Abstract
Superoxide (O2− ) enhances tubuloglomerular feedback (TGF) by scavenging nitric oxide at the macula densa (MD). The primary source of O2− in the MD during TGF is NADPH oxidase, which is activated by membrane depolarization. While Rac, a small GTP-binding protein, has been shown to enhance NADPH oxidase activity, its role in O2− generation by the MD is unknown. We hypothesized that depolarization of the MD leads to translocation of Rac to the apical membrane, and its activation, in turn, augments O2− generation during TGF. We tested this by measuring membrane potential and increased O2− levels during TGF responses in isolated, perfused tubules containing the intact MD plaque. Switching tubular NaCl from 10 to 80 mM, which induces TGF, depolarized membrane potential by 28.4 ± 4.5% from control (P < 0.05) and O2− levels from 124 ± 19 to 361 ± 27 U/min. This NaCl-induced depolarization and O2− generation were blocked by a Cl− channel blocker, 5-nitro-2(3-phenylpropylamino) benzoic acid (NPPB; 10−6 M). Inhibition of Rac blunted NaCl-induced O2− generation by 47%. When the NaCl content of the MD perfusate was increased from 10 to 80 mM, immunointensity of Rac on the apical side increased from 32 ± 3.1 to 46 ± 2.5% of the total immunofluorescence in the MD, indicating that high NaCl induces the translocation of Rac to the apical membrane. This NaCl-induced Rac translocation was blocked by a Cl− channel blocker, NPPB, indicating that depolarization of the MD induced Rac translocation. In conclusion, we found that depolarization of the MD during TGF leads to translocation of Rac to the apical membrane, which enhances O2− generation by the MD.
Keywords: glomerular filtration rate, NADPH oxidase, NaCl, translocation
the macula densa (MD) is a group of specialized epithelial cells located at the distal segment of the thick ascending limb (TAL) that serve as a sensor of luminal NaCl levels. A change in NaCl delivery to the MD results in an adjustment to the tone of the glomerular arterioles, and consequently glomerular filtration rate (GFR), through a mechanism called tubuloglomerular feedback (TGF). The TGF response is initiated by an increase in tubular flow (and consequently in NaCl delivery) to the MD, which in turn initiates a signaling cascade that constricts the afferent arterioles and thereby reduces GFR (3, 22, 29). This fall in GFR helps restore tubular flow toward normal, thus preventing excessive changes that could lead to perturbations of extracellular sodium and volume (14, 21, 26, 30). Thus TGF is one of the main mechanisms regulating the renal microcirculation, NaCl excretion, and ultimately blood pressure.
Our previous studies have shown that superoxide (O2−) enhances TGF by scavenging nitric oxide (NO) at the MD (18). The primary source of O2− in the MD during TGF is NADPH oxidase, which is activated by membrane depolarization and enhanced by elevated intracellular pH (15, 16). Using laser capture microdissection to isolate MD cells from frozen rat kidneys, we found that the MD expresses the NOX2 and NOX4 isoforms of NADPH oxidase, and that NOX2 was mostly responsible for NaCl-induced O2− (32). However, the mechanisms that modulate the activity of NOX2 during NaCl-induced activation are incompletely understood. In the present study we evaluate the role of Rac, a small GTP-binding protein that acts as a molecular switch, which activates NADPH oxidase (9), in modulating O2− generation by the MD during TGF. We hypothesized that depolarization of the MD leads to activation and translocation of Rac to the apical membrane, which in turn enhances O2− generation during TGF.
MATERIALS AND METHODS
All experiments were approved by the University of Mississippi Animal Care and Use Committee prior to performing any procedures on animals.
Isolation and microperfusion of the TAL and attached MD.
