Abstract
In sickle cell disease (SCD), the events originating from hemoglobin S polymerization and intravascular sickling lead to reperfusion injury, hemolysis, decreased nitric oxide (NO) bioavailability, and oxidative stress. Oxidative stress is implicated as a contributing factor to multiple organ damage in SCD. We hypothesize that inhibition of sickling by genetic manipulation to enhance antisickling fetal hemoglobin (HbF) expression will have an ameliorating effect on oxidative stress by decreasing intravascular sickling and hemolysis and enhancing NO bioavailability. We tested this hypothesis in BERK (Berkeley) mice expressing exclusively human α- and βS-globins and varying levels of HbF, i.e., BERK (<1% HbF), BERKγM (20% HbF) and BERKγH (40% HbF). Intravascular sickling showed a distinct decrease with increased expression of HbF, which was accompanied by decreased hemolysis and increased NO metabolites (NOx) levels. Consistent with decreased intravascular sickling and increased NO bioavailability, BERKγM and BERKγH mice showed markedly decreased lipid peroxidation accompanied by increased activity/levels of antioxidants [superoxide dismutase (SOD), catalase, glutathione peroxidase (GPx), and reduced glutathione (GSH)] in the muscle, kidney, and liver compared with BERK mice (P < 0.05–0.0001). NOx levels showed a strong inverse correlation with hemolytic rate and oxidative stress. Decreased oxidative stress in the presence of elevated HbF levels led to an anti-inflammatory effect as evidenced by decreased peripheral leukocyte counts. These results show that the protective effect of HbF is mediated primarily by decreasing intravascular sickling resulting in decreased oxidative stress and increased NO bioavailability.
Keywords: sickle cell disease, hemolysis, inflammation, reperfusion, multiple-organ damage
central to the vaso-occlusive pathophysiology of sickle cell disease (SCD) is polymerization of hemoglobin S (HbS) and intravascular sickling under deoxygenated conditions, the consequences of which can lead to vaso-occlusion, reperfusion injury, hemolysis, decreased nitric oxide (NO) bioavailability, and oxidative stress (15, 22, 39, 46, 48). Oxidative stress in SCD results from hypoxia-reperfusion (vaso-occlusive events) and inactivation of anti-inflammatory nitric oxide (NO) by oxidants and plasma heme (hemolysis). Chronic oxidative stress is implicated as a critical factor in endothelial activation, inflammatory effects (e.g., higher peripheral leukocyte counts), and multiple organ damage in SCD (22).
Because intravascular sickling is considered etiologic to the pathophysiology of SCD, attempts have been made to employ antisickling strategies to alleviate pathophysiology of this disease. In view of the antisickling properties of fetal hemoglobin (HbF), recent therapies have been designed to elevate HbF levels in sickle patients. For example, hydroxyurea has been commonly used to induce higher levels of HbF (7, 53). However, the mechanism of hydroxyurea action is somewhat controversial since hydroxyurea also results in increased NO production and thereby in decreased inflammation (i.e., diminished leukocyte counts) prior to the elevation of HbF (10). Recent reports suggest that hydroxyurea enhances HbF levels in sickle patients by an NO-derived mechanism (9, 10). However, it is relevant to address the issue whether genetic manipulation to increase antisickling HbF (i.e., without any pharmacological intervention) will decrease intravascular sickling, hemolysis, vaso-occlusive events (reperfusion injury), and consequently decrease oxidative stress and improve NO bioavailability.
We have previously shown that introduction of antisickling HbF in transgenic-knockout (Berkeley, BERK) mice (expressing exclusively human α- and βS-globins) results in improved hematologic and microvascular flow parameters (27). Both human SCD and transgenic sickle mouse models are characterized by decreased NO bioavailability (4, 29, 49). The decreased NO bioavailability in BERK mice is associated with compensatory up-regulation of cyclooxygenase-2 (COX-2), a vasodilatory species, and impaired vascular reactivity to NO-mediated vasoactive stimuli (27). In contrast, BERK mice expressing 20% HbF show markedly improved microvascular reactivity to NO-mediated vasodilators and decreased COX-2 expression (27). These effects were attributed to antisickling effect of HbF.
Intravascular sickling and associated transient vaso-occlusive events (reperfusion injury) contribute to chronic oxidative stress in SCD, resulting in multiple organ damage. Among the sources of excessive superoxide (O2.−) generation in human SCD and transgenic sickle mice are plasma membrane NADPH oxidase (21, 57), endothelial NO synthase decoupling (2, 23), and increased plasma xanthine oxidase (4). Our recent studies have shown that arginine supplementation of BERK mice significantly decreases lipid peroxidation and increases the activity/level of endogenous antioxidants secondary to enhanced NO production (13). The availability of BERK mice expressing varying levels of HbF provides a unique opportunity to address the relationship between intravascular sickling, hemolysis, NO bioavailability, and organ oxidative stress.
In the present studies, we have used BERK mice expressing >1%, 20% and 40% HbF levels (BERK, BERKγM, and BERKγH mice, respectively) to resolve the following issues: 1) What is the effect of elevated HbF levels on intravascular sickling, hemolysis, and NO bioavailability?; 2) Is increased NO bioavailability in HbF-expressing sickle mice associated with decreased organ oxidative stress?; and 3) What is the relationship between intravascular hemolysis, NO bioavailability, and organ oxidative stress?
In our studies, we have evaluated the effect of HbF on lipid peroxidation and selected antioxidants. Lipid peroxidation is implicated in cellular injury and organ damage. The first line of cellular defense against oxidative insults consists of the antioxidant SOD that converts O2.− into H2O2, whereas the antioxidants catalase and glutathione peroxidase (GPx) convert H2O2 into water. Reduced GSH is the most abundant nonenzymatic antioxidant in the cell, and its protective action is based on the thiol group of its cysteine, with the formation of oxidized GSSG (5, 14, 38).
The current study reveals that the introduction of antisickling HbF in BERK mice significantly alleviates oxidative stress, and this effect is mediated by antisickling property of HbF, which leads to increased NO bioavailability and decreased organ oxidative stress.
MATERIALS AND METHODS
Chemicals
Butylated hydroxytoluene (BHT), deferoxamine (DFO), DTNB, GSH, GSSG, pyrogallol, triton X-100, EDTA, BSA, thiobarbituric acid (TBA), NADH, and NADPH were obtained from Sigma/Aldrich (St. Louis, MO).
Transgenic Mice
Berkeley (BERK) mice expressing cointegrated 6.4-kb miniLCR, a 1.5-kb PstI fragment of human α1 gene, and a 39-kb KpnI fragment containing human GγAγδ and βS globin genes were generated as described previously (44). These mice are homozygous for the mouse α-knockout (45) and homozygous for mouse β-knockout (52) and express exclusively human hemoglobin via the hemizygous copy of the BERK transgene (44). Two different constructs generated by Gilman (20) (M and H), as previously described, were used for generation of HbF-expressing BERKγM and BERKγH mice. The M (G203) construct was identical to the γH construct, except that it included a PCR-generated 4.3-kb fragment (HumHBB 912 to 5200) containing HS 3 and 4 of the LCR instead of HS4. Injection of these constructs into fertilized mouse oocytes resulted in production of several founder mice. To breed mice on a day-to-day basis, the following schemes are used: 1) The BERK H mice (γH construct) breed successfully with each other. 2) For BERK M (γM or G203 construct) mice, fully knocked out healthy females expressing neutral hemoglobins such as HbA (miniLCRA Hba0//Hba0 Hbb0//Hbb0) or HbC and a BERK M male in which the M is homozygous were used. This leads to a projected breeding efficiency similar to that for the BERK mice. The globin composition in adult transgenic knockout mice was determined by HPLC, as described previously (17). These mice were maintained in a specific pathogen-free facility at Albert Einstein College of Medicine. These knockout mice were extensively backcrossed (an average of eight generations) onto C57BL/6J background. We used BERK mice expressing >1.0%, ∼20%, and ∼40% HbF (termed BERK, BERKγM, and BERKγH mice, respectively). Hematological characteristics of these mice have been described (17, 27).
