Abstract
Hepatocellular carcinoma (HCC) is the third leading cause of cancer deaths worldwide, with a mortality rate approximating its incidence. Understanding the biology of these tumors, as well as treatment modalities, has been challenging. The opioid growth factor (OGF; [Met5]-enkephalin) and the OGF receptor (OGFr) form an endogenous growth-regulating pathway in homeostasis and neoplasia. In this investigation, we examined the relationship of the OGF-OGFr axis in HCC and define its presence, function, and mechanism. Using SK-HEP-1, Hep G2, and Hep 3B human HCC cell lines, we found that OGF and OGFr were present and functional. Exogenous OGF was observed to have a dose-dependent, reversible, and receptor-mediated inhibitory action on cell proliferation. Endogenous OGF was found to be constitutively produced and tonically active on cell replicative activities, with neutralization of this peptide accelerating cell proliferation. Silencing of OGFr using siRNA stimulated cell replication, even when exogenous OGF was added to the cultures, documenting its importance in mediating OGF activity. The mechanism of OGF-OGFr action on cell number was related to inhibition of DNA synthesis and not to apoptotic or necrotic pathways. Both OGF and OGFr were detected in surgical specimens of HCC, and no quantitative differences were recorded in peptide or receptor between pathological and normal specimens. These data are the first to report that the OGF-OGFr system is a native biological regulator of cell proliferation in HCC. The findings may provide important insight in designing treatment strategies for this deadly disease.
Keywords: hepatocellular carcinoma, cell proliferation, tissue culture, siRNA, therapy
hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide and the third leading cause of cancer mortality (20). HCC is refractory to conventional cytotoxic chemotherapy and radiotherapy (25) and has a mortality rate that approximates its incidence (12, 21). Transplantation, surgical resection, and ablation are currently the only treatment for achieving long-term survival (13). However, due to advanced stages of disease at the time of diagnosis and associated liver dysfunction, most patients are not candidates for surgical resection (1), and even with surgical treatment, the incidence of recurrence in patients with underlying cirrhosis is very high due to multicentric carcinogenesis (22). There is an urgent medical need to not only understand the biology of HCC, but to use this knowledge to devise innovative and alternative therapies for this lethal disease.
The opioid growth factor (OGF), chemically termed [Met5]-enkephalin, is an endogenous opioid peptide that is an important regulator in the onset and progression of a variety of human cancers (2, 15–17, 27, 34). OGF interacts with the OGF receptor (OGFr) to delay the G1/S interface of the cell cycle by modulating cyclin-dependent kinase inhibitory pathways (4–6). Attenuation of the OGF-OGFr axis in cancer cells through: 1) disruption of OGF-OGFr interfacing by continuous exposure to opioid antagonists [e.g., naltrexone (NTX)] (2, 15, 34), 2) neutralization of OGF by antibodies to the peptide (15), or 3) a decrease in OGFr by antisense cDNA or siRNA for OGFr (26, 37), stimulates cell proliferation. An increase in OGF-OGFr activity in cancer cells by 1) addition of exogenous OGF (2, 15, 34); 2) treatment with imidazoquinoline compounds, such as imiquimod and resiquimod (26); or 3) transfection of sense cDNA for OGFr (18, 35), depresses cell proliferation.
Opioid receptors have been detected in surgical samples taken from human neoplasms of the liver (29). The relationship of the OGF-OGFr axis to human HCC, however, is unknown. The present investigation explores the question of whether the OGF-OGFr axis is present and functions in human HCC and studies the mechanism(s) underlying these pathways.
MATERIALS AND METHODS
Cell culture.
HCC cell lines, SK-HEP-1 (7), Hep G2 (10), and Hep 3B (10) were kindly donated by Dr. Max Schmidt from the University of Indiana (Indianapolis, IN). Cultures were grown in MEM supplemented with 10% fetal calf serum, 2 mM l-glutamine, and 1.2% sodium bicarbonate. All media contained antibiotics (100 Units/ml penicillin, 100 μg/ml streptomycin, 100 μg/ml kanamycin), unless otherwise indicated. All cells were grown in a humidified atmosphere of 5% CO2-95% air at 37°C.
Surgical specimens.
All tumor specimens were obtained from patients who had undergone surgery at The Milton S. Hershey Medical Center (Hershey, PA) between January and July 2009. This included samples from the following patients with HCC: a 70-yr-old male, well-differentiated tumor, stage 2; an 81-yr-old male, moderately differentiated tumor, stage 3A; and a 62-yr-old female, well-differentiated tumor, stage 3A. Tissues were obtained as rapidly as possible following removal. Ulcerated and necrotic tissues were dissected from the samples, and tissues were frozen. Tumor characterization was confirmed by an experienced pathologist (Dr. H. Crist). All protocols were approved by the Institutional Review Board of The Milton S. Hershey Medical Center and written permission to use the tissues was received from all patients.
Immunohistochemistry.
Log phase SK-HEP-1 and Hep G2 cells were plated onto 22-mm round cover glasses and cultured for 72 h. Frozen tissue samples were sectioned on a cryostat at 10 μm and transferred to gelatin-coated slides. Cells and tissues were fixed and permeabilized in 95% EtOH and acetone and processed for immunohistochemistry, according to previously published procedures (2, 27, 32–34). Polyclonal antibodies to OGF and OGFr were generated in the laboratory and have been fully characterized (31). Controls (Co) included cells incubated only with secondary antibodies. At least two cover glasses and two samples/specimen for each antibody were examined.
To evaluate the level of OGF and OGFr in surgical specimens of HCC and margins (considered normal by pathological analysis), semiquantitative immunohistochemistry was utilized. For quantification of OGF and OGFr, images were taken at the same exposure time with special care not to photobleach samples. The mean intensity of staining was determined from at least four sections from HCC and the margins of the surgical specimens.