We used methods similar to those described previously to isolate and microperfuse the TAL and attached MD (14, 16, 17). Briefly, Sprague-Dawley rats were anesthetized with ketamine (50 mg/kg im) and xylazine (50 mg/kg ip). The kidneys were removed and sliced along the corticomedullary axis. Slices were placed in ice-cold MEM (GIBCO, Grand Island, NY) containing 5% BSA (Sigma, St. Louis, MO) and dissected under a stereomicroscope (model SMZ 1500; Nikon). A single superficial intact glomerulus was microdissected together with its adherent tubular segments consisting of the TAL, MD, and early distal tubule. With the use of a micropipette, the sample was transferred to a temperature-regulated chamber mounted on an inverted microscope (Eclipse Ti; Nikon). The TAL was cannulated with an array of glass pipettes. Another pipette was used to hold and stabilize the glomerulus. The sample was arranged so that each MD cell could be clearly visualized on the edge of the glomerulus. The MD was perfused with physiological saline comprising (in mM) 10 HEPES, 1.0 CaCO3, 0.5 K2HPO4, 4.0 KHCO3, 1.2 MgSO4, 5.5 glucose, 0.5 Na acetate, 0.5 Na lactate, and either 80 (high NaCl) or 10 NaCl (low NaCl). The pH of the solution was 7.4. The bath consisted of MEM and was exchanged continuously at a rate of 1 ml/min at 37°C. The imaging system consisted of a microscope (Eclipse Ti; Nikon), digital charge-coupled device camera (CoolSnap; Photometrics), xenon light (LB-LS/30; Shutter Instruments), and optical filter changer (Lambda 10–3; Shutter Instruments). Images were displayed and analyzed with NIS-Elements imaging software (Nikon).
Measurement of O2− with dihydroethidium.
We used a O2−-sensitive fluorescent dye, dihydroethidium, to detect O2− levels as we recently described (15, 16). Once the TAL was perfused, MD cells were loaded with 10 μM dihydroethidium in 0.1% DMSO plus 0.1% pluronic acid from the lumen for 30 min and then washed for 20 min. The loaded MD cells were exposed to 380 and 490 nm light to excite dihydroethidium and oxyethidium, respectively. Emitted fluorescence from dihydroethidium was recorded using a 420-nm dichroic mirror with a 460/50 nm band-pass filter; for oxyethidium we used a 565-nm dichroic mirror with a 605/55 nm band-pass filter. Square-shaped regions of interest were set inside the cytoplasm of MD cells, and their mean intensity was recorded every 5 s for 2 min. We recorded and calculated the rate of the changes for oxyethidium/dihydroethidium as an indicator of O2− production (8, 33). Since we previously found that increased luminal NaCl activates neuronal NOS and induces NO release by the MD (14), we added the NOS inhibitor Nω-nitro-l-arginine methyl ester (l-NAME; 10 −4 M) to the bath and lumen while measuring O2− to eliminate its reaction with NO.
Measurement of membrane potential.
To study depolarization of the MD during TGF, we measured membrane potential using a voltage-sensitive fluorescent dye, bis-(1,3-dibutylbarbituric acid) trimethine oxonol (DiBAC) in the isolated, perfused MD. Once the TAL was perfused, MD cells were loaded with 5 μM DiBAC in 0.1% DMSO from the lumen at 37 ± 1°C for 15 min. DiBAC was present in the lumen throughout the experiment. Intracellular dye was excited at 490 nm, and fluorescence emissions were recorded at 510 nm. Square-shaped regions of interest were set inside the cytoplasm of MD cells close to the apical or basolateral membrane, and mean intensity within them was recorded every 5 s for 2 min. Cell membrane potential was expressed as relative changes in DiBAC fluorescence intensity compared with basal.
Measurement of Rac immunofluorescence in perfused MD.
The TAL was cannulated and perfused for 30 min at either 10 or 80 mM NaCl solution. The MD was then fixed by adding 4% paraformaldehyde in PBS to both bath and lumen for 30 min and washed for 10 min by adding Tris-buffered saline Tween-20 to the bath and lumen. The sample was permeabilized and blocked with 0.1% Triton X-100 and 3% BSA for 60 min. The MD was incubated with an anti-Rac1 primary antibody (1:1,000) for 60 min from the lumen, followed by incubation with Alexa Fluor 488 rabbit anti-mouse IgG secondary antibody (1:400) for 30 min. Rac immunofluorescence was visualized under a fluorescent microscope. To measure Rac1 translocation, each MD cell was evenly divided into three equal sections: apical, middle, and basal. Rac1 translocation was calculated based on the differences in immunointensity of Rac1 in each section at 10 or 80 mM NaCl. The pH of all the solutions used in tubule was adjusted to 7.4 and the osmolality was adjusted to 180 mOsm with mannitol.