Control C57BL mice were maintained on a standard diet and drank water ad libitum. Sickle mice were maintained on “sickle chow” developed by Paszty et al. (44) without added arginine and obtained from Purina (Purina Mills, St. Louis, MO) as diet no. 5740C and had access to Nestlets (Anacare, Bellmore, NY) nesting material. All experimental protocols were approved by the institutional animal studies committee.
Measurement of Globin Chains
The globin composition was determined by denaturing HPLC as previously described (16). The percent HbF was calculated as the percent of all β-like globins.
Intravascular Sickling
To determine intravascular sickling, blood samples were drawn from the tail vein into airtight syringes containing 2.5% glutaraldehyde solution in 0.1 mol/l cacodylate buffer, pH 7.4, as described previously (28).
Measurement of Hemolysis
For plasma free hemoglobin, blood samples were drawn from the abdominal aorta, and determinations made using a tetramethyl-benzidine-based assay kit (Catachem , Bridgeport, CT) that measures plasma free hemoglobin and other heme-containing proteins present in plasma.
NOx Determination
For the determination of NO metabolites, NOx, the blood was drawn from bifurcating abdominal aorta using EDTA as an anticoagulant. Total NOx concentration in plasma was determined by nitrate/nitrite colorimetric assay kit (Cayman Chemical, Ann Arbor, MI) using manufacturer's instructions (13, 29).
Preparation of Homogenates, Cytosol, and Microsome Fractions
Mice were killed by cervical dislocation, and the entire liver was then perfused immediately with cold 0.9% NaCl and thereafter carefully removed, trimmed free of extraneous tissue, and rinsed in chilled 0.15M Tris-KCl buffer (0.15M KCl + 10 mM Tris·HCL, pH 7.4). The liver was then blotted dry, weighed quickly and homogenized in ice-cold 0.15 M Tris·KCl buffer (pH 7.4) to yield 10% (wt/vol) homogenate. An aliquot of this homogenate (0.5 ml) was used for assaying reduced glutathione levels, while the remainder was centrifuged at 10,000 rpm for 30 min. The resultant supernatant was transferred into precooled ultracentrifugation tubes and centrifuged at 105,000 g for 60 min in a Beckman ultracentrifuge (model no. L870M). The supernatant (cytosol fraction), after discarding any floating lipid layer and appropriate dilution, was used for the assay of antioxidant enzymes, whereas the pellet representing microsomes was suspended in homogenizing buffer and used for assaying lipid peroxidation. The kidney and the muscle from the legs were carefully removed, along with the liver, trimmed free of extraneous tissue and rinsed in chilled 0.15 M Tris·KCl (pH 7.4). The organs were then blotted dry, weighed quickly, and homogenized in ice-cold 0.15 M Tris-KCl buffer (pH 7.4) to yield a 10% (wt/vol) homogenate. A 0.5-ml aliquot of this homogenate was used for assaying reduced glutathione. The rest of the homogenate was processed as described for the liver.
Assay Methods
Lipid peroxidation.
Lipid peroxidation (LPO) in the microsomes was estimated spectrophotometrically by thiobarbituric acid reactive substances (TBARS) method, as described by Ohkawa et al. (42) and is expressed in terms of malondialdehyde formed per milligram protein. LPO was measured using an iron chelator (DFO) and an antioxidant butylated hydroxytoluene (BHT) in the microsomal preparation as described previously (13). DFO prevents further iron-catalyzed TBARS formation (6), and BHT is used to prevent further lipid peroxidation (19).
Glutathione.
Glutathione (GSH) was estimated as total nonprotein sulfhydryl group by the method as described by Moron et al. (37).
SOD.
Total SOD was assayed utilizing the technique of Marklund and Marklund (35), which involves inhibition of pyrogallol autoxidation at pH 8.0. A single unit of enzyme was defined as the quantity of superoxide dismutase required to produce 50% inhibition of autoxidation.
Catalase.
Catalase was estimated at 240 nm by monitoring the disappearance of H2O2 as described by Aebi (1). The reaction mixture (1 ml, vol) contained 0.02 ml of suitably diluted cytosol in phosphate buffer (50 mM, pH 7.0) and 0.1 ml of 30 mM H2O2 in phosphate buffer. Catalase enzyme activity has been expressed as moles of H2O2 reduced per minute per milligram protein.
Glutathione peroxidase.
GPx activity was measured by the coupled assay method as described by Paglia and Valentine (43). One unit of enzyme activity has been defined as nanomoles of NADPH consumed per minute per milligram protein based on an extinction coefficient of 6.22 mM−1 cm−1.
Peripheral leukocyte counts.
Peripheral leukocyte counts on blood samples obtained from mice were measured using the Bayer Advia 120 (Tarrytown, NY).
Statistical analysis.
Statistical analysis of hematological parameters was performed using one-way ANOVA, followed by Student-Newman-Keuls multiple comparisons. Comparisons between groups were made using Student's t-test. P < 0.05 was considered significant. Regression analysis was done using a linear model Y = a + bX. Statistical analysis was performed using Statgraphics plus 5.0 program for Windows (Manugistics, Rockville, MD). Plasma NOx calculation was performed using the analysis spreadsheet provided by Cayman chemical at http://www.caymanchem.com/app/template/Home.vm.
RESULTS
Hematological parameters, intravascular sickling, and hemolysis. As depicted in Table 1, BERK mice (HbF < 1.0) show low hematocrit level (20.0% ± 2.0%), high reticulocyte counts (39.5% ± 1.5%), and maximal intravascular (venous) sickling (19.9% ± 1.9%), and hemolysis (7.8 ± 0.4 μmol measured as plasma heme); these associations reflect increased red cell destruction in BERK mice. In contrast, with an increase in HbF expression in BERK mice, percent sickled red cells in venous samples showed a distinct decrease as follows: BERKγM (∼20% HbF) − 13.3% ± 0.9%; BERKγH (∼40% HbF) − 6.0% ± 1.0% (P < 0.05 vs. BERK mice, multiple comparisons by ANOVA). The decreased sickling in BERKγM and BERKγH mice resulted in increased hematocrit levels and decreased reticulocyte counts, as well as in decreased hemolysis compared with BERK mice (P < 0.05, multiple comparisons by ANOVA) (Table 1). BERKγH mice (40% HbF) showed maximal reduction in anemia as evidenced by higher hematocrit (40.8% ± 1.1%) and a concomitant decrease in percent reticulocytes compared with C57BL, BERK, and BERKγM mice (P < 0.05 vs. BERK, multiple comparisons). However, in BERKγH mice, plasma hemoglobin levels (5.3 ± 0.2 μmol), although lower than in BERK mice, remained higher compared with C57BL mice (3.0 ± 0.2 μmol, P < 0.01), indicating persistence of hemolysis.
