Abstract
Behavioral and physiological rhythms can be entrained by daily restricted feeding (RF), indicating the existence of a food-entrainable oscillator (FEO). One manifestation of the presence of FEO is anticipatory activity to regularly scheduled feeding. In the present study, we tested if intact ghrelin signaling is required for FEO function by studying food anticipatory activity (FAA) in preproghrelin knockout (KO) and wild-type (WT) mice. Sleep-wake activity, locomotor activity, body temperature, food intake, and body weight were measured for 12 days in mice on a RF paradigm with food available only for 4 h daily during the light phase. On RF days 1–3, increases in arousal occurred. This response was significantly attenuated in preproghrelin KO mice. There were progressive changes in sleep architecture and body temperature during the subsequent nine RF days. Sleep increased at night and decreased during the light periods while the total daily amount of sleep remained at baseline levels in both KO and WT mice. Body temperature fell during the dark but was elevated during and after feeding in the light. In the premeal hours, anticipatory increases in body temperature, locomotor activity, and wakefulness were present from RF day 6 in both groups. Results indicate that the preproghrelin gene is not required for the manifestation of FAA but suggest a role for ghrelinergic mechanisms in food deprivation-induced arousal in mice.
Keywords: food-entrainable oscillator, food anticipatory activity, electroencephalographic slow-wave activity, food deprivation, hypothermia
interactions between energy homeostasis, feeding, and sleep are well-documented. For example, meal size correlates with the subsequent duration of sleep (8). Starvation induces sleep loss in rats (16). These sleep-loss effects develop more rapidly if the initial energy levels of the body are low (3, 10). In contrast, refeeding after food deprivation increases sleep in rats (3, 33). Rats kept on a calorie-rich “cafeteria diet” tend to overeat and have an increased amount of sleep (7, 15). Intravenous infusion of a variety of nutrients differently modulates sleep in rats (9). Chronic sleep deprivation leads to hyperphagia and weight loss in rats (30), whereas chronic short sleep duration is associated with obesity in humans (34, 41). When food availability is restricted to the light period, it alters the diurnal distribution of sleep in rats (29, 31). These findings indicate a strong relationship between sleep and feeding.
The diurnal rhythm of sleep and feeding is driven by biological clock(s). The biological clock within the suprachiasmatic nucleus (SCN) is entrained to a 24-h rhythm by light. In addition to light, another potent entraining signal is periodic feeding. The biological clock that is affected by feeding is called the food-entrainable oscillator (FEO), which is located outside the confines of the SCN. One manifestation of the activity of the FEO is food-anticipatory activity (FAA). FAA is characterized by increases in behavioral activity, corticosterone secretion, and body temperature 1–4 h before scheduled feeding time when food availability is restricted to a few hours a day.
The site of the FEO remains elusive. Lesions of hypothalamic and extrahypothalamic areas (19, 22, 23) or subdiaphragmatic vagotomy (28) all failed to abolish FAA in rats. Recently, the dorsomedial hypothalamus (DMH) was posited to be an integrator of light- and food-entrainable circadian rhythms (12, 13); these findings, however, remain controversial (18, 20, 26). Genetic deletion (17, 24, 46) or postnatal lesions (e.g., Refs. 1, 21, and 25) of feeding-related signals in mice or rats have also failed to eliminate FAA. Clock genes are expressed in various peripheral organs, including the gastrointestinal system, suggesting the possibility that the FEO is located in the gastrointestinal system. Irrespective whether the FEO is peripheral or central, gut-to-brain signaling is required either as an output signal from a peripheral clock to the brain to elicit behavioral responses or as an afferent signal from the gut to a centrally located oscillator. Gastrointestinal hormones, the secretions of which are phase-locked to feeding, are likely candidates to serve as such a signal.