OGFr binding assays.
Receptor binding assays for OGFr were performed using log phase SK-HEP-1 and Hep G2 cells according to previous reports (18, 26, 35) and custom-synthesized [3H]-[Met5]-enkephalin (52.7 Ci/mmol; Perkin Elmer-New England Nuclear). Independent assays were performed at least three times.
Cell growth.
Cells were plated and counted 24 h later (time 0) to determine seeding efficiency. OGF or other compounds were added at time 0, and media and compounds were replaced daily. All drugs were prepared in sterile water, and dilutions represent final concentrations of the compounds. An equivalent volume of sterile water was added to control wells. At designated times, cells were harvested with 0.25% trypsin/0.53 mM EDTA, centrifuged, stained with trypan blue, and counted with a hemacytometer. At least 2 aliquots per well of at least two wells per treatment per time point were sampled.
Specificity of endogenous OGF.
The specificity of endogenous OGF for cell growth was determined by treating cells with a polyclonal antibody to OGF (1:200; Co172); preimmune rabbit serum (1:200) served as a control. Serum and media were changed daily, and cells were counted after 72 h of treatment. Cell viability was determined by trypan blue staining with at least 2 aliquots/well, and two wells per treatment were evaluated.
Specificity of OGFr: knockdown with OGFr-siRNA.
The OGFr-targeted siRNA (antisense, 5′-uagaaacucagguuuggcg-3′; sense, 5′- cgccaaaccugaguuucua-3′) was designed and obtained as a ready-annealed, purified, duplex probe from Ambion (Austin, TX). Then 5 × 104 cells/well were seeded in six-well plates containing 1 ml of media without antibiotics. Cells were transfected with either 20 nM OGFr-siRNA or scrambled siRNA (Ambion) solutions with Oligofectamine reagent (Invitrogen, Carlsbad, CA) in serum and antibiotic-free media. Cells were incubated for 4 h at 37°C before the addition of 10−6 M OGF or NTX. Cultures were incubated for an additional 20 h, and fresh, complete media (1 ml) either lacking or containing OGF or NTX was added. At 72 h, cells were harvested and cell numbers counted, or RNA was collected for Northern blot experiments. Two independent experiments were conducted.
Total RNA was extracted using the Paris Kit (Ambion), separated on an agarose gel, and transferred to a nylon membrane (Immobilon; Bio-Rad Laboratories, Hercules, CA). Membranes were probed with 32P-dCTP-OGFr cDNA. To control for equal loading, blots were stripped and reprobed with radiolabeled GAPDH, and optical density of each band was determined and analyzed by QuickOne (Bio-Rad Laboratories). Each value was normalized to GAPDH from the same blot. Means ± SE were determined from at least two independent experiments.
To evaluate the level of OGFr protein knockdown, semiquantitative immunohistochemistry was utilized on a subset of cultures that were seeded and transfected with siRNAs on round coverslips. For quantification of OGFr protein levels, images were taken at the same exposure time with special care not to photobleach samples. The mean intensity of staining was determined for at least 100 cells/group, and at least two coverslips per group.
Mechanisms of OGF-modulated growth inhibition: DNA synthesis, apoptosis, and necrosis.
The effect of OGF on DNA synthesis (BrdU incorporation), apoptosis [terminal deoxyneucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL)], and necrosis (trypan blue) of human HCC cells was evaluated. Cells were seeded onto 22-mm diameter cover glasses placed in six-well plates (5 × 104 cells/cover glass), and treated with compounds for 72 h, with media and drugs replaced daily. Three hours prior to fixing cells, 30 μM BrdU (Sigma Chemicals, Indianapolis, IN) was added to some cultures. Cells were fixed in 10% neutral buffered formalin for 10 min and either stained with antibodies to BrdU (anti-BrdU-BOD, Invitrogen) or processed for TUNEL (Trevigen, Gaithersburg, MD) to assess DNA synthesis and apoptosis, respectively. Positive and negative controls for apoptosis were included in the TUNEL kit. DNA synthesis and apoptosis were assessed from 2–4 coverslips/treatment with at least 300 cells evaluated for each treatment.
Statistical analysis.
All data were analyzed (GraphPad Prism software) using one-way ANOVA, with subsequent comparisons made using Newman-Keuls tests. Data for the semiquantitative densitometric analysis was evaluated with a Student's t-test.
RESULTS
OGF and OGFr are present in human HCC cells.
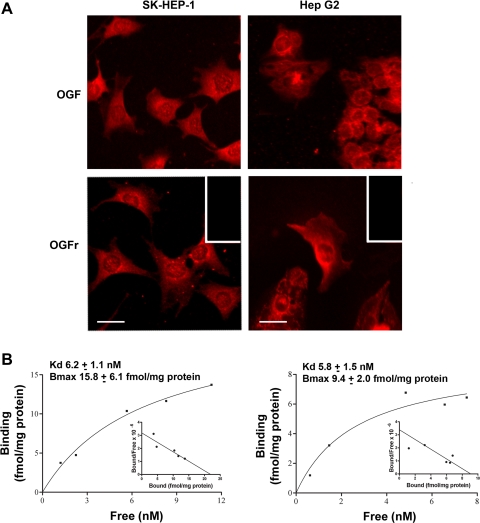
Immunoreactive OGF and OGFr were localized to the cytoplasm of SK-HEP-1 and Hep G2 cells, with speckling of immunoreactivity noted in the nucleus (Fig. 1A). No staining was recorded in specimens processed with secondary antibody only.
Fig. 1.