Table 1.
The effect of HbF on hematological parameters, intravascular sickling, and hemolysis (plasma hemoglobin) in knockout sickle mice
| Mouse | HbF, % | Hematocrit, % | Retics, % | Intravascular Sickling, % | Plasma Hemoglobin, μmol heme |
|---|---|---|---|---|---|
| C57BL/6J | 0 | 46.0 ± 1.4 (9)* | 1.6 ± 0.3 (6)* | 0 | 3.0 ± 0.2 (5)* |
| BERK | <1.0 | 20.0 ± 2.0 (9)* | 39.5 ± 1.9 (5)* | 19.9 ± 1.9 (4)* | 7.8 ± 0.4 (8)* |
| BERKγM | 21.0 | 36.3 ± 0.9 (11)* | 31.9 ± 1.9 (5)* | 13.3 ± 0.9 (4)* | 5.5 ± 0.4 (6)* |
| BERKγH | 40.0 | 40.8 ± 1.1 (6)† | 9.1 ± 1.6 (4)* | 6.0 ± 1.0 (4)* | 5.3 ± 0.2 (5)† |
Values are expressed as means ± SE; the numbers in parentheses represent the number of mice used in these measurements. Retics, reticulocytes.
P < 0.05 among the groups indicated;
P < 0.05 vs. C57BL and knockout sickle (BERK) mice (multiple comparisons by ANOVA).
Plasma NO metabolites (NOx) levels.
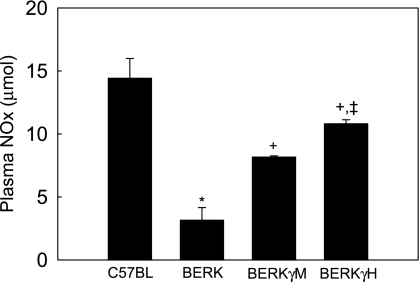
Since NO is effectively inactivated by plasma hemoglobin, we measured NO metabolites (NOx) as a measure of NO. Consistent with maximal hemolysis (Table 1), BERK mice showed marked 78% decrease in NOx levels compared with C57BL mice (each n = 4; P < 0.001) (Fig. 1). In contrast, BERKγM and BERKγH mice expressing 20% and 40% γ-globin levels, respectively (each n = 4), showed marked increases in NOx levels compared with BERK mice. While BERKγM mice showed 2.6-fold in NOx values compared with BERK mice (P < 0.001), maximal NOx levels were observed in BERKγH mice that were 1.3-fold greater than in BERKγM mice (P < 0.001) and 3.42-fold greater than in BERK mice (P < 0.001). The resulting NOx levels in BERKγH mice were almost 75% of the control C57BL values.
Fig. 1.
Plasma NOx levels in C57BL, BERK, BERKγM, and BERKγH mice: the effect of fetal hemoglobin (HbF). BERK mice showed minimal NOx levels compared with other groups, while BERKγH mice had 3.42-fold greater NOx levels compared with BERK mice. The NOx values in BERKγH mice were almost 75% of the control C57BL values. *P < 0.001 vs. C57BL controls; +P < 0.001 vs. BERK mice; ‡P < 0.001 vs. BERKγM mice.
Lipid peroxidation.
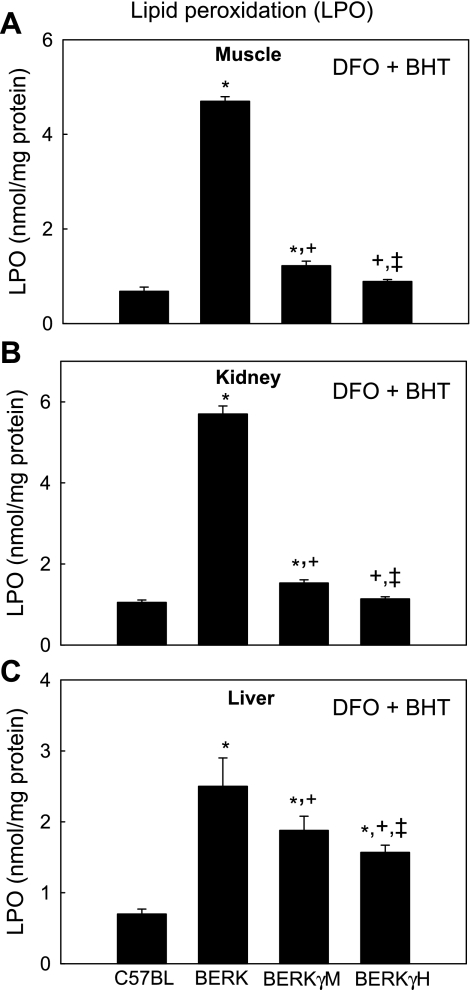
TBARS assay was performed in the presence of DFO and BHT to prevent further lipid peroxidation during the procedure as described in the Methods. Figure 2 depicts the protective effect of HbF on LPO in various tissues (muscle, liver, and kidney) of BERK mice. Evidently, in any given tissue, BERK mice (n = 4) showed marked increase in LPO compared with C57BL mice [i.e., 5.4- to 6.9-fold increase vs. C57BL (n = 6), P < 0.01]. In contrast, BERKγM (n = 6) and BERKγH (n = 4) showed marked 73.2% to 81% decreases in LPO, respectively, in muscle and kidney tissues (P < 0.001), but a smaller (24.8% and 37.2%) decrease in the liver (P < 0.001) when compared with BERK mice. LPO values in BERKγH mice showed further but modest 17% to 27% decreases in these tissues when compared with BERKγM mice (P < 0.05–0.001). The resulting LPO values of BERKγH mice were not significantly different from control C57BL baseline levels in muscle and kidney but remained significantly (2.4-fold) higher in liver.
Fig. 2.
The effect of HbF on lipid peroxidation (LPO) in muscle, kidney and liver of C57BL, BERK, BERKγM, and BERKγH mice. TBARS assay was performed in the presence of deferoxamine (DFO) and butylated hydroxytoluene (BHT) to prevent further lipid peroxidation during the procedure as described in Methods. Lipid peroxidation levels in BERKγH mice showed almost complete recovery to the control levels in the muscle and kidney tissues, and nearly 40% reduction in the liver compared with BERK mice *P < 0.01–<0.001 vs. C57BL controls; +P < 0.001 vs. BERK mice; ‡P < 0.05–0.001 vs. BERKγM mice.
Antioxidants
Glutathione.