Chemicals.
Dihydroethidium, DiBAC, DMSO, pluronic acid, and Alexa Fluor 488 rabbit anti-mouse IgG secondary antibody were obtained from Invitrogen (Eugene, OR). Anti-Rac1 primary antibody was from Abcam. All other chemicals were purchased from Sigma.
Statistics.
Data were collected as repeated measures over time under different conditions. We tested only the effects of interest, using a paired t-test or ANOVA for repeated measures. Significance was judged as P < 0.05.
RESULTS
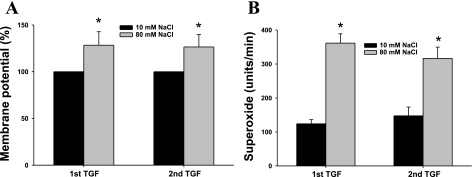
We first measured changes in membrane potential and the associated changes in O2− production during TGF responses in isolated, perfused MD. Inducing a TGF response by increasing the tubular NaCl concentration from 10 to 80 mM (15, 25), caused the membrane potential to depolarize by 28.4 ± 4.5% from the control. This was associated with an increase in O2− levels from 124 ± 19 to 361 ± 27 U/min. After obtaining the measurements, the luminal NaCl was switched back to 10 mM for 30 min, and then a second TGF response was induced by again increasing the luminal NaCl to 80 mM. This second TGF response was again associated with membrane depolarization (by 26.5 ± 3.5% from its control value; P < 0.05, n = 6, Fig. 1A), and an increase in O2− from 147 ± 25 to 316 ± 32 U/min (P < 0.01, n = 5, Fig. 1B), demonstrating that NaCl-induced TGF responses are associated with reproducible depolarization of the membrane potential and increases in O2− levels.
Fig. 1.
Increases in luminal NaCl induce macula densa (MD) depolarization and superoxide (O2−) generation. A: when the luminal NaCl was switched from 10 to 80 mM, a maneuver that initiates tubuloglomerular feedback (TGF), membrane potential was depolarized by 28.4 ± 4.5% from the control. Repeating of the switch induced similar changes in membrane potential (*P < 0.05, 10 vs. 80 mM NaCl; n = 6). B: increasing luminal NaCl from 10 to 80 mM significantly enhanced O2− generation by the MD. Repetitive switching of luminal NaCl induced similar O2− generation (*P < 0.01, 10 vs. 80 mM NaCl; n = 5).
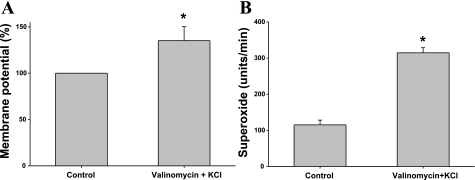
In our next series of experiments, we determined whether depolarization was contributing to NaCl-induced increases in O2− levels. For this, we first determined whether depolarizing the MD, via a different mechanism other than increasing NaCl, also elevated O2− levels in the MD. In these experiments, we depolarized the MD using valinomycin (a K-selective ionophore)+KCl, which has been shown to induce TGF (23), and measured whether O2− levels changed. Adding valinomycin (10−6 M) and 25 mM KCl (maintaining NaCl at 10 mM), caused the MD to depolarize by 35.2 ± 5.1% from the control (P < 0.05, n = 6, Fig. 2A), and O2− levels to increase from 115 ± 13 to 314 ± 14 U/min (P < 0.01, n = 5, Fig. 2B). These data indicate that depolarizing the MD by either increasing luminal NaCl or by adding valinomycin+KCl increased MD-induced O2− production.
Fig. 2.
Valinomycin+KCl induces MD depolarization and O2− generation. A: when valinomycin (10−6 M), a K-selective ionophore, and 25 mM KCl were added in tubule perfusate without changing luminal NaCl concentration, MD membrane potential depolarized significantly (*P < 0.05; n = 6). B: when valinomycin (10−6 M) and 25 mM KCl were added in tubule perfusate without changing luminal NaCl concentration, O2− significantly increased (*P < 0.01, n = 5).