Presence and distribution of opioid growth factor (OGF) and OGF receptor (OGFr) in human hepatocellular carcinoma (HCC) cells. A: immunohistochemistry of log phase SK-HEP-1 and Hep G2 cells stained with polyclonal, ammonium sulfate-purified antibodies (1:100) to [Met5]-enkephalin (OGF) or OGFr. Rhodamine-conjugated IgG (1:1,000) served as the secondary antibody. Immunoreactivity was associated with the cytoplasm, and a speckling of stain was noted in cell nuclei. Immunostaining was not detected in cell preparations incubated with secondary antibodies only (inset). Scale bar = 10 μm. B: representative saturation isotherm of specific binding of [3H]-[Met5]-enkephalin to nuclear homogenates of SK-HEP-1 (left) and Hep G2 (right) cells. Mean ± SE binding affinity (Kd) and maximal binding capacity (Bmax) from at least 3 independent assays performed in duplicate are shown. Representative Scatchard plot (inset) of specific binding of radiolabeled [Met5]-enkephalin to SK-HEP-1 and Hep G2 proteins revealed a 1-site model of binding for each cell line.
Receptor binding analysis of nuclear protein from log phase SK-HEP-1 and Hep G2 cells revealed site-specific and a one site model of saturable binding (Fig. 1B). The binding capacities (Bmax) for SK-HEP-1 and Hep G2 were 15.8 ± 6.1 and 9.4 ± 2.0 fmol/mg protein, respectively, whereas the binding affinities (Kd) for SK-HEP-1 and Hep G2 were 6.2 ± 1.1 and 5.8 ± 1.5 nM, respectively.
OGF depresses growth of HCC cells.
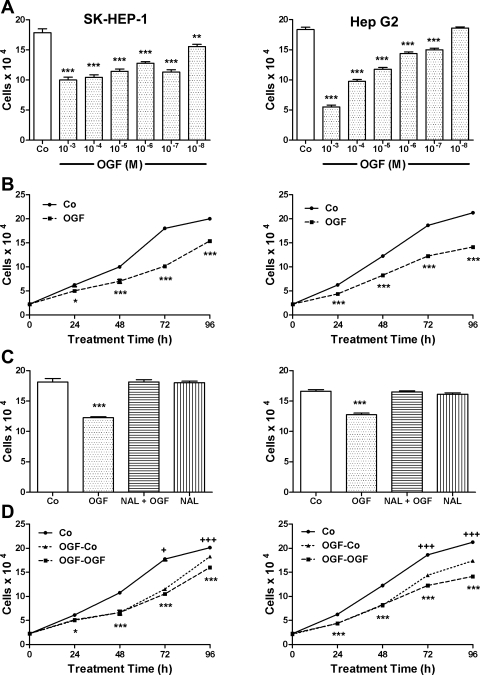
The effects of OGF in concentrations ranging from 10−3 M to 10−8 M at 72 h revealed a dose-dependent inhibitory effect on cell number (Fig. 2A). Dosages of 10−3 M to 10−8 M, reduced cell number in SK-HEP-1 cultures by 51% to 13%, and dosages of 10−3 M to 10−7 M, but not 10−8 M, inhibited cell number in Hep G2 cultures by 70% to 18%, from vehicle-treated groups. Over a 96-h period, cell number was reduced significantly in SK-HEP-1 cultures treated with 10−6 M OGF by 20–45%, and in Hep G2 cultures by 30% to 34%, compared with control cultures (Fig. 2B).
Fig. 2.
OGF inhibits growth of human HCC cells in a dose-dependent, temporal, receptor-mediated, and reversible manner. A: growth of SK-HEP-1 and Hep G2 cells subjected to various concentrations of OGF for 72 h. OGF or an equivalent volume of sterile water [control (Co)] was added 24 h after seeding 100,000 cells/well, and media and OGF were replaced daily. B: growth of SK-HEP-1 and Hep G2 cells treated with OGF (10−6 M) or an equivalent volume of sterile water over a 96-h period. OGF or water (Co) was added 24 h (0 h) after cells were seeded at 100,000 cells/well; media and OGF were changed daily. C: opioid receptor mediation of the growth inhibitory effects of OGF in SK-HEP-1 and Hep G2 cells. Cell cultures were subjected to OGF (10−6 M), the opioid antagonist naloxone (Nal; 10−8 M), Nal + OGF, or sterile water (Co) for 72 h. Cells were seeded at 100,000 cells/well, and media and compounds were replaced daily. D: reversibility of the growth-inhibitory effects on SK-HEP-1 and Hep G2 cells treated with 10−6 M OGF or sterile water (Co). Cells were seeded at 100,000 into 6-well plates and treated with OGF or sterile water for 48 h; cells were counted at 0, 24, 48, 72, and 96 h. At 48 h, one-half of the culture plates continued to receive OGF for an additional 48 h, and one-half of the plates were treated with sterile water for 48 h. Control cultures received sterile water throughout the 96 h. Compounds and media were replaced daily. For all experiments, data represent means ± SE for at least 2 aliquots/well from at least 2 wells/group. Data significantly different from respective controls: *P < 0.05, **P < 0.01, ***P < 0.001. OGF-Co group significantly different from OGF-OGF group: +P < 0.05; +++P < 0.01.
To determine whether OGF activity was mediated by an opioid receptor, cells were grown in the presence of OGF, and the short-acting nonselective opioid receptor antagonist naloxone (Nal) at a concentration of Nal that did not influence cell proliferation (Fig. 2C). Cell number was reduced by 32% and 23% in SK-HEP-1 and Hep G2 cultures, respectively, treated with 10−6 M OGF compared with vehicle-treated cultures, cultures receiving both OGF and Nal, or Nal alone.