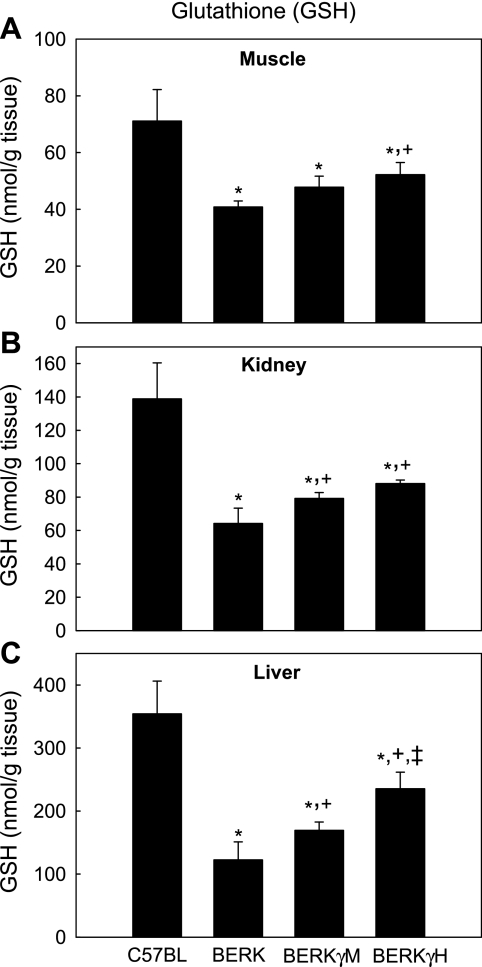
As shown in Fig. 3, in each group of mice (n = 4–6), glutathione (GSH) levels were maximal in the liver followed by kidney and the muscle tissues in that order (P < 0.05, multiple comparisons by ANOVA). Furthermore, for any given tissue, GSH levels were lowest in BERK mice and ∼43–65% lower than those in control C57BL mice (n = 6) (P < 0.001). In BERK mice expressing HbF, GSH levels increased significantly in any given tissue compared with BERK mice (BERKγM, % increase: muscle ∼17%, kidney 23%, and liver 38%, P < 0.05–0.01 vs. BERK; BERKγH, % increase: 28%, 37.2%, and 92% in that order, P < 0.01–0.001 vs. BERK) (Fig. 3). In BERKγH mice, the greater increase in GSH levels was clearly associated with higher expression of HbF, and the values approached almost 63% to 74% of the C57BL baseline values in the tissues examined.
Fig. 3.
GSH in C57BL, BERK, BERKγM, and BERKγH mice: the effect of HbF. In BERKγH mice, GSH values approached almost 63% to 74% of the C57BL baseline levels in these tissues. *P < 0.001 vs. C57BL controls; +P < 0.05–0.001 vs. BERK mice; ‡P < 0.01 vs. BERKγM mice.
SOD.
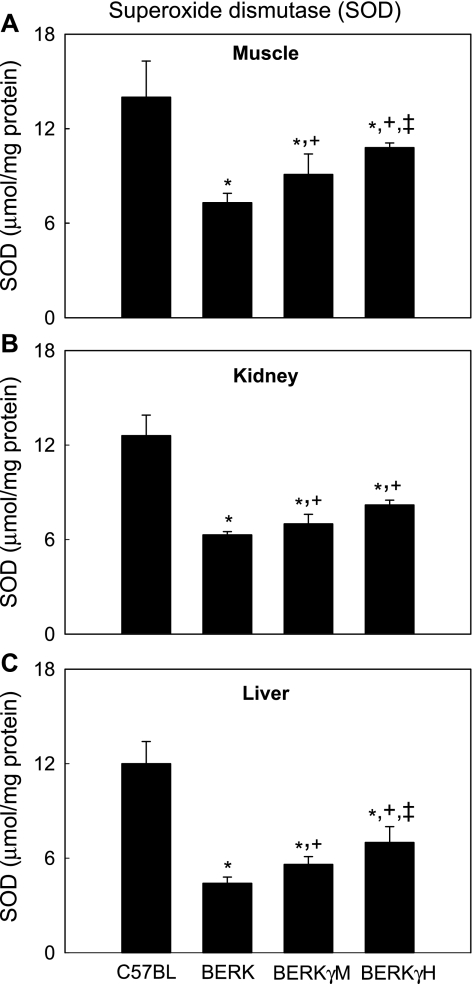
As shown in Fig. 4, BERK mice showed lowest total SOD activity in any given tissue compared with other groups. Compared with C57BL controls, BERK mice tissues showed a marked ∼48–63.3% decrease in SOD activity (P < 0.001). Compared with BERK mice, SOD activity was significantly augmented in BERKγM and BERKγH mice. In BERKγM mice, SOD activity increased by ∼25%, 11%, and 27% in the muscle, kidney, and the liver, respectively, (P < 0.05-<0.001 vs. BERK), while in BERKγH mice, the increase was more pronounced, i.e., 48%, 30%, and 59% in the corresponding tissues (P < 0.001 vs. BERK). Compared with BERKγM mice, BERKγH mice exhibited significant 18.7% and 25% increases in SOD activity in the muscle and liver, respectively (P < 0.05 and P < 0.001), although the resulting values remained lower than in control C57BL mice.
Fig. 4.
SOD activity in C57BL, BERK, BERKγM, and BERKγH mice: the effect of HbF. *P < 0.01–0.001 vs. C57BL controls; +P < 0.05–0.001 vs. BERK mice; ‡P < 0.05–0.001 vs. BERKγM mice in the muscle and kidney.
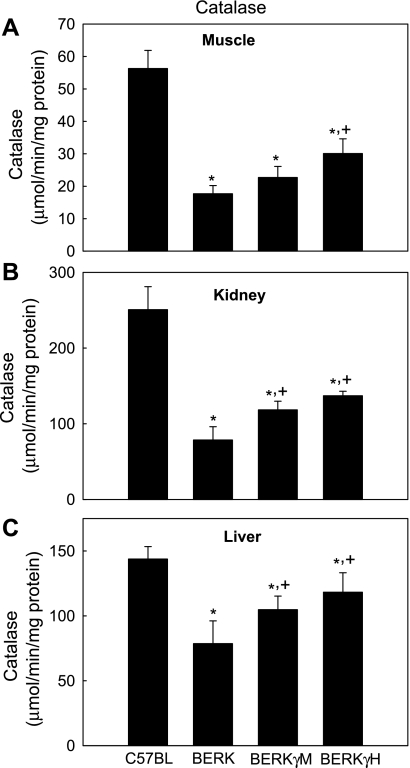
Catalase.
In the tissues examined, BERK mice exhibited a marked decrease in catalase activity (Fig. 5). The muscle and kidney catalase showed maximal decrease (68.4% and 68.7%, decrease, respectively, each P < 0.001 vs. C57BL mice) followed by 32% decrease in the liver (P < 0.001 vs. C57BL). In contrast, BERKγM expressing 20% HbF levels showed significant 28.2% and 50.5% increases in catalase activity in the kidney and liver, respectively (P < 0.05 and P < 0.01 vs. BERK), but no significant change in the muscle. On the other hand, marked increase in catalase activity was observed in any given tissue of BERKγH mice (muscle, 70.0%; kidney, 74.3%; liver 22.4%; P < 0.01, P < 0.001, and P < 0.01, respectively, vs. BERK).
Fig. 5.
Catalase activity in C57BL, BERK, BERKγM, and BERKγH mice: the effect of HbF. *P < 0.05–0.001 vs. C57BL controls, +P < 0.05–0.001 vs. BERK mice.