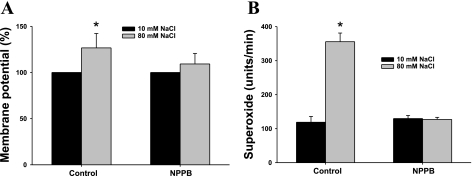
To further establish the role of depolarization on TGF-induced increases in O2− levels, we examined the role of the basolateral Cl− channel on TGF-induced depolarization and O2− generation. TGF is initiated by an increase in luminal NaCl with the subsequent enhancement in luminal Na+-K+-2Cl− cotransporter (NKCC2) activity and an increase in intracellular NaCl in the MD. The increased intracellular Cl− might stimulate basolateral Cl− efflux and result in cell depolarization (2, 13). Therefore, we determined whether blocking the Cl− channel prevents NaCl-induced depolarization of the MD as well as the associated increase in O2− production. As shown in Fig. 3, increasing luminal NaCl from 10 to 80 mM depolarized the MD by 26.7 ± 5.6% from the control and increased O2− from 119 ± 17 to 355 ± 26 U/min. Repeating this TGF response in the perfused MD after treating the MD with the Cl− channel blocker, 5-nitro-2(3-phenylpropylamino) benzoic acid (NPPB, 10−6 M, in the luminal perfusate for 30 min) prevented the depolarization (the membrane potential was depolarized by 9.3 ± 1.4% from the control; n = 5, Fig. 3A) and the increase in O2− (129 ± 9 vs. 127 ± 6 U/min; n = 6, Fig. 3B). These data provide further evidence that depolarization plays an important role in NaCl-induced increases in MD O2−.
Fig. 3.
Cl− channel inhibition prevents MD depolarization and O2− generation. A: when the luminal NaCl solution was switched from 10 to 80 mM, membrane potential depolarized significantly. The luminal NaCl was then switched to 10 mM, and a Cl− channel blocker, 5-nitro-2(3-phenylpropylamino) benzoic acid (NPPB; 10−6 M) was added to the lumen for 30 min. When luminal NaCl was increased to 80 mM again, the NaCl-induced depolarization was blocked (*P < 0.05, 80 mM in control vs. 80 mM in NPPB; n = 5). B: inhibition of Cl− channels with NPPB (10−6 M) blocked NaCl-induced O2− generation (*P < 0.01, 80 mM in control vs. 80 mM in NPPB; n = 6).
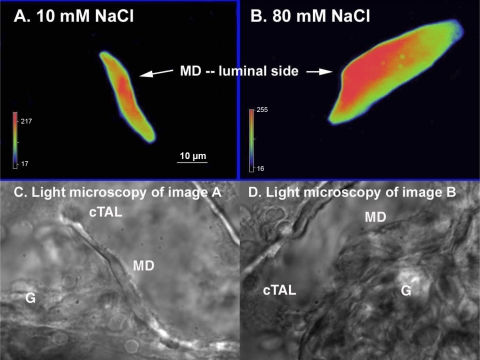
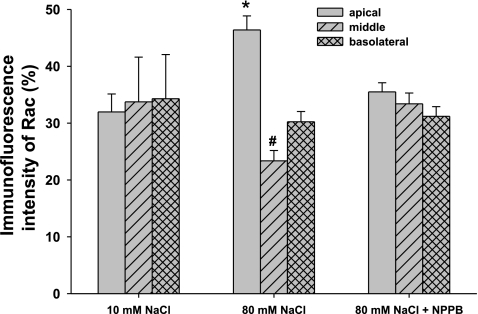
We next evaluated the role of Rac1 on depolarization-induced O2− production by the MD. To study whether triggering a TGF response with high NaCl leads to translocation of Rac to the apical membrane, we measured Rac1 immunofluorescence in a perfused MD during a TGF response. Fig. 4 shows representative images that show the intracellular distribution of Rac1 in a MD cell, before and after a TGF response. During perfusion with 10 mM NaCl, Rac was evenly distributed in the MD cells (Fig. 4A). In contrast, when the NaCl was increased to 80 mM to induce TGF, the immunointensity of Rac on the apical side increased (Fig. 4B). The average data is depicted in Fig. 5. The immunointensity for Rac on the apical membrane of the MD, which was 32 ± 3.1% of the total immunofluorescence during perfusion with 10 mM NaCl, increased to 46 ± 2.5% during perfusion with 80 mM NaCl (P < 0.05, n = 5). This was associated with a decrease in the immunointensity in the middle, from 34 ± 7.9 to 23 ± 1.8% of the total immunofluorescence (P < 0.05, n = 5). This increase in the translocation of Rac during high NaCl was due to depolarization; translocation was blocked by NPPB, the Cl− channel blocker. Immunointensity of Rac on the apical side remained at 35 ± 1.6% of the total immunofluorescence in the MD during 80 mM NaCl perfusate in MD treated with 10−6 M of NPPB (n = 5, Fig. 5, right). These data indicate that NaCl-induced depolarization of the MD causes translocation of Rac to the apical membrane.