Ghrelin is an orexigenic peptide, produced mainly by the stomach. Its secretion is locked to feeding activity; ghrelin levels are elevated during fasting and suppressed after eating (2, 6, 43). In an experimental restricted feeding (RF) paradigm, plasma ghrelin levels increase in parallel to FAA (11). Ghrelin is also produced by the brain; ghrelin-immunoreactive cell bodies are present in the hypothalamic area between the arcuate nucleus (ARC), paraventricular nucleus (PVN), DMH, and lateral hypothalamus (5). Ghrelin stimulates food intake via the activation of neuropeptide Y (NPY)-ergic neurons in the hypothalamic ARC. Intracerebroventricular administration or hypothalamic microinjections of ghrelin promote wakefulness in rats (37, 38). Ghrelin receptors are expressed in the ARC, SCN, DMH, and PVN of the hypothalamus (14, 27, 47), all of which are implicated in feeding and sleep regulation.
Based on these observations, we hypothesized that ghrelin might be an integrator of feeding- and arousal-related signals directed to the FEO, or ghrelinergic neurons may be part of the FEO itself. To test this hypothesis, we studied the short (1–3 days)- and longer (8–12 days)-term effects of RF on sleep, locomotor activity, and body temperature in preproghrelin knockout [KO (originally named as ghrelin−/−; see Ref. 36)] and wild-type (WT) mice. The wake-promoting effects of short-term RF were attenuated in preproghrelin KO animals, but they displayed normal FAA, suggesting that ghrelin is not required for the normal function of the FEO but it plays a significant role in arousal responses to fasting.
MATERIALS AND METHODS
Animals and surgery.
Breeding pairs of preproghrelin KO and WT mice with a C57BL6J/129SvEv genetic background, backcrossed to C57BL6J for 10 generations, were generated at Baylor College of Medicine (Houston, TX), and further bred at Washington State University. The procedures used to generate the mutant mice were described previously (36). Each mouse used was genotyped (Transnetyx, Cordova, TN) from tail-tip samples. Male mice, 4 mo of age at the time of surgery, were maintained, under veterinary supervision, in animal quarters approved by the Association for Assessment and Accreditation of Laboratory Animal Care. The procedures employed were approved by the Washington State University Institutional Animal Use and Care Committee. Mice were anesthetized with an intraperitoneal injection of ketamine-xylazine mixture (87 and 13 mg/kg, respectively). The animals were implanted with cortical electroencephalographic (EEG) electrodes, placed over the frontal and parietal cortexes and stainless steal electromyographic (EMG) electrodes in the dorsal neck muscles. The EEG and EMG electrodes were connected to a pedestal that was fixed to the skull with dental cement. Wax-coated telemetry transmitters (model XM; Mini Mitter, Bend, OR) were implanted intraperitoneally in each animal. After surgery, mice were housed individually in standard plastic cages and maintained at 29 ± 1°C (a thermoneutral ambient temperature for mice) in sound-attenuated environmental chambers on a 12:12-h light-dark cycle (light on at 9:00 A.M.). During a 7- to 10-day recovery period, the animals were handled daily and connected to the recording cables for habituation to the experimental conditions. Water was available ad libitum throughout the experiment.
Experimental protocol.
Baseline body temperature, food intake, and sleep-wake activity data were obtained from the animals over a 48-h period (baseline days 1 and 2), when food was available ad libitum. From the 3rd day, mice were restricted to a 4-h daily meal, beginning 4 h after lights-on, for 12 consecutive days (RF). Body weight of mice was assessed daily at the beginning of the feeding period, and then preweighed amounts of food pellets were placed in the cages. The leftover was collected and weighed after 4 h. The RF condition was followed by a 2-day recovery period during which mice had ad libitum access to food.
Biotelemetry and sleep-wake recordings.