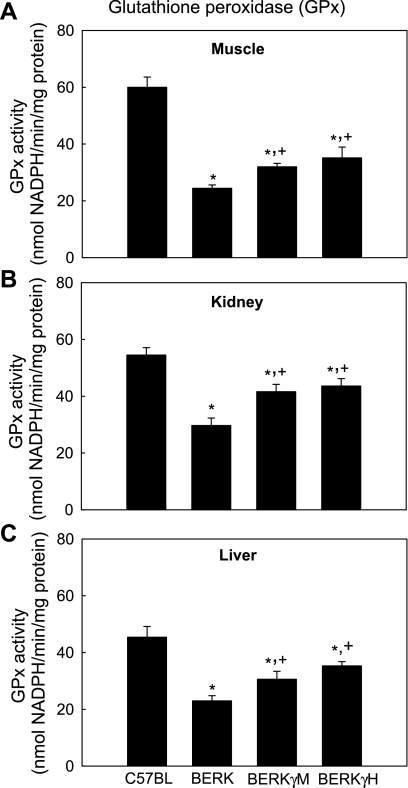
Glutathione peroxidase.
In the tissues examined, glutathione peroxidase (GPx) activity showed marked 45.5–59.3% decrease (P < 0.01) in BERK mice compared with C57BL controls (Fig. 6). In contrast, introduction of HbF resulted in 31.1–40.0% increases in GPx activity in BERKγM mice (P < 0.001 vs. BERK), while maximal increase (43.8–53.5%) was observed for BERKγH mice (P < 0.001 vs. BERK). Although BERKγH mice showed marked increases in GPx activity over BERK mice, the resulting values did not reach the level of C57BL baseline values.
Fig. 6.
Glutathione peroxidase (GPx) activity in C57BL, BERK, BERKγM, and BERKγH mice: the effect of HbF. *P < 0.01–0.001 vs. C57BL controls; +P < 0.001 vs. BERK mice.
Correlates of Oxidative Stress
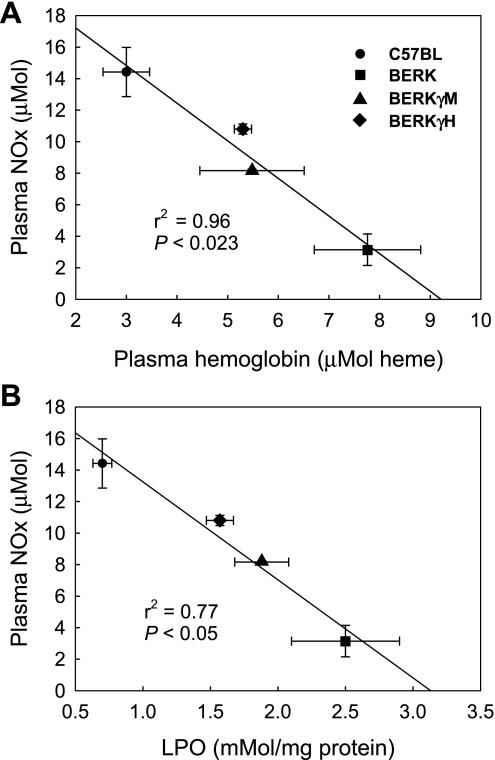
Next, we analyzed our data to ascertain whether the decreased organ oxidative stress in HbF expressing BERK mice was a consequence of decreased hemolysis and increased NO bioavailability. As shown in Table 1, the decrease in intravascular sickling paralleled percent HbF expression in BERKγM and BERKγH mice, and it was associated with increased hematocrit and decreased reticulocyte counts. Importantly, linear regression analysis of the data showed a strong relationship between plasma hemoglobin and plasma NOx levels as NO bioavailability increased commensurate with the decrease in plasma hemoglobin (Fig. 7A). Similarly, a strong inverse relationship was noted when plasma NOx levels were plotted against the LPO values in the liver tissue (Fig. 7B). Thus, in BERK mice (HbF, <1.0%), lowest plasma NOx values were associated with maximal lipid peroxidation; a similar relationship between NOx levels and lipid peroxidation was observed in the muscle and kidney (data not shown). Conversely, with the introduction of antisickling HbF, the marked increase in NOx levels was associated with decreased lipid peroxidation.
Fig. 7.
A: relationship between plasma heme and NOx values. Linear regression revealed a strong correlation between plasma heme and NOx levels, indicating that a decrease in hemolysis was associated with increased NO bioavailability. B: relationship between liver LPO and plasma NOx values showed a strong correlation between the two parameters. With an increase in plasma NOx, the LPO values showed a distinct decrease in BERKγM, BERKγH, and control C57BL mice. These results indicate that a reduction in hemolysis results in increased NO bioavailability that, in turn, has an antioxidant effect.
HbF Decreases Peripheral Leukocyte Counts
To ascertain whether the decrease in oxidative stress observed in the presence of HbF was associated with attenuated inflammation, we measured peripheral leukocyte counts in C57BL, BERK, and BERKγH mice. Compared with C57BL mice (n = 20), BERK mice (n = 7) showed 3.2-fold greater peripheral leukocyte counts (10,915 ± 1,190 vs. 35,064 ± 3,255 counts/μl, P < 0.001). In contrast, compared with BERK mice, BERKγH mice (n = 14; HbF ∼40%) showed marked 3-fold decrease in peripheral leukocyte counts (11,542 ± 1,085 counts/μl, each P < 0.001 vs. BERK), and the resulting values were not significantly different from the control C57BL values.
DISCUSSION
The multiple (pleiotropic) effects of the βS gene include intravascular sickling, dense red cell generation, reticulocytosis, abnormal cation transport, hemolysis, reperfusion injury, and multiple organ damage. On the other hand, the presence of modifier (epistatic) genetic factors such as HbF, a product of γ-globin genes (Gγ and Aγ genes), significantly alter disease severity and progression by inhibiting HbS polymerization and intravascular sickling, which is the primary defect in SCD. In the present study, we show that the presence of HbF in transgenic-knockout sickle (BERK) mice significantly ameliorates several expressed abnormalities. In this mouse model of SCD, increasing HbF levels results in commensurate decrease in intravascular sickling-decreased hemolysis, enhanced NO production and, importantly, decreased organ oxidative stress. Finally, the results show a strong relationship between NO bioavailability and hemolysis on one hand, and between NO bioavailability and organ oxidative stress on the other, clearly indicating that events originating from the primary defect (i.e., HbS polymerization and sickling) lead to increased organ oxidative stress.
Oxidative damage in SCD and mouse models of SCD has been attributed to oxygen free radicals resulting from reperfusion injury and heme-mediated peroxidase chemistry (22, 26, 32, 54). BERK mice, expressing exclusively human α- and βS-globins, show maximal intravascular sickling that can intermittently disrupt blood flow to the tissues and result in reperfusion injury and oxidant generation. BERK mice also show maximal hemolysis and minimal NO metabolites (NOx) compared with other groups of mice examined. We believe that contribution of oxidative stress to NO consumption in BERK mice will be significant and even independent of hemolysis, particularly during episodes of transient vaso-occlusive events and reperfusion injury. NO inactivation by plasma ferrous hemoglobin, as shown by Gladwin and colleagues (49), is a major contributor to oxidative stress. Nitric oxide synthase (NOS) itself can be decoupled by oxidation of the essential cofactor tetrahydrobiopterin (BH4) (2, 11), and decoupled NOS produces superoxide in place of nitric oxide. Previous studies indicate that oxidative stress is modulated by NO bioavailability and vice versa (18, 25, 55).
