12: 1– 13, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Knuepfer MM, Branch CA. Cardiovascular responses to cocaine are initially mediated by the central nervous system in rats. J Pharmacol Exp Ther 263: 734– 741, 1992 [PubMed] [Google Scholar]
- 28.Lee HB, Blaufox MD. Blood volume in the rat. J Nucl Med 25: 72– 76, 1984 [PubMed] [Google Scholar]
- 29.Lee Y, Lee CH, Oh U. Painful channels in sensory neurons. Mol Cells 20: 315– 324, 2005 [PubMed] [Google Scholar]
- 30.Liu X, Vaupel DB, Grant S, London ED. Effect of cocaine-related environmental stimuli on the spontaneous electroencephalogram in polidrug abusers. Neuropsychopharmacology 1910–17, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Lukas SE, Mendelson JH, Amass L, Benedikt R. Behavioral and EEG studies of acute cocaine administration: comparisons with morphine, amphetamine, pentobarbital, nicotine, ethanol and marijuana. NIDA Res Monogr 95: 146– 152, 1990 [PubMed] [Google Scholar]
- 32.Luoh HF, Kuo TB, Chan SH, Pan WH. Power spectral analysis of electroencephalographic desynchronization induced by cocaine in rats: correlation with microdialysis evaluation of dopaminergic neurotransmission at the medial prefrontal cortex. Synapse 16: 29– 35, 1994 [DOI] [PubMed] [Google Scholar]
- 33.Matsuzaki M, Spingler PJ, Whitlock EG, Misra AL, Mule SJ. Comparative effects of cocaine and pseudococaine on EEG activities, cardiorespiratory functions, and self-administration behavior in the rhesus monkey. Psychopharmacology (Berlin) 57: 12– 20, 1978 [DOI] [PubMed] [Google Scholar]
- 34.McClung R, Reilly E, Dafny N. Urethane modification of EEG-like activity and acoustically evoked field potentials recorded from deep nuclei. Appl Neurophysiol 39: 11– 26, 1976. –1977 [DOI] [PubMed] [Google Scholar]
- 35.Michaelis M, Goder R, Habler HJ, Janig W. Properties of afferent nerve fibers supplying the saphenous vein in the cat. J Physiol 474: 233– 243, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neve KA, Neve RL. Molecular biology of dopamine receptors. In: The Dopamine Receptors, edited by Neve KA, Neve RL. Totowa, NJ: Humana, 1997, p. 27– 76 [Google Scholar]
- 37.Pan WH, Chen Lai YJ NH, Luoh HF. Differential effects of chloral hydrate and pentobarbital sodium on cocaine-induced electroencephalographic desynchronization at the medial prefrontal cortex in rats. Life Sci 54: PL419– PL424, 1994 [DOI] [PubMed] [Google Scholar]
- 38.Paxinos J, Watson C. The Rat Brain in Stereotaxic Coordinates Orlando, FL: Academic, 1998 [Google Scholar]
- 39.Piao LH, Fujita T, Jiang CY, Liu T, Yue HY, Nakatsuka T, Kumamoto E. TRPA1 activation by lidocaine in nerve terminals results in glutamate release increase. Biochem Biophys Res Commun 379: 980– 984, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Pitts DK, Udom CE, Marwah J. Cardiovascular effects of cocaine in anesthetized and conscious rats. Life Sci 40: 1099– 1111, 1987 [DOI] [PubMed] [Google Scholar]
- 41.Poon J, van den Buuse M. Autonomic mechanisms in the acute cardiovascular effects of cocaine in conscious rats. Eur J Pharmacol 363: 147– 152, 1998 [DOI] [PubMed] [Google Scholar]
- 42.Premkumar LS. Block of a Ca2+-activated potassium channel by cocaine. J Membr Biol 204: 129– 136, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Reith ME. Cocaine receptors on monoamine transporters and sodium channels. NIDA Res Monogr 88: 23– 43, 1988 [PubMed] [Google Scholar]
- 44.Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors of dopamine transporters are related to self-administration of cocaine. Science 237: 1219– 1223, 1987 [DOI] [PubMed] [Google Scholar]
- 45.Ritz MC, Cone EJ, Kuhar MJ. Cocaine inhibition of ligand binding at dopamine, norephinephrine and serotonin transporters: a structure-activity study. Life Sci 46: 635– 645, 1990 [DOI] [PubMed] [Google Scholar]