To examine the reversibility of OGF inhibition on cell number, cultures of SK-HEP-1 and Hep G2 were exposed for 48 h to 10−6 M OGF, and cell number was decreased by 38% and 32%, respectively, from control levels. Media was removed at 48 h, and fresh media added with no OGF (OGF-Co); some cultures continued to receive a daily change of media and OGF (OGF-OGF). At 24 h after OGF-containing media was replaced with fresh media, cell number in the OGF-Co group in SK-HEP-1 and Hep G2 cultures was increased 9% and 17%, respectively, from cultures continuing to receive OGF (i.e., OGF-OGF). At 48 h after OGF-containing media was replaced with fresh media, cell number in the OGF-Co group in SK-HEP-1 and Hep G2 cultures was increased 12% and 23%, respectively, from cultures continuing to receive OGF (i.e., OGF-OGF) (Fig. 2D).
The endogenous opioid specific for growth inhibition of HCC cells is OGF.
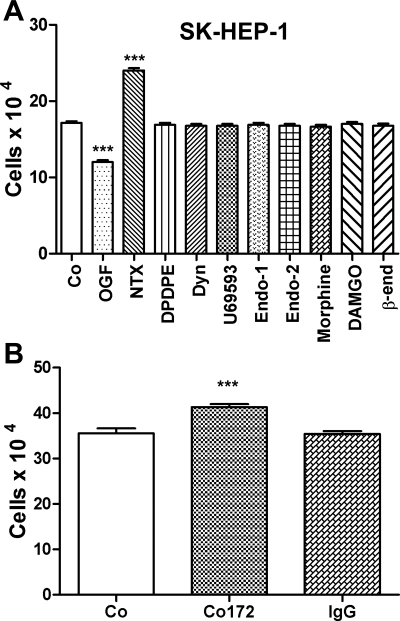
To determine whether endogenous or exogenous opioids other than OGF modulate the growth of HCC, SK-HEP-1 cultures were treated daily for 48 h with 10−6 M concentrations of natural and synthetic opioid-related compounds, many specific for μ, δ, and κ opioid receptors (Fig. 3A). Under the same conditions and concentrations whereby OGF markedly decreased cell number, all other opioid-related peptides had no effect on the proliferation.
Fig. 3.
OGF is the specific opioid peptide involved in the growth inhibition of human HCC cells. A: effects of various endogenous and exogenous opioids on SK-HEP-1 cell number. Cells were seeded at 50,000/well into 24-well plates and treated daily with 10−6 M concentrations of a variety of opioids for 48 h. B: SK-HEP-1 cells were treated with a polyclonal antibody specific for OGF (Co172) or with preimmune serum (IgG). Compounds were replaced daily. Cell number was measured at 48 h. Data represent means ± SE for at least 2 aliquots/well from at least 2 wells/group. ***Significantly different from sterile water-treated cells (Co) at P < 0.001.
Persistent opioid receptor blockade between OGF and OGFr with the general opioid receptor antagonist NTX (10−6 M) was also evaluated for its effect on cell growth of human HCC cells. SK-HEP-1 cell number was increased 38% from control values (Fig. 3A).
The specificity of endogenous OGF's inhibitory action was investigated by neutralizing native OGF with a polyclonal antibody. SK-HEP-1 cultures exposed to the OGF antibody had 16% more cells than control cultures; cultures treated with sterile water (Co) and those receiving preimmune serum (IgG) had a similar number of cells (Fig. 3B).
Silencing of OGFr in human HCC cells blocks the inhibitory action of endogenous and exogenous OGF and the stimulatory action of NTX.
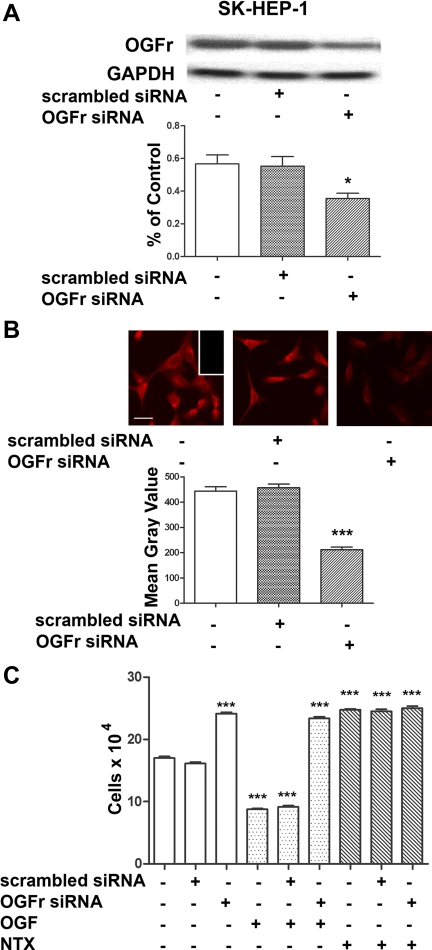
The requirement of the OGFr for OGF's inhibitory action on cell proliferation was evaluated at the molecular level using siRNA technology. OGFr siRNA transfected SK-HEP-1 cells had 47% less OGFr mRNA levels relative to cells not transfected (Fig. 4A). Cells exposed to scrambled siRNA were comparable in OGFr mRNA levels to untransfected cells. Semiquantitative immunohistochemistry revealed that OGFr siRNA transfected SK-HEP-1 cells have 48% of OGFr protein levels than cells not transfected (Fig. 4B). SK-HEP-1 cells transfected with OGFr siRNA had 28% more cells than cultures that were not transfected (Fig. 4C). The addition of exogenous OGF or NTX had no inhibitory or stimulatory, respectively, effects on cells transfected with OGFr siRNA compared with cultures transfected with OGFr siRNA and treated with sterile water.
Fig. 4.