In BERK mice, decreased NO bioavailability will contribute to oxidative stress in a manner similar to that observed after inhibiting NO synthase activity in transgenic (NY1DD) sickle mice that have mild pathology (13). In fact, the severe pathology of BERK mice is associated with intense organ oxidative stress, as evidenced by marked increase in lipid peroxidation, a marker of peroxidation of membrane lipids by peroxides, and diminished activity of antioxidants, which is in accord with our previous studies (13). BERK mice show 5.4- to 6.9- fold increases in lipid peroxidation in the tissues examined. Lipid peroxidation causes damage to the cell membrane and mitochondria and is a likely contributor to multiple organ damage in SCD. In BERK mice, depleted levels of tissue antioxidants such as GSH, total SOD, catalase and GPx will result in diminished capacity to quench oxygen radicals and thereby enhance lipid peroxidation (8, 12). Moreover, during reperfusion (i.e., transient vaso-occlusive events), overproduction of oxygen radicals is implicated in degradation and consumption of antioxidants (40). Hence, failure to replenish antioxidants will exacerbate oxidative stress and contribute to inflammation and multiple organ damage in SCD.
Our studies show that introduction of HbF in BERK mice resulted in marked decrease in intravascular sickling leading to recovery of hematological parameters and decreased hemolysis in BERKγM and BERKγH mice, respectively expressing 20% and 40% HbF (balance HbS). Compared with BERK mice, BERKγH mice showed >100% increase in Hct and reduced erythropoietic stress as evidenced by ∼85% decrease in peripheral reticulocytes counts (see Table 1). Decreased sickling is likely to diminish transient vaso-occlusive events and oxidant generation. Decreased hemolysis was evident by 32% decrease in cell-free plasma hemoglobin, which will also result in decreased release of arginase that consumes arginine, the substrate of NOS (23). Importantly, BERKγM and BERKγH mice showed a significant increase in NO metabolite (NOx) levels, which is indicative of decreased NO consumption/inactivation secondary to diminished plasma heme levels and global oxidative stress in these mice. Although NOx values in BERKγH mice did not reach the C57BL baseline levels, the increase was 3.42-fold over the BERK values and within 75% of the C57BL levels.
In conformity with increased NO bioavailability, BERKγM and BERKγH mice showed marked, 73.2% and 81%, decrease in lipid peroxidation in muscle and kidney tissues (see Fig. 2). In contrast to 5.4- to 6.9-fold increase in lipid peroxidation in the tissues of BERK mice over the control C57BL values, lipid peroxidation levels in BERKγH mice showed almost complete recovery to the control levels in muscle and kidney, and nearly 40% decrease in liver compared with BERK mice, demonstrating a marked ameliorating effect of antisickling HbF in these mice. These observations are consistent with previous reports that termination of lipid peroxidation, involving the reaction of NO with lipid-oxy and -peroxy radicals, is one of the most important antioxidant properties of NO (41, 55, 56).
Consistent with attenuated lipid peroxidation, BERK mice expressing HbF, show distinct increase in the level/activity of antioxidants (both nonenzymatic and enzymatic). Because the decrease in antioxidants in BERK mice suggests consumption/inactivation of antioxidants by excessive generation of reactive oxygen species, the decreased oxidative stress in BERKγM and BERKγH mice, as exemplified by diminished lipid peroxidation, is likely to result in the observed recovery of antioxidants. Nonenzymatic GSH, which was depleted by ∼43–65% in the tissues of BERK mice compared with C57BL mice, showed a distinct rebound in BERKγH mice with the values approaching 63.5% to 73.4% of the C57BL baseline levels (see Fig. 3). GSH makes major contributions to the recycling of other antioxidants that become depleted due to excessive oxidative stress (3, 36). GSH effectively scavenges reactive oxygen species directly and indirectly by enzymatic reactions (58). Moreover, GPx catalyzes GSH-dependent reduction of H2O2 and other peroxides (34). Increased oxidant generation and depleted GSH and glutamine levels have been associated with compromised red cell integrity, hemolysis, and decreased NO bioavailability, all factors that might as well contribute to the pathogenesis of this disease (23, 38). Oxidant generation is also associated with damage to protein 4.1 binding ability (51) and damage to red cell flipase (33), which will contribute to phosphatidylserine exposure and rapid removal from the circulation.
Increased O2.− generation not only results in endothelial dysfunction and inflammation in BERK mice, but also in excessive nitrotyrosine formation that is the result of protein nitration by peroxynitrite (ONOO−) (27, 29), a molecule that is implicated in lipid peroxidation (47, 50) and impaired vascular responses (27, 30, 31). ONOO− formation and nitration of tyrosine residues of proteins (i.e., formation of nitrotyrosine) may also be driven by plasma heme-mediated peroxidase chemistry (32, 54). Importantly, decreased sickling in the BERK mice expressing 20% HbF is associated with diminished nitrotyrosine expression (27), and NO replenishment by arginine in this mouse model increases NO production, decreases lipid peroxidation and increases endogenous antioxidants (13).
The antioxidant effect of HbF is further evident by significant increases in antioxidant enzymes SOD, catalase, and GPx. With an increase in HbF levels, BERKγM and BERKγH mice show corresponding increases in these antioxidants in all the tissues examined, although the resulting values remained lower than in control C57BL mice (see Figs. 4–6). This incomplete recovery is likely due, in part, to persistent albeit reduced hemolysis in HbF expressing BERK mice, and may reflect heme-mediated oxidative stress and an incomplete recovery of NOx levels (i.e., 75% of C57BL baseline) as observed in BERKγH mice expressing 40% HbF. The persistent hemolysis in BERK mice expressing HbF is a condition comparable to that observed in sickle patients on hydroxyurea therapy (24). Nevertheless, the observed antisickling effect of HbF in BERK mice is likely to decrease sickling-induced vaso-occlusive events and result in reduced hemolysis, increased plasma NOx production, and reduced oxidative stress.
In the present studies, we show that reduction of intravascular sickling by introduction of HbF has wide-ranging effects leading to an increase in NO bioavailability and decreased organ oxidative stress. A strong relationship between plasma NOx levels and lipid peroxidation (see Fig. 7) further supports a role of NO in decreased organ oxidative stress. Decreased oxidative stress is accompanied by a significant decrease in peripheral leukocyte counts, demonstrating the anti-inflammatory effect of HbF. Thus, replenishment of NO by genetic manipulation to elevate HbF levels would result in decreased disease severity by attenuating oxidative stress and inflammation. We have previously shown that NO replenishment by arginine significantly elevates NO metabolites in transgenic-knockout sickle mice, accompanied by attenuated lipid peroxidation and enhanced activity of antioxidants (13), suggesting a role for NO in modulating oxidative stress in SCD. Future studies will be needed to examine whether combining HbF elevation with arginine therapy will have a greater therapeutic effect in this disease. Nevertheless, the results of this study show that the protective effects of antisickling HbF are derived from its ability to decrease oxidative stress and enhance NO bioavailability.