- 46.Rothman RB, Baumann MH. Monoamine transporters and psychostimulant drugs. Eur J Pharmacol 479: 23– 40, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Sasaki H, Coffey P, Villegas-Perez MP, Vidal-Sanz M, Young MJ, Lund RD, Fukuda Y. Light induced EEG desynchronization and behavioral arousal in rats with restored retinocollicular projection by peripheral nerve graft. Neurosci Lett 218: 45– 48, 1996 [DOI] [PubMed] [Google Scholar]
- 48.Servan-Shreiber D, Perlstein WM, Cohen JD, Mintun M. Selective pharmacological activation of limbic structures in human volunteers: a positron emission tomography study. J Neuropsychiatry 10: 148– 159, 1998 [DOI] [PubMed] [Google Scholar]
- 49.Sherer MA, Kumor KM, Jaffe JH. Effects of intravenous cocaine are partially attenuated by haloperidol. Psychiatry Res 27: 117– 125, 1989 [DOI] [PubMed] [Google Scholar]
- 50.Shriver DA, Long IP. A pharmacological comparison of some quaternary derivatives of cocaine. Arch Int Pharmacodyn 189: 198– 208, 1971 [PubMed] [Google Scholar]
- 51.Solomon GF, Moos RH, Stone GC, Fessel WJ. Peripheral vasoconstriction induced by emotional stress in rats. Angiology 15: 362– 365, 1964 [DOI] [PubMed] [Google Scholar]
- 52.Steriade M. Brain stem activation of thalamocortical system. Brain Res Bull 50: 391– 392, 1999 [DOI] [PubMed] [Google Scholar]
- 53.Steriade M, McCarley RW. Brain Control of Wakefulness and Sleep New York: Kluver Academic/Plenum, 2005 [Google Scholar]
- 54.Stojicić S, Milutinović-Smiljanić S, Sarenac O, Milosavljević S, Paton JF, Murphy D, Japundzić-Zigon N. Blockade of central vasopressin receptors reduces the cardiovascular response to acute stress in freely moving rats. Neuropharmacology 54: 824– 836, 2008 [DOI] [PubMed] [Google Scholar]
- 55.Tsibulsky VL, Norman AB. Real time computations of in vivo drug levels during drug self-administration experiments. Brain Res Protoc 15: 38– 45, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vongpatanasin W, Mansour Y, Chavoshan B, Arbique D, Victor RG. Cocaine stimulates the human cardiovascular system via a central mechanism of action. Circulation 100: 497– 502, 1999 [DOI] [PubMed] [Google Scholar]
- 57.Wee S, Specio SE, Koob GF. Effects of dose and session duration on cocaine self-administration in rats. J Pharmacol Exp Ther 320: 1134– 1143, 2007 [DOI] [PubMed] [Google Scholar]
- 58.Wellman PJ, Nation JR, Davis KW. Impairment of acquisition of cocaine self-administration in rats maintained on a high-fat diet. Pharmacol Biochem Behav 88: 89– 93, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilcox KM, Paul IA, Ordway GA, Woolverton WL. Role of the dopamine transporter and the sodium channel on the cocaine-like discriminative stimulus effects of local anesthetics in rats. Psychopharmacology 157: 260– 268, 2001 [DOI] [PubMed] [Google Scholar]
- 60.Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev 94: 469– 492, 1987 [PubMed] [Google Scholar]
- 61.Woodward JJ, Compton DM, Balster RL, Martin BR. In vitro and in vivo effects of cocaine and selected local anesthetics on the dopamine transporter. Eur J Pharmacol 13: 7– 13, 1995 [DOI] [PubMed] [Google Scholar]
- 62.Wu SN, Chang HD, Sung BJ. Cocaine-induced inhibition of ATP-sensitive K+ channels in rat ventricular myocytes and in heart-derived H9c2 cells. Basic Clin Pharmacol Toxicol 98: 510– 517, 2006 [DOI] [PubMed] [Google Scholar]
- 63.Zernig G, Giacomuzzi S, Riemer Y, Wakonig G, Sturm K, Saria A. Intravenous drug injection habits: drug users’ self-reports versus researchers’ perception. Pharmacology 68: 49– 56, 2003 [DOI] [PubMed] [Google Scholar]
- 64.Zhang S, Rajamani S, Chen Y, Gong Q, Rong Y, Zhou Z, Ruoho A, January CT. Cocaine blocks HERG, but not KvLQT1+minK, potassium channel. Molec Pharmacol 59: 1069– 1076, 2001 [DOI] [PubMed] [Google Scholar]