Core body temperature, locomotor activity, and EEG data were collected simultaneously from each animal with the exception of three preproghrelin KO mice from which only body temperature and activity data were obtained (sleep: n = 8 for both WT and KO; temperature and activity: n = 8 for WT and n = 11 for KO). The transmitter-emitted signals were converted to temperature and locomotor activity data using VitalView Series 3000 data acquisition software. Body temperature and locomotor activity values were collected in every 10 s or 10 min, respectively, and were averaged into 1-h time blocks for each day. Animals were connected to recording cables 3 days after surgery for habituation to the experimental conditions. Recording cables were attached to commutators that were connected to amplifiers. The amplified EEG and EMG signals were digitized and recorded by computer using SleepWave Software (Biosoft Studio). EEG was filtered below 0.1 Hz and above 40 Hz. EMG activity was used for the vigilance state determination and was not further analyzed. The vigilance states [i.e., wakefulness, non-rapid-eye movement sleep (NREMS), and rapid-eye movement sleep (REMS)] were visually determined off-line in 10-s epochs by using the conventional criteria as described previously (37). Briefly, low-amplitude, mixed-frequency EEG accompanied by high EMG activity was defined as wakefulness. NREMS was identified by high-amplitude EEG waves, predominant EEG power in the delta range (0.5–4 Hz), and low EMG activity. REMS was identified by highly regular low-amplitude EEG, the dominance of theta activity, and minimal EMG activity. Power density values in the delta range during NREMS [also known as EEG slow-wave activity (SWA) and used as a measure of NREMS intensity] were averaged across the entire 48-h baseline recording period to obtain a reference value for each mouse. EEG SWA values are expressed as a percentage of this reference value in 2-h time blocks. The total number of wakefulness, NREMS, and REMS episodes and the average length of these episodes were determined for each day. Time spent in wakefulness, NREMS and REMS, body temperature, and locomotor activity were expressed in 1-h time blocks. Data from the first (days 1, 2, and 3), middle (days 4 and 6), and the last (days 8 and 12) days of RF were collapsed and are referred to as RF days 1–3, RF days 4–6, and RF days 8–12, respectively.
Statistical analyses.
Two-way ANOVA for repeated measures (factors: time and day) was performed to compare the durations of wake, NREMS and REMS, EEG SWA during NREMS, body temperature, and locomotor activity between baseline and RF days within the same genotype. When significant day effect or day × time interaction was found, a paired t-test was performed post hoc. Body weight and food intake were analyzed by ANOVA (repeated measure: day, independent factor: genotype) followed by paired t-test for comparisons between days within a genotype. For the comparison of the number and duration of wake, NREMS, and REMS episodes between genotypes, two-tailed Student's t-test was used. Body temperature during the 4-h period before feeding on RF days and during the corresponding hours on the baseline and recovery days were further analyzed by 1) computing linear regression daily for each genotype (dependent variable: temperature, independent variable: time) and 2) testing for parallelism between the regression lines of WT and preproghrelin KO groups. Increasing body temperature (hence the slope of the regression lines) in these hours is indicative of FAA. Significant differences in parallelism mean different slopes and would indicate differences in temperature-defined food anticipation. P values <0.05 were considered significant.
RESULTS
Wakefulness, NREMS, and REMS.
Under baseline conditions, both groups of mice showed characteristic nocturnal sleep-wake patterns; the amounts of NREMS and REMS were greater during daylight hours and less during the dark. There was no significant difference between genotypes in the time spent in the vigilance states under ad libitum feeding conditions (Figs. 1–4 and Table 1).