OGFr is required for OGF's inhibitory action on growth on HCC cells. A: Northern blot analysis and semiquantitative densitometry demonstrating the specificity and level of OGFr knockdown in SK-HEP-1 cells. Log phase cells were transfected for 24 h with either scrambled siRNA or OGFr siRNA. Forty-eight hours after transfection, cells were harvested and RNA isolated. Data (%OGFr/GAPDH ratio) represent means ± SE for 2 blots from independent experiments. *Significantly different from nontransfected cultures at P < 0.05. B: photomicrographs of log phase SK-HEP-1 cells stained with a polyclonal antibody to OGFr (B0344) demonstrating the extent of OGFr protein knockdown. Cells were transfected for 24 h with OGFr siRNA or scrambled siRNA, and incubated in media for an additional 48 h. Photomicrographs of cells stained with OGFr were taken at the same exposure time. Semiquantitative measurement of the OGFr immunohistochemistry demonstrating the level of protein knockdown in SK-HEP-1 cells. Decreased OGFr staining intensity (mean gray value) is indicative of decreased OGFr protein expression. Data represent means ± SE. ***Significantly different from nontransfected cells at P < 0.001. C: growth of SK-HEP-1 cultures transfected with OGFr siRNA or scrambled siRNA for 24 h and treated with either OGF (10−6 M), naltrexone (NTX; 10−6 M), or an equivalent volume of sterile water for 72 h; compounds and media were changed daily. Values represent means ± SE cell counts for at least 2 aliquots/well and least 2 wells/treatment. ***Significantly different at P < 0.001 from cultures that were not transfected and treated with sterile water.
OGF alters DNA synthesis but not apoptosis or necrosis.
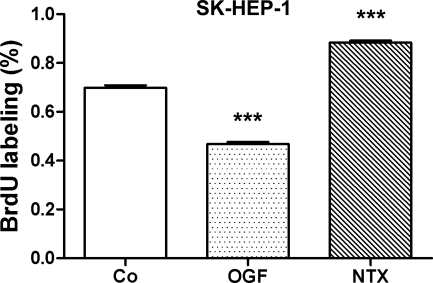
To evaluate the mechanism by which OGF inhibits human HCC cell growth, DNA synthesis of SK-HEP-1 cultures exposed to OGF, NTX, or sterile water was measured (Fig. 5). The proportion of BrdU-labeled cells in SK-HEP-1 cultures exposed to OGF for 72 h was decreased by 47% compared with cultures receiving sterile water, whereas cells given NTX increased by 21%, from cultures receiving sterile water.
Fig. 5.
OGF‘s inhibitory effects on DNA synthesis and NTX’s stimulatory effects on DNA synthesis of HCC cells. SK-HEP-1 cells were seeded on to 22-mm coverslips and treated for 72 h with 10−6 M concentrations of OGF, NTX, or an equivalent volume of sterile water, and 3 h with 30 μM BrdU. Data represent the %BrdU positive cells (means ± SE) for at least 2 coverslips/treatment group and a total of at least 300 cells/treatment group. ***Significantly different from water-treated cells at P < 0.001.
Examination of apoptosis or necrosis in at least 300 SK-HEP-1 cells treated with either OGF, NTX, or sterile water for 72 h revealed no (≤1/1,000 cells) apoptosis or necrosis; positive controls for the TUNEL kit had ≥ 95% positive cells.
The OGF-OGFr axis is present and functions in a variety of HCC cells.
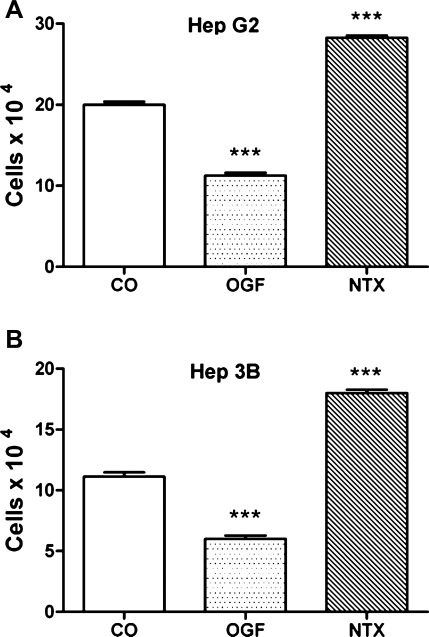
The ubiquity of the OGF-OGFr axis in human HCC cells was examined by evaluating a total of three cell lines: SK-HEP-1, Hep G2, and Hep 3B. After 72 h of treatment with OGF, Hep G2 and Hep 3B cells were reduced in number by 43% and 46%, respectively, from their controls. Exposure to NTX increased cell number in Hep G2 and Hep 3B cultures by 41%, and 61%, respectively, relative to cells treated with sterile water (Fig. 6.).
Fig. 6.
The OGF-OGFr axis ubiquitously inhibits growth of human HCC cells. A: growth of Hep G2 cells subjected to 10−6 M concentrations of OGF or NTX, or an equivalent volume of sterile water for 72 h. B: growth of Hep 3B cells subjected to 10−6 M concentrations of OGF or NTX, or an equivalent volume of sterile water for 72 h. For all experiments, compounds were added 24 h after seeding 100,000 cells/well, and media and compounds were replaced daily. Data represent means ± SE for at least 2 aliquots/well from at least 2 wells/group. ***Significantly different from respective controls at P < 0.001.
OGF and OGFr are present in human HCC specimens.
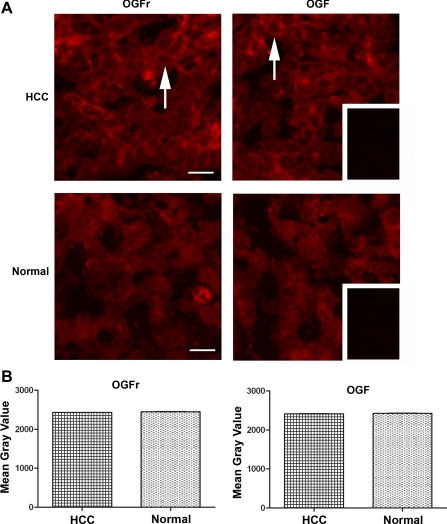
The presence of OGF and OGFr in surgical specimens of HCC from three patients was determined by immunohistochemistry. Both OGF and OGFr were detected in the cytoplasm and nucleus of HCC cells that were characteristic of these cancers (e.g., large and irregular nuclei) (Fig. 7A)
Fig. 7.