Perspectives and Significance
The events originating from the primary defect of SCD (i.e., hemoglobin S polymerization and intravascular sickling) lead to multiple pathologies that include red cell abnormalities, vaso-occlusive events (reperfusion injury), and hemolysis, which contribute to increased oxidative stress and decreased NO bioavailability. Increased oxidative stress and NO deficiency in this disease are a major source of endothelial dysfunction, inflammation, vascular pathobiology, and multiple organ damage. The interaction between NO and oxidative stress includes but is not limited to peroxynitrite (OONO−) formation; oxidative decoupling of endothelial nitric oxide synthase (eNOS); red cell hemolysis due to oxidative stress leading to release of arginase and the consumption of arginine, the substrate of NOS; and release of cell-free hemoglobin. Thus, the pathophysiology of sickle cell disease is driven by both increased oxidative stress and reduced NO bioavailability.
The introduction of antisickling HbF into BERK mice significantly alleviates oxidative stress and increases NO production, primarily by decreasing intravascular sickling and its consequences. We have previously demonstrated that arginine supplementation increases NO production, decreases oxidative stress and improves microvascular flow in transgenic sickle mice (13, 29). Taken together, these results support the contention that the interaction of oxidative stress and NO deficiency impact multiple aspects of the pathophysiology of SCD. Depleted levels of antioxidants likely result in increased susceptibility to lipid peroxidation. Lipid peroxidation causes damage to the cell and mitochondrial membranes and will contribute to multiple organ damage in this disease. Degradation and consumption of antioxidants by the release of oxidants during reperfusion has been recognized (40). Our observations support the concept that multiple-organ damage in SCD is associated with increased oxidative stress, and elevated levels of antisickling HbF lead to an ameliorating effect.
GRANTS
This study was supported by National Institutes of Health grants HL070047 (to D. K. Kaul) and HL092183 (to M. E. Fabry).
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank Sandra Suzuka for genotyping of the transgenic mice used in these studies.
REFERENCES
- 1.Aebi H. Catalase in vitro. Methods Enzymol 105: 121–126, 1984 [DOI] [PubMed] [Google Scholar]
- 2.Alp NJ, Channon KM. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol 24: 413–420, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Anderson ME. Glutathione and glutathione delivery compounds. Adv Pharmacol 38: 65–78, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Aslan M, Ryan TM, Adler B, Townes TM, Parks DA, Thompson JA, Tousson A, Gladwin MT, Patel RP, Tarpey MM, Batinic-Haberle I, White CR, Freeman BA. Oxygen radical inhibition of nitric oxide-dependent vascular function in sickle cell disease. Proc Natl Acad Sci USA 98: 15215–15220, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baguley BC, Wakelin LP, Jacintho JD, Kovacic P. Mechanisms of action of DNA intercalating acridine-based drugs: how important are contributions from electron transfer and oxidative stress? Curr Med Chem 10: 2643–2649, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Braughler JM, Duncan LA, Chase RL. The involvement of iron in lipid peroxidation. Importance of ferric to ferrous ratios in initiation. J Biol Chem 261: 10282–10289, 1986 [PubMed] [Google Scholar]
- 7.Charache S, Barton FB, Moore RD, Terrin ML, Steinberg MH, Dover GJ, Ballas SK, McMahon RP, Castro O, Orringer EP. Hydroxyurea and sickle cell anemia. Clinical utility of a myelosuppressive “switching” agent. The Multicenter Study of Hydroxyurea in Sickle Cell Anemia. Medicine 75: 300–326, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Chiu D, Lubin B. Abnormal vitamin E and glutathione peroxidase levels in sickle cell anemia: evidence for increased susceptibility to lipid peroxidation in vivo. J Lab Clin Med 94: 542–548, 1979 [PubMed] [Google Scholar]
- 9.Cokic VP, Andric SA, Stojilkovic SS, Noguchi CT, Schechter AN. Hydroxyurea nitrosylates and activates soluble guanylyl cyclase in human erythroid cells. Blood 111: 1117–1123, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cokic VP, Smith RD, Beleslin-Cokic BB, Njoroge JM, Miller JL, Gladwin MT, Schechter AN. Hydroxyurea induces fetal hemoglobin by the nitric oxide-dependent activation of soluble guanylyl cyclase. J Clin Invest 111: 231–239, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crabtree MJ, Smith CL, Lam G, Goligorsky MS, Gross SS. Ratio of 5,6,7,8-tetrahydrobiopterin to 7,8-dihydrobiopterin in endothelial cells determines glucose-elicited changes in NO vs. superoxide production by eNOS. Am J Physiol Heart Circ Physiol 294: H1530–H1540, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Das SK, Nair RC. Superoxide dismutase, glutathione peroxidase, catalase and lipid peroxidation of normal and sickled erythrocytes. Br J Haematol 44: 87–92, 1980 [DOI] [PubMed] [Google Scholar]
- 13.Dasgupta T, Hebbel RP, Kaul DK. Protective effect of arginine on oxidative stress in transgenic sickle mouse models. Free Radic Biol Med 41: 1771–1780, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dobashi K, Ghosh B, Orak JK, Singh I, Singh AK. Kidney ischemia-reperfusion: modulation of antioxidant defenses. Mol Cell Biochem 205: 1–11, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Fabry ME, Nagel RL. The effect of deoxygenation on red cell density: significance for the pathophysiology of sickle cell anemia. Blood 60: 1370–1377, 1982 [PubMed] [Google Scholar]
- 16.Fabry ME, Sengupta A, Suzuka SM, Costantini F, Rubin EM, Hofrichter Christoph G, Manci E, Culberson D, Factor SM. A second generation transgenic mouse model expressing both hemoglobin S (HbS) and HbS-Antilles results in increased phenotypic severity. Blood 86: 2419–2428, 1995 [PubMed] [Google Scholar]
- 17.Fabry ME, Suzuka SM, Weinberg RS, Lawrence C, Factor SM, Gilman JG, Costantini F, Nagel RL. Second generation knockout sickle mice: the effect of HbF. Blood 97: 410–418, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Gaboury J, Woodman RC, Granger DN, Reinhardt P, Kubes P. Nitric oxide prevents leukocyte adherence: role of superoxide. Am J Physiol Heart Circ Physiol 265: H862–H867, 1993 [DOI] [PubMed] [Google Scholar]
- 19.Garcia YJ, Rodriguez-Malaver AJ, Penaloza N. Lipid peroxidation measurement by thiobarbituric acid assay in rat cerebellar slices. J Neurosci Methods 144: 127–135, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Gilman JG. Developmental changes of human Gγ and Aγ and mouse embryonic εy1, εy2, and βh in transgenic mice with HS4-Gγ-Aγ. Blood 86: 648a, 1995 [Google Scholar]
- 21.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res 86: 494–501, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Hebbel RP, Osarogiagbon R, Kaul D. The endothelial biology of sickle cell disease: inflammation and a chronic vasculopathy. Microcirculation 11: 129–151, 2004 [PubMed] [Google Scholar]