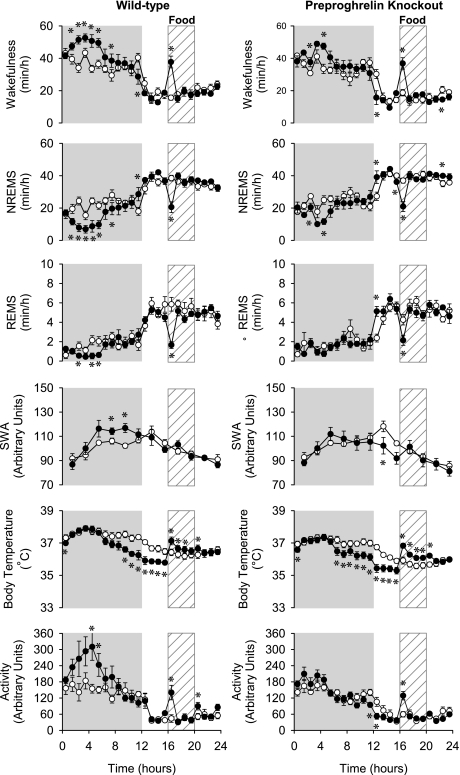
Fig. 1.
The diurnal rhythm of wakefulness, non-rapid-eye-movement sleep (NREMS), rapid-eye-movement sleep (REMS), electroencephalographic (EEG) slow-wave activity (SWA) during NREMS, body temperature, and locomotor activity of wild-type [WT (left), n = 8] and preproghrelin knockout [KO (right); sleep data: n = 8 ; temperature and activity data: n = 11] mice on the baseline days (open symbols) and on the restricted feeding (RF, filled symbols) days 1–3. Data are averaged across RF days 1, 2, and 3 and expressed in 1-h bins for each parameter except for EEG SWA where data points represent 2-h averages. Error bars, SE; gray shaded area, dark phase of the day. *Significant difference between baseline days and treatment days (univariate tests of significance for planned comparison, P < 0.05).
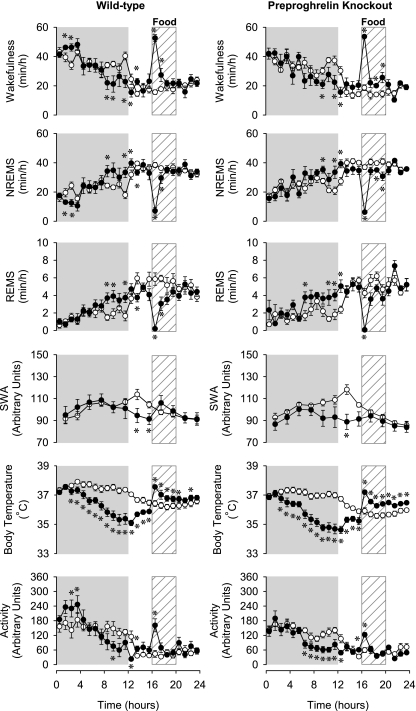
Fig. 4.
The diurnal rhythm of wakefulness, NREMS, REMS, EEG SWA, body temperature, and locomotor activity of WT (left) and preproghrelin KO (right) mice on the baseline days (open symbols) and on RF days 8–12. Data are averaged across RF days 8 and 12. *Significant difference between baseline days and treatment days (univariate tests of significance for planned comparisons, P < 0.05). See legend for Fig. 1 for details.
Table 1.