Presence and quantitative analysis of OGF and OGFr in surgical specimens of HCC and normal tissue. A: HCC resected from a 72-yr-old male with moderately differentiated cancer (Stage 3A) and stained with antibodies to OGF or OGFr. Note the immunoreactivity for OGF and OGFr adjacent to the nucleus and throughout the cytoplasm (arrows), as well as a speckling of immunoreactivity in the nucleus. Tissues processed with secondary antibody showed no staining (inset). Bar = 20 μm. B: semiquantitative densitometric analysis of OGF and OGFr from surgical specimens of 2 patients with HCC that compare tumor with normal (margin) tissues. Data represent means ± SE for at least 4 sections/specimen.
To inquire as to whether there were differences in the quantity of OGF and OGFr in HCC compared with normal tissues (margins considered to be normal by pathology), semiquantitative densitometry was performed. No differences in OGF or OGFr quantities were recorded (Fig. 7B).
DISCUSSION
The presence of endogenous opioid receptors in surgical specimens of human liver cancer was previously detected by Zagon et al. (29). This observation prompted two questions: 1) Does the OGF-OGFr axis exist in human HCC? and 2) Can cell proliferation in these tumor cells be modulated by the OGF-OGFr pathway? Using a tissue culture model, both OGFr and OGF were detected by immunohistochemistry, and receptor binding studies revealed that the OGFr was capable of binding OGF. Cell proliferation assays with SK-HEP-1 and Hep G2 cell lines ascertained that OGF depressed cell number in a dose-dependent fashion. OGF inhibitory activity was rapid, persistent, and did not exhibit tolerance, being detected as early as 24 h after initiating peptide administration and extending for 4 days. The effects of OGF on HCC cell replication were receptor mediated as they were blocked by Nal in the absence of an effect of Nal alone. Moreover, the effect of OGF on HCC cells was reversible, indicating a cytostatic but not toxic action. Cells subjected to a wide variety of synthetic and natural opioid receptors, including those specific for μ (DAMGO, endomorphin 1 and 2), δ (DPDPE), and κ (U69593) opioid receptors, showed that none of these compounds had any effect on growth at a concentration (10−6 M) of OGF that markedly depressed cell proliferation. Additionally, the present study revealed that the OGF-OGFr axis functions in at least three different human HCC cell lines, indicating the ubiquity of the system with respect to this disease. Finally, both OGF and OGFr were detected in surgical specimens from patients undergoing resection for HCC, indicating that the presence and function of the OGF-OGFr system defined in tissue culture was relevant to HCC in situ. However, no differences in the quantity of OGF or OGFr were observed between HCC and normal liver, suggesting that the OGF-OGFr axis was intact and capable of being modulated by exogenous OGF. Thus, these results reveal for the first time, the presence and physiological significance of a native biological pathway regulating cell proliferation in human HCC.
Although OGF was the only opioid peptide to inhibit HCC cell proliferation, and OGF was detected in these cells by immunohistochemistry, the question can be raised as to whether endogenous OGF is a functional native peptide related to growth. Two pieces of evidence support this to be the case. First, cells exposed to the general opioid antagonist NTX accelerated in growth, indicating that the opioid related to cell proliferation is constitutively expressed and tonically active. Second, and more specific to OGF, neutralization of OGF by an antibody to this peptide stimulated cell replication. Thus, depletion of endogenous OGF in these cultures removed the inhibitory influence on these cells that establishes an equilibrium in cell proliferation.
An extensive literature search shows that OGF interacts with OGFr to regulate cell proliferation (2, 4–6, 14–16, 18, 26–28, 34–36), implying that effects on the control of cell number by endogenous OGF, as well as exogenously administered peptide, are mediated by this receptor. OGFr was detected in HCC cells by immunohistochemistry and receptor binding techniques. However, this opioid peptide also serves in neurotransmission and is known to bind to classical opioid receptors such as μ, δ, and κ as well (11). To examine the specificity of OGF for OGFr with respect to regulation of cell proliferation, the effect of silencing OGFr using siRNA was undertaken. Cells treated with OGFr siRNA were observed to have an increase in cell number, suggesting that attenuating OGFr compromised the action of OGF. Moreover, exogenously administered OGF, which depresses cell number in log phase cultures, did not have any effect when the cells were transfected with OGFr siRNA. Indeed, there was a greater number of cells in cultures treated with OGFr siRNA and exposed to OGF than in transfected cultures given vehicle or scrambled siRNA. All of these data support OGFr as the receptor mediating OGF action. Taken together with the knowledge that OGF is the peptide involved with modulating cell number of human HCC cells, it is clear that proliferation of these carcinoma cells is regulated by the OGF-OGFr axis. Whether OGF and OGFr are related to the pathophysiology of HCC remains to be determined.
A decrease in cell number as seen by treatment with OGF could be due to a mechanism involving a decrease in cell survival because of either programmed cell death, necrosis, and/or a reduction in DNA synthesis and subsequent cell replication. Our data show that neither apoptosis nor necrosis are involved with OGF activity in HCC cells, a result consistent with a previous publication examining apoptosis/necrosis in a variety of cancer cells in culture (30). However, DNA synthesis in HCC cultures treated with OGF exhibited a markedly diminished cell number relative to control levels, whereas blockade of the OGF-OGFr interaction by NTX elevated DNA synthesis. Consonant with previous studies, these data indicate that the mechanism of OGF involves the regulation of cell proliferation (2, 4–6, 18, 26, 27, 34, 35). The action of OGF on cell proliferation has been reported to involve the cellular interaction of OGF and OGFr (32, 33), trafficking from cytoplasm to the nucleus (3, 32, 33), and inhibition of the cell cycle (4–6). Thus, OGF, an autocrine and paracrine-derived peptide (36), is known to interact with OGFr on the outer nuclear envelope (32, 33) and, together, transit into the paranuclear cytoplasm and into the nucleus with the aid of nuclear localization signals in OGFr (3, 32, 33). In the nucleus, the OGF-OGFr complex induces an upregulation of two cyclin-dependent kinase inhibitors, p16 and p21, which lead to a depression in the phosphorylation of Rb and a retardation of cells in the G1-S phase of the cell cycle (4–6). Further studies are required to investigate OGF-OGFr modulation of HCC with regard to the cell cycle.