Fig. 4.
Representative experiment of NaCl-induces Rac translocation in the MD. A: when tubule was perfused with 10 mM NaCl, Rac was evenly distributed in the MD cells. B: when tubule was perfused with 80 mM NaCl, immunointensity of Rac on apical side increased. C and D: images of light microscopy of A and B, respectively. G, glomerulus; cTAL, cortical thick ascending limb.
Fig. 5.
Depolarization induces Rac translocation in the MD. When the MD was perfused with 10 mM NaCl, immunointensity of Rac among apical, middle, and basolateral sides were not significantly different. When the MD was perfused with 80 mM NaCl, immunointensity of Rac on the apical side was increased from 32 ± 3.1 to 46 ± 2.5% of the total immunofluorescence in the MD, and the middle was decreased from 34 ± 7.9 to 23 ± 1.8% (P < 0.05 vs. 10 mM NaCl, n = 5). In the presence of a Cl− channel blocker to prevent depolarization NPPB (10−6 M), when the MD was perfused with 80 mM NaCl, translocation of Rac was blocked (*P < 0.01, 10 vs. 80 mM NaCl; #P < 0.05, 10 vs. 80 mM NaCl; n = 5).
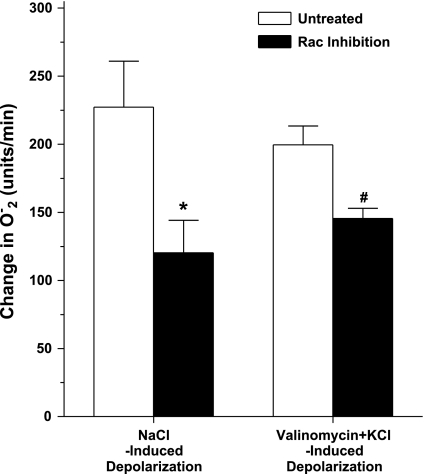
Finally, we determined the role of Rac in modulating depolarization-induced increases in O2− in the MD. For this we investigated whether a Rac1 inhibitor would alter O2− levels during NaCl and valinomycin+KCl-induced depolarization of MD cells (Fig. 6). Switching from the low- to high-NaCl perfusate caused O2− to increase from 121 ± 15 to 349 ± 33 U/min. This increase was blunted by 47% after the MD was treated with the Rac1 inhibitor, NSC23766 (50 μM intraluminally for 30 min); O2− increased from 127 ± 12 to 247 ± 23 U/min (P < 0.05 vs. control, n = 5). Likewise, the Rac inhibitor also blunted valinomycin+KCl-induced increases in O2− by 31%; O2− increased from 124 ± 13 to 271 ± 8 U/min (P < 0.05 vs. without Rac inhibition, n = 5). These results may suggest that while Rac may not be necessary for depolarization-induced O2− production, it augments depolarization-induced increases in O2− in the MD.
Fig. 6.
The effect of Rac1 inhibition on O2− generation by the MD in response to NaCl- and valinomycin+KCl-induced depolarization. Switching from the low- to high-NaCl perfusate caused O2− to increase by 228 ± 23 U/min (from 121 ± 15 to 349 ± 33 U/min). This increase was blunted by 47% after the MD was treated with the Rac1 inhibitor, NSC23766 (*P < 0.05 vs. control, n = 5). When valinomycin (10−6 M) and 25 mM KCl were added in tubule perfusate without changing luminal NaCl concentration, O2− increased by 199 ± 14 U/min (from 115 ± 13 to 314 ± 14 units/min). The Rac inhibitor also blunted valinomycin+KCl-induced increases in O2− by 31% (#P < 0.05 vs. without Rac inhibition, n = 5).