The amounts of wakefulness, NREMS, and REMS during the 12 h of dark and light phases and the 24-h amounts of each vigilance state on the baseline and RF day 8 in preproghrelin WT and KO mice
| WT (n = 8) |
KO (n = 8) |
|||
|---|---|---|---|---|
| Baseline | RF Day 8 | Baseline | RF Day 8 | |
| Wakefulness, min | ||||
| Dark | 434.2 ± 16.6 | 366.2 ± 18.4* | 416.7 ± 17.6 | 331.6 ± 9.4* |
| Light | 233.8 ± 11.0 | 319.2 ± 13.2* | 205.6 ± 12.5 | 313.0 ± 18.2* |
| Daily | 668.0 ± 26.4 | 685.4 ± 23.2 | 616.1 ± 21.8 | 643.4 ± 15.3 |
| NREMS, min | ||||
| Dark | 265.0 ± 15.8 | 317.4 ± 16.4* | 282.2 ± 15.9 | 340.1 ± 7.5* |
| Light | 424.8 ± 13.2 | 352.1 ± 12.6* | 454.8 ± 10.9 | 357.7 ± 14.1* |
| Daily | 689.8 ± 27.6 | 669.5 ± 23.0 | 742.0 ± 21.6 | 699.1 ± 11.1 |
| REMS, min | ||||
| Dark | 20.7 ± 1.8 | 37.4 ± 2.8* | 20.8 ± 3.2 | 45.3 ± 4.2* |
| Light | 61.3 ± 3.9 | 48.3 ± 3.6* | 59.1 ± 4.1 | 49.2 ± 4.3* |
| Daily | 82.0 ± 5.3 | 85.7 ± 3.8 | 81.0 ± 4.1 | 95.2 ± 5.9 |
Values are means ± SE; n, no. of mice. WT, wild type; KO, knockout; RF, restricted feeding; NREMS, non-rapid-eye movement sleep; REMS, rapid-eye movement sleep. There was no significant difference between genotypes in the time spent in the vigilance states under ad libitum feeding conditions and on RF day 8. RF led to decreased sleep during the day and increased sleep during the dark period in both genotypes. The daily, 24-h amounts of NREMS, REMS, and wakefulness remained at baseline levels in both groups.
Significant difference between baseline and RF day 8 within the same genotype (paired t-test, P < 0.05).
RF elicited progressive changes in the diurnal distribution of sleep-wake activity in both groups (see Table 2 for statistics). On RF days 1–3 (Fig. 1), wakefulness during the first 8 h of the dark period was significantly elevated above baseline in both genotypes; these increases were significantly higher in WT mice compared with KO [79.7 ± 15.4 min above baseline in WT mice compared with 43.2 ± 11.5 min in KO mice (Student's t-test, P < 0.05)]. Simultaneously, NREMS was significantly suppressed in both groups, whereas REMS was suppressed only in the WT animals. On the first 3 days of RF, neither group of mice showed an anticipatory increase in wakefulness before the scheduled feeding time (Fig. 2B). In the first hour of the daily meal time, wakefulness was significantly increased and NREMS and REMS suppressed in both preproghrelin KO and WT mice.
Table 2.
Results of statistical analyses [2-way ANOVA for repeated measures (factors: time and day)] performed on wakefulness, NREMS, REMS, SWA body temperature, and locomotor activity data separately for WT and KO mice
| Treatment |
Time |
Interaction |
||||
|---|---|---|---|---|---|---|
| F value | P | F value | P | F value | P | |
| WT RF 1–3 | ||||||