- 23.Hsu LL, Champion HC, Campbell-Lee SA, Bivalacqua TJ, Manci EA, Diwan BA, Schimel DM, Cochard AE, Wang X, Schechter AN, Noguchi CT, Gladwin MT. Hemolysis in sickle cell mice causes pulmonary hypertension due to global impairment in nitric oxide bioavailability. Blood 109: 3088–3098, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jison ML, Munson PJ, Barb JJ, Suffredini AF, Talwar S, Logun C, Raghavachari N, Beigel JH, Shelhamer JH, Danner RL, Gladwin MT. Blood mononuclear cell gene expression profiles characterize the oxidant, hemolytic, and inflammatory stress of sickle cell disease. Blood 104: 270–280, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jourd‘heuil D, Jourd’heuil FL, Kutchukian PS, Musah RA, Wink DA, Grisham MB. Reaction of superoxide and nitric oxide with peroxynitrite. Implications for peroxynitrite-mediated oxidation reactions in vivo. J Biol Chem 276: 28799–28805, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Kaul DK, Hebbel RP. Hypoxia/reoxygenation causes inflammatory response in transgenic sickle mice but not in normal mice [see comments]. J Clin Invest 106: 411–420, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaul DK, Liu XD, Chang HY, Nagel RL, Fabry ME. Effect of fetal hemoglobin on microvascular regulation in sickle transgenic-knockout mice. J Clin Invest 114: 1136–1145, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaul DK, Xue H. Rate of deoxygenation and rheologic behavior of blood in sickle cell anemia. Blood 77: 1353–1361, 1991 [PubMed] [Google Scholar]
- 29.Kaul DK, Zhang X, Dasgupta T, Fabry ME. Arginine therapy of transgenic-knockout sickle mice improves microvascular function by reducing non-nitric oxide vasodilators, hemolysis, and oxidative stress. Am J Physiol Heart Circ Physiol 295: H39–H47, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kooy NW, Lewis SJ. Nitrotyrosine attenuates the hemodynamic effects of adrenoceptor agonists in vivo: relevance to the pathophysiology of peroxynitrite. Eur J Pharmacol 310: 155–161, 1996 [DOI] [PubMed] [Google Scholar]
- 31.Kooy NW, Lewis SJ. The peroxynitrite product 3-nitro-l-tyrosine attenuates the hemodynamic responses to angiotensin II in vivo. Eur J Pharmacol 315: 165–170, 1996 [DOI] [PubMed] [Google Scholar]
- 32.Krajewski ML, Hsu LL, Gladwin MT. The proverbial chicken or the egg? Dissection of the role of cell-free hemoglobin versus reactive oxygen species in sickle cell pathophysiology. Am J Physiol Heart Circ Physiol 295: H4–H7, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuypers FA, de Jong K. The role of phosphatidylserine in recognition and removal of erythrocytes. Cell Mol Biol (Noisy -le-grand) 50: 147–158, 2004 [PubMed] [Google Scholar]
- 34.Lei XG. In vivo antioxidant role of glutathione peroxidase: evidence from knockout mice. Methods Enzymol 347: 213–225, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47: 469–474, 1974 [DOI] [PubMed] [Google Scholar]
- 36.Meister A. Glutathione-ascorbic acid antioxidant system in animals. J Biol Chem 269: 9397–9400, 1994 [PubMed] [Google Scholar]
- 37.Moron MS, DePierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta 582: 67–78, 1979 [DOI] [PubMed] [Google Scholar]
- 38.Morris CR, Suh JH, Hagar W, Larkin S, Bland DA, Steinberg MH, Vichinsky EP, Shigenaga M, Ames B, Kuypers FA, Klings ES. Erythrocyte glutamine depletion, altered redox environment, and pulmonary hypertension in sickle cell disease. Blood 111: 402–410, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagel RL. The challenge of painful crisis in sickle cell disease. JAMA 286: 2152–2153, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Nita DA, Nita V, Spulber S, Moldovan M, Popa DP, Zagrean AM, Zagrean L. Oxidative damage following cerebral ischemia depends on reperfusion—a biochemical study in rat. J Cell Mol Med 5: 163–170, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Donnell VB, Chumley PH, Hogg N, Bloodsworth A, Darley-Usmar VM, Freeman BA. Nitric oxide inhibition of lipid peroxidation: kinetics of reaction with lipid peroxyl radicals and comparison with alpha-tocopherol. Biochemistry 36: 15216–15223, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95: 351–358, 1979 [DOI] [PubMed] [Google Scholar]
- 43.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70: 158–169, 1967 [PubMed] [Google Scholar]
- 44.Paszty C, Brion CM, Manci E, Witkowska HE, Stevens ME, Mohandas N, Rubin EM. Transgenic knockout mice with exclusively human sickle hemoglobin and sickle cell disease [see comments]. Science 278: 876–878, 1997 [DOI] [PubMed] [Google Scholar]
- 45.Paszty C, Mohandas N, Stevens ME, Loring JF, Liebhaber SA, Brion CM, Rubin EM. Lethal alpha-thalassaemia created by gene targeting in mice and its genetic rescue. Nat Genetics 11: 33–39, 1995 [DOI] [PubMed] [Google Scholar]
- 46.Persons DA, Hargrove PW, Allay ER, Hanawa H, Nienhuis AW. The degree of phenotypic correction of murine beta-thalassemia intermedia following lentiviral-mediated transfer of a human gamma-globin gene is influenced by chromosomal position effects and vector copy number. Blood 101: 2175–2183, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys 288: 481–487, 1991 [DOI] [PubMed] [Google Scholar]
- 48.Reed W, Vichinsky EP. New considerations in the treatment of sickle cell disease. Annu Rev Med 49: 461–474, 1998 [DOI] [PubMed] [Google Scholar]
- 49.Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, III, Schechter AN, Gladwin MT. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med 8: 1383–1389, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Rubbo H, Radi R, Trujillo M, Telleri R, Kalyanaraman B, Barnes S, Kirk M, Freeman BA. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J Biol Chem 269: 26066–26075, 1994 [PubMed] [Google Scholar]
- 51.Schwartz RS, Rybicki AC, Heath RH, Lubin BH. Protein 4.1 in sickle erythrocytes. Evidence for oxidative damage. J Biol Chem 262: 15666–15672, 1987 [PubMed] [Google Scholar]
- 52.Shehee WR, Oliver P, Smithies O. Lethal thalassemia after insertional disruption of the mouse major adult beta-globin gene. Proc Natl Acad Sci USA 90: 3177–3181, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steinberg MH, Lu ZH, Barton FB, Terrin ML, Charache S, Dover GJ. Fetal hemoglobin in sickle cell anemia: determinants of response to hydroxyurea. Multicenter Study of Hydroxyurea. Blood 89: 1078–1088, 1997 [PubMed] [Google Scholar]
- 54.Thomas DD, Espey MG, Vitek MP, Miranda KM, Wink DA. Protein nitration is mediated by heme and free metals through Fenton-type chemistry: an alternative to the NO/O2- reaction. Proc Natl Acad Sci USA 99: 12691–12696, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wink DA, Miranda KM, Espey MG, Pluta RM, Hewett SJ, Colton C, Vitek M, Feelisch M, Grisham MB. Mechanisms of the antioxidant effects of nitric oxide. Antioxid Redox Signal 3: 203–213, 2001 [DOI] [PubMed] [Google Scholar]
- 56.Wink DA, Mitchell JB. Chemical biology of nitric oxide: Insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic Biol Med 25: 434–456, 1998 [DOI] [PubMed] [Google Scholar]
- 57.Wood KC, Hebbel RP, Granger DN. Endothelial cell NADPH oxidase mediates the cerebral microvascular dysfunction in sickle cell transgenic mice. FASEB J 19: 989–991, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr 134: 489–492, 2004 [DOI] [PubMed] [Google Scholar]