DISCUSSION
TGF is initiated by increasing luminal NaCl and activating the luminal NKCC2, which increases intracellular NaCl in the MD. The increased intracellular Cl− stimulates basolateral Cl− efflux and results in cell depolarization (1, 13). Depolarization of the MD during TGF has been measured with a micro electrode (2) and, as we did previously and in the present study, using membrane potential-sensitive fluorescence dye. Thus, these observations raise the possibility that depolarization may play an important role in TGF. This contention is supported by our previous findings that Cl− channel inhibitors prevent NaCl-induced membrane depolarization and TGF (23). Moreover, depolarizing the MD with valinomycin+KCl induces TGF (23). Thus, these data suggest that depolarization of the MD plays an essential role in TGF. In our present study, we found that NaCl- and valinomycin+KCl-induced depolarization/TGF were associated with enhanced O2− production in the MD, which confirms our previous study, which provided evidence that the source of the O2− in the MD was NADPH oxidase and that this regulates the responsiveness of TGF (16). Therefore, it is important to identify the factors that may influence NADPH oxidase activity in the MD.
NOX2 in the MD is the primary source for NaCl-induced O2− generation (32). Activation of NOX2 requires two specialized cytosolic proteins, p47phox and p67phox, and the small GTP-binding protein Rac, all of which are translocated to the membrane to form the active enzyme (7, 24). While there are several isoforms, Rac1 and Rac2 appear to be the most important in regulating NADPH oxidase. Rac1 is expressed ubiquitously and is the main Rac GTPase for NADPH oxidase activation in nonphagocytic cells (10). Rac2 is mainly expressed in phagocytes. Activation of Rac is associated with recruitment of p67phox (12), which associates with p47phox and the cytochrome of the NOX2 complex (5, 6). NOX1 and NOX3 also require Rac for optimal activity (4, 20). However, regulation by Rac GTPase does not appear to be required for the function of NOX4, NOX5, or the Duox family members (11, 19). Thus several pieces of information raise the possibility that Rac may play an important role in regulating O2− generation in the MD. First, NaCl-induced O2− generation in the MD is mediated primarily via NOX2 (32), which may be regulated by Rac as NOX2 in other kind of cells are. Second, depolarization, which is an important component of TGF, can activate Rac (27, 28). However, it is not clear whether Rac is involved in O2− generation in the MD. In the present study, we provide evidence that depolarizing the MD with either high NaCl or valinomycin promotes translocation of Rac from the cytosol to the apical membrane. In addition, this translocation is associated with an increase in O2− production during depolarization. Blocking depolarization with a Cl− channel blocker prevented the translocation of Rac as well as the increase in O2−, suggesting that depolarization may have been the stimuli for Rac translocation.
While blocking Rac1 blunted depolarization-induced increases in O2− , the effect was modest. There are several possible explanations for this. The first is that other sources of O2− (NOX4, xanthine oxidase, COX-2, uncoupled NOS, cytochrome P-450, and mitochondria) may be more important during TGF than Rac-dependent isoforms of NADPH oxidase. We consider this unlikely for several reasons: 1) depolarization-induced O2− is completely abolished by apocynin (a NADPH oxidase inhibitor); 2) inhibitors of other potential sources (xanthine oxidase, COX2, NOS) did not significantly alter O2− levels; and 3) blocking NOX2 in a MD-like cell line (MMDD1 cells, which are very similar to MD cells isolated via laser capture microdissection) with siRNA completely blocked NaCl-induced increases in O2−, suggesting that most (if not all) of the increase in O2−, was dependent on NOX2 (16). Taken together, the data suggests that depolarization-induced increases in O2− are via NOX2, although we cannot completely rule out a role for Rac-independent oxidases.
A second potential reason for this partial effect of the Rac-1 inhibitor on NOX-derived increases in O2− is that Rac may be a catalyst that augments depolarization-induced NOX activity in the MD, rather than an essential component for the activation of NOX. Indeed, there are studies suggesting that Rac only mediates part of the effect of NOX (31). Finally, it is possible that we may not have achieved complete blockade of Rac1 within the time constraints imposed by our experimental preparation. However, despite the modest effect of Rac1 blockade on O2− levels, we did achieve complete blockade of depolarization-induced translocation of Rac. This suggests that the increase in NOX activity during Rac1 blockade was due to Rac-independent activation of NOX and not because of incomplete blockade of Rac.