Previous reports on the relationship of HCC to opioids have documented that there is an elevation in nociceptin/orphanin FQ in the plasma of patients with this disease (8, 24). A subsequent study by Spadaro et al. (23) corroborated that nociception/orphanin FQ were increased in patients with chronic liver disease, but this peptide was of low utility as a diagnostic tool for liver cirrhosis. Using a tissue culture model of HCC, Jairaj et al. (9) observed that 2 × 10−4 M morphine or its analogs had no effect on cell viability. Notas et al. (19) showed that 6 days of treatment with the synthetic opioids ethylketocyclazocine, [d-Ala2,d-Leu5] enkephalin, and DAMGO, repressed the cell number of Hep G2 cells and report that the inhibitory effect of ethylketocyclazocine on cell number was related to apoptosis. Although transcripts for κ, but not for μ or δ, opioid receptors were detected, receptor binding assays with radiolabeled ethylketocyclazocine revealed no specific binding sites. Notas et al. (19) conclude that opioids modulate the number of HCC cells independent of opioid receptors. The present investigation did not record any change in the number of SK-HEP-1 cells with 10−6 M concentrations of μ (DAMGO, endomorphin-1, endomorphin-2), δ (DPDPE), or κ (dynorphin, U69,593) ligands. Moreover, these opioids did not alter cell survival. The reason(s) for this discrepancy in results is(are) unclear. However, some major differences between these studies include 1) the longer treatment time of drug exposure [i.e., 6 days by Notas et al. (19) and 3 days herein], 2) the cell line utilized [i.e., Hep G2 for Notas et al. (19) and SK-HEP-1 herein], and 3) the assay used to determine cell number [i.e., MTT by Notas et al. (19) and cell counts herein]. Moreover, Notas et al. (19) did not include any statistical analyses of their data, in contrast to the rigorous statistical analysis utilized in the present study.
Perspectives and Significance
These results reveal for the first time the presence and functional significance of a native biological pathway regulating cell proliferation in human HCC. A critical question that needs to be addressed in future studies is whether OGF has efficacy in modulating the incidence and progression of human HCC in vivo. Preclinical experiments are needed to establish whether this agent can alter the course of this lethal cancer. If preclinical studies are successful, these results would warrant initiation of clinical trials.
GRANTS
This work was supported, in part, by National Cancer Institute Grant KO8-CA-100094 (to K. F. Staveley-O'Carroll).
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank Dr. Henry Crist, Department of Pathology, The Pennsylvania State University, College of Medicine, for confirmation of hepatocellular carcinoma in surgical specimens. Tumor specimens were obtained from the Tissue Bank at the Penn State Hershey Cancer Institute (PSHCI) with the generous help of Dr. C. Bart Rountree (Department of Pediatrics, The Pennsylvania State University, College of Medicine) and Mr. Daniel Beard (PSHCI).
REFERENCES
- 1.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol 27: 1485–1491, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bisignani GJ, McLaughlin PJ, Ordille SD, Jarowenko MJ, Zagon IS. Human renal cell proliferation in tissue culture is tonically inhibited by opioid growth factor. J Urol 162: 2186–2191, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Cheng F, McLaughlin PJ, Verderame MF, Zagon IS. Dependence on nuclear localization signals of the opioid growth factor receptor in the regulation of cell proliferation. Exp Biol Med (Maywood) 234: 532–541, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Cheng F, Zagon IS, Verderame MF, McLaughlin PJ. The OGF-OGFr axis utilizes the p16 pathway to inhibit progression of human squamous cell carcinoma of the head and neck. Cancer Res 67: 10511–10518, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Cheng F, McLaughlin PJ, Verderame MF, Zagon IS. The OGF-OGFr axis utilizes the p21 pathway to restrict progression of human pancreatic cancer. Mol Cancer 7: 5–17, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng F, McLaughlin PJ, Verderame MF, Zagon IS. The OGF-OGFr axis utilizes the p16INK4a and p21WAF1/CIP1 pathways to restrict normal cell proliferation. Mol Biol Cell 20: 319–327, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fogh J, Trempe G. New human tumor cell lines. In: Human Tumor Cells in Vitro, edited by Fogh J. New York: Plenum, 1975, p. 115–159 [Google Scholar]
- 8.Horvath A, Folhoffer A, Lakatos PL, Halosz J, Illyes G, Schaff Z, Hantos MB, Tekes K, Szalay F. Rising plasma nociceptin level during development of HCC: a case report. World J Gastroenterol 10: 152–154, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jairaj M, Watson DG, Grant MH, Skellern GG. The toxicity of opiates and their metabolites in HepG2 cells. Chem Biol Interact 146: 121–129, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Knowles BB, Howe CC, Aden DP. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science 209: 497–499, 1980 [DOI] [PubMed] [Google Scholar]
- 11.Leslie FM. Methods used for the study of opioid receptors. Pharmacol Rev 39: 197–249, 1987 [PubMed] [Google Scholar]