In conclusion, we found that depolarization, by either a high-NaCl perfusate or valinomycin+KCl, leads to translocation of Rac to the apical membrane of the MD. This translocation is associated with enhancement of depolarization-induced O2− generation by the MD. The source of the depolarization-induced O2− in the MD is not completely established, but is likely NOX2.
Perspectives and Significance
Depolarization-induced activation of RAC/NOX2 augments O2− generation by the MD. This increase in O2− may, in turn, quench NO at the MD and thus enhance TGF responses, raising the possibility that Rac-enhanced increases in O2− during TGF responses may lead to increased TGF sensitivity and abnormal sodium handling observed during certain conditions associated with elevated oxidative stress, such as during hypertension, heart failure, acute renal failure, and cirrhosis. Therefore, it will be important to further delineate the regulation of this pathway (including interactions with Rac2) to determine whether abnormalities in it contribute to pathologic conditions and perhaps to identify novel therapeutic targets.
GRANT
This work was supported by the National Heart, Lung, and Blood Institute Grant RO1-HL086767.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Bell PD, Lapointe JY, Peti-Peterdi J. Macula densa cell signaling. Annu Rev Physiol 65: 32.1–32.20, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Bell PD, Lapointe JY, Cardinal J. Direct measurement of basolateral membrane potentials from cells of the macula densa. Am J Physiol Renal Fluid Electrolyte Physiol 257: F463–F468, 1989 [DOI] [PubMed] [Google Scholar]
- 3.Briggs JP, Schnermann J. Macula densa control of renin secretion and glomerular vascular tone: evidence for common cellular mechanisms. Renal Physiol (Basel) 9: 193–203, 1986 [DOI] [PubMed] [Google Scholar]
- 4.Cheng G, Diebold BA, Hughes Y, Lambeth JD. Nox1-dependent reactive oxygen generation is regulated by Rac1. J Biol Chem 281: 17718–17726, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Dang PM, Cross AR, Quinn MT, Babior BM. Assembly of the neutrophil respiratory burst oxidase: a direct interaction between p67PHOX and cytochrome b558 II. Proc Natl Acad Sci USA 99: 4262–4265, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dang PM, Johnson JL, Babior BM. Binding of nicotinamide adenine dinucleotide phosphate to the tetratricopeptide repeat domains at the N-terminus of p67PHOX, a subunit of the leukocyte nicotinamide adenine dinucleotide phosphate oxidase. Biochemistry 39: 3069–3075, 2000 [DOI] [PubMed] [Google Scholar]
- 7.DeLeo FR, Yu L, Burritt JB, Loetterle LR, Bond CW, Jesaitis AJ, Quinn MT. Mapping sites of interaction of p47-phox and flavocytochrome b with random-sequence peptide phage display libraries. Proc Natl Acad Sci USA 92: 7110–7114, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fink B, Laude K, McCann L, Doughan A, Harrison DG, Dikalov S. Detection of intracellular superoxide formation in endothelial cells and intact tissues using dihydroethidium and an HPLC-based assay. Am J Physiol Cell Physiol 287: C895–C902, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Hassanain HH, Goldschmidt-Clermont PJ. Rac, superoxide, and signal transduction. In: Antioxidant and Redox Regulation of Genes, edited by Sen SK, Sies H, Bauerle PA. San Diego, CA: Academic, 1999, p. 47–79 [Google Scholar]
- 10.Hordijk PL. Regulation of NADPH oxidases: the role of Rac proteins. Circ Res 98: 453–462, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Kamiguti AS, Serrander L, Lin K, Harris RJ, Cawley JC, Allsup DJ, Slupsky JR, Krause KH, Zuzel M. Expression and activity of NOX5 in the circulating malignant B cells of hairy cell leukemia. J Immunol 175: 8424–8430, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Koga H, Terasawa H, Nunoi H, Takeshige K, Inagaki F, Sumimoto H. Tetratricopeptide repeat (TPR) motifs of p67(phox) participate in interaction with the small GTPase Rac and activation of the phagocyte NADPH oxidase. Biol Chem 274: 25051–25060, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Lapointe JY, Bell PD, Hurst AM, Cardinal J. Basolateral ionic permeabilities of macula densa cells. Am J Physiol Renal Fluid Electrolyte Physiol 260: F856–F860, 1991 [DOI] [PubMed] [Google Scholar]