- 12.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 362: 1907–1917, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, Sherman M, Schwartz M, Lotze M, Talwalkar J, Gores GJ. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst 100: 698–711, 2008 [DOI] [PubMed] [Google Scholar]
- 14.McLaughlin PJ, Kreiner S, Morgan CR, McLaughlin PJ. Prevention and delay in progression of human squamous cell carcinoma of the head and neck in nude mice by stable overexpression of the opioid growth factor receptor (OGFr). Int J Oncol 4: 751–757, 2008 [PubMed] [Google Scholar]
- 15.McLaughlin PJ, Levin RJ, Zagon IS. Regulation of human head and neck squamous cell carcinoma growth in tissue culture by opioid growth factor. Int J Oncol 14: 991–998, 1999 [DOI] [PubMed] [Google Scholar]
- 16.McLaughlin PJ, Stack BC, Braine KM, Ruda JD, Zagon IS. Opioid growth factor (OGF) inhibition of a human squamous cell carcinoma of the head and neck in nude mice: dependency on the route of administration. Int J Oncol 24: 227–232, 2004 [PubMed] [Google Scholar]
- 17.McLaughlin PJ, Stack BC, Levin RJ, Fedok F, Zagon IS. Defects in the OGF receptor (OGFr) in human squamous cell carcinoma of the head and neck. Cancer 97: 1701–1710, 2003 [DOI] [PubMed] [Google Scholar]
- 18.McLaughlin PJ, Verderame MF, Hankins JL, Zagon IS. Overexpression of the opioid growth factor receptor downregulates cell proliferation of human squamous carcinoma cells of the head and neck. Int J Mol Med 19: 421–428, 2007 [PubMed] [Google Scholar]
- 19.Notas G, Kampa M, Nifli AP, Xidakis K, Papasava D, Thermos K, Kouroumalis E, Castanas E. The inhibitory effect of opioids on HepG2 cells is mediated via interaction with somatostatin receptors. Eur J Pharmacol 555: 1–7, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: GLOBOCAN 2000. Int J Cancer 94: 153–156, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 55: 74–108, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Shimizu M, Takai K, Moriwaki H. Strategy and mechanism for the prevention of hepatocellular carcinoma: phosphorylated retinoid X receptor α is a critical target for hepatocellular carcinoma chemoprevention. Cancer Sci 100: 369–374, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spadaro A, Ajello A, Luigiano C, Morace C, Resta ML, Berlinghieri G, Campo S, Scisca C, Alibrandi A, D'Arrigo G, Alessi N, Ferrau O, Freni MA. Low utility of plasma nociceptin/orphanin FQ in the diagnosis of hepatocellular carcinoma. World J Gastroenterol 12: 16–20, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szalay F, Hantos MB, Horvath A, Lakatos PL, Folhoffer A, Dunkel K, Hegedus D, Tekes K. Increased nociceptin/orphanin FQ plasma levels in hepatocellular carcinoma. World J Gastroenterol 10: 42–45, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka S, Arii S. Molecularly targeted therapy for hepatocellular carcinoma. Cancer Sci 100: 1–8, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zagon IS, Donahue RN, Rogosnitzky M, McLaughlin PJ. Imiquimod upregulates the opioid growth factor receptor to inhibit cell proliferation independent of immune function. Exp Biol Med (Maywood) 8: 968–979, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Zagon IS, Hytrek SD, McLaughlin PJ. Opioid growth factor tonically inhibits human colon cancer cell proliferation in tissue culture. Am J Physiol Regul Integr Comp Physiol 271: R511–R518, 1996 [DOI] [PubMed] [Google Scholar]
- 28.Zagon IS, Kreiner S, Heslop JJ, Conway AB, Morgan CR, McLaughlin PJ. Prevention and delay in progression of human pancreatic cancer by overexpression of the opioid growth factor receptor (OGFr). Int J Mol Med 33: 317–323, 2008 [PubMed] [Google Scholar]
- 29.Zagon IS, McLaughlin PJ, Goodman SR, Rhodes RE. Opioid receptors and endogenous opioids in diverse human and animal cancers. J Natl Cancer Inst 79: 1059–1065, 1987 [PubMed] [Google Scholar]
- 30.Zagon IS, McLaughlin PJ. Opioids and the apoptotic pathway in human cancer cells. Neuropeptides 37: 79–88, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Zagon IS, McLaughlin PJ. Production and characterization of polyclonal and monoclonal antibodies to the zeta (ζ) opioid receptor. Brain Res 630: 295–302, 1993 [DOI] [PubMed] [Google Scholar]
- 32.Zagon IS, Ruth TB, Leure-duPree AE, Sassani JW, McLaughlin PJ. Immunoelectron microscopic localization of the opioid growth factor receptor (OGFr) and OGF in the cornea. Brain Res 967: 37–47, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Zagon IS, Ruth TB, McLaughlin PJ. Nucleocytoplasmic distribution of opioid growth factor (OGF) and its receptor (OGFr) in tongue epithelium. Anat Rec A Discov Mol Cell Evol Biol 282: 24–37, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Zagon IS, Smith JP, McLaughlin PJ. Human pancreatic cancer cell proliferation in tissue culture is tonically inhibited by opioid growth factor. Int J Oncol 14: 577–584, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Zagon IS, Verderame MF, Hankins JL, McLaughlin PJ. Overexpression of the opioid growth factor receptor potentiates growth inhibition in human pancreatic cancer cells. Int J Oncol 30: 775–783, 2007 [PubMed] [Google Scholar]
- 36.Zagon IS, Verderame MF, McLaughlin PJ. The biology of the opioid growth factor receptor (OGFr). Brain Res Rev 38: 351–376, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Zagon IS, Verderame MF, McLaughlin PJ. The expression and function of the OGF-OGFr axis–a tonically active negative regulator of growth–in COS cells. Neuropeptides 37: 290–297, 2003 [DOI] [PubMed] [Google Scholar]