- 14.Liu R, Carretero OA, Ren Y, Garvin JL. Increased intracellular pH at the macula densa activates nNOS during tubuloglomerular feedback. Kidney Int 67: 1837–1843, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Liu R, Carretero OA, Ren Y, Wang H, Garvin JL. Intracellular pH regulates superoxide production by the macula densa. Am J Physiol Renal Physiol 295: F851–F856, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu R, Garvin JL, Ren Y, Pagano PJ, Carretero OA. Depolarization of the macula densa induces superoxide production via NAD(P)H oxidase. Am J Physiol Renal Physiol 292: F1867–F1872, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Liu R, Pittner J, Persson AE. Changes of cell volume and nitric oxide concentration in macula densa cells caused by changes in luminal NaCl concentration. J Am Soc Nephrol 13: 2688–2696, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Liu R, Ren Y, Garvin JL, Carretero OA. Superoxide enhances tubuloglomerular feedback by constricting the afferent arteriole. Kidney Int 66: 268–274, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal 18: 69–82, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Miyano K, Sumimoto H. Role of the small GTPase Rac in p22phox-dependent NADPH oxidases. Biochimie 89: 1133–1144, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Navar LG, Bell PD, Thomas CE, Ploth DW. Influence of perfusate osmolality on stop-flow pressure feedback responses in the dog. Am J Physiol Renal Fluid Electrolyte Physiol 235: F352–F358, 1978 [DOI] [PubMed] [Google Scholar]
- 22.Ren Y, Garvin JL, Carretero OA. Role of macula densa nitric oxide and cGMP in the regulation of tubuloglomerular feedback. Kidney Int 58: 2053–2060, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Ren Y, Yu H, Wang H, Carretero OA, Garvin JL. Nystatin and valinomycin induce tubuloglomerular feedback. Am J Physiol Renal Physiol 281: F1102–F1108, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Rotrosen D, Yeung CL, Leto TL, Malech HL, Kwong CH. Cytochrome b558: The flavin-binding component of the phagocyte NADPH oxidase. Science 256: 1459–1462, 1992 [DOI] [PubMed] [Google Scholar]
- 25.Schnermann J. Adenosine mediates tubuloglomerular feedback. Am J Physiol Regul Integr Comp Physiol 283: R276–R277, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Schnermann J, Persson AE, Agerup B. Tubuloglomerular feedback. Nonlinear relation between glomerular hydrostatic pressure and loop of Henle perfusion rate. J Clin Invest 52: 862–869, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res 91: 406–413, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Sohn HY, Keller M, Gloe T, Morawietz H, Rueckschloss U, Pohl U. The small G-protein Rac mediates depolarization-induced superoxide formation in human endothelial cells. J Biol Chem 275: 18745–18750, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Welch WJ, Tojo A, Wilcox CS. Roles of NO and oxygen radicals in tubuloglomerular feedback in SHR. Am J Physiol Renal Physiol 278: F769–F776, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Wilcox CS, Welch WJ, Murad F, Gross SS, Taylor G, Levi R, Schmidt HHW. Nitric oxide synthase in macula densa regulates glomerular capillary pressure. Proc Natl Acad Sci USA 89: 11993–11997, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamauchi A, Marchal CC, Molitoris J, Pech N, Knaus U, Towe J, Atkinson SJ, Dinauer MC. Rac GTPase isoform-specific regulation of NADPH oxidase and chemotaxis in murine neutrophils in vivo. Role of the C-terminal polybasic domain. J Biol Chem 280: 953–964, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Zhang R, Harding P, Garvin JL, Juncos R, Peterson E, Juncos LA, Liu R. Isoforms and functions of NADPH Oxidase at the macula densa. Hypertension 53: 556–563, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vásquez-Vivar J, Kalyanaraman B. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic Biol Med 34: 1359–1368, 2003 [DOI] [PubMed] [Google Scholar]