| Wake | F(1,7) = 12.2 | <0.05 | F(23,161) = 30.4 | <0.05 | F(23,161) = 6.1 | <0.05 |
| NREMS | F(1,7) = 7.4 | <0.05 | F(23,161) = 26.6 | <0.05 | F(23,161) = 6.5 | <0.05 |
| REMS | F(1,7) = 6.9 | <0.05 | F(23,161) = 30.8 | <0.05 | F(23,161) = 2.6 | <0.05 |
| SWA | F(1,7) = 0.9 | NS | F(11,77) = 9.9 | <0.05 | F(11,77) = 5.4 | <0.05 |
| Body temperature | F(1,7) = 9.8 | <0.05 | F(23,161) = 30.9 | <0.05 | F(23,161) = 12.5 | <0.05 |
| Activity | F(1,7) = 5.2 | <0.05 | F(23,161) = 16.5 | <0.05 | F(23,161) = 2.6 | <0.05 |
| KO RF 1–3 | ||||||
| Wake | F(1,7) = 6.5 | <0.05 | F(23,161) = 31.9 | <0.05 | F(23,161) = 4.4 | <0.05 |
| NREMS | F(1,7) = 6.9 | <0.05 | F(23,161) = 28.1 | <0.05 | F(23,161) = 4.3 | <0.05 |
| REMS | F(1,7) = 0.4 | NS | F(23,161) = 25.9 | <0.05 | F(23,161) = 2.4 | <0.05 |
| SWA | F(1,7) = 0.3 | NS | F(11,77) = 14.6 | <0.05 | F(11,77) = 3.5 | <0.05 |
| Body temperature | F(1,7) = 16.0 | <0.05 | F(23,161) = 32.5 | <0.05 | F(23,161) = 16.9 | <0.05 |
| Activity | F(1,11) = 0.01 | NS | F(23,253) = 24.1 | <0.05 | F(23,253) = 3.2 | <0.05 |
| WT RF 4 and 6 | ||||||
| Wake | F(1,7) = 1.2 | NS | F(23,161) = 13.2 | <0.05 | F(23,161) = 5.9 | <0.05 |
| NREMS | F(1,7) = 0.4 | NS | F(23,161) = 12 | <0.05 | F(23,161) = 5.6 | <0.05 |
| REMS | F(1,7) = 1.8 | NS | F(23,161) = 12.3 | <0.05 | F(23,161) = 5.3 | <0.05 |
| SWA | F(1,7) = 0.03 | NS | F(11,77) = 3.5 | <0.05 | F(11,77) = 4.0 | <0.05 |
| Body temperature | F(1,7) = 21.7 | <0.05 | F(23,161) = 16.3 | <0.05 | F(23,161) = 21.9 | <0.05 |
| Activity | F(1,7) = 0.5 | NS | F(23,161) = 13.3 | <0.05 | F(23,161) = 4.0 | <0.05 |
| KO RF 4 and 6 | ||||||
| Wake | F(1,7) = 0.3 | NS | F(23,161) = 12.7 | <0.05 | F(23,161) = 6.3 | <0.05 |
| NREMS | F(1,7) = 0.7 | NS | F(23,161) = 11.5 | <0.05 | F(23,161) = 6.0 | <0.05 |
| REMS | F(1,7) = 2.7 | NS | F(23,161) = 12.4 | <0.05 | F(23,161) = 4.3 | <0.05 |
| SWA | F(1,7) = 2.7 | NS | F(11,77) = 6.2 | <0.05 | F(11,77) = 5.2 | <0.05 |
| Body temperature | F(1,7) = 75.4 | <0.05 | F(23,161) = 16.8 | <0.05 | F(23,161) = 34.5 | <0.05 |
| Activity | F(1,11) = 13.1 | <0.05 | F(23,253) = 14.5 | <0.05 | F(23,253) = 3.1 | <0.05 |
| WT RF 8 & 12 | ||||||
| Wake | F(1,7) = 0.1 | NS | F(23,161) = 17.0 | <0.05 | F(23,161) = 13.5 | <0.05 |
| NREMS | F(1,7) = 0.2 | NS | F(23,161) = 15.4 | <0.05 | F(23,161) = 13.3 | <0.05 |
| REMS | F(1,7) = 0.7 | NS | F(23,161) = 13.4 | <0.05 | F(23,161) = 6.9 | <0.05 |
| SWA | F(1,7) = 3.5 | NS | F(11,77) = 4.4 | <0.05 | F(11,77) = 7.5 | <0.05 |
| Body temperature | F(1,7) = 40.1 | <0.05 | F(23,161) = 28.9 | <0.05 | F(23,161) = 58.1 | <0.05 |
| Activity | F(1,7) = 0.03 | NS | F(23,161) = 10.5 | <0.05 | F(23,161) = 5.2 | <0.05 |
| KO RF 8 & 12 | ||||||
| Wake | F(1,7) = 0.0 | NS | F(23,161) = 14.5 | <0.05 | F(23,161) = 11.0 | <0.05 |
| NREMS | F(1,7) = 0.1 | NS | F(23,161) = 14.1 | <0.05 | F(23,161) = 10.1 | <0.05 |
| REMS | F(1,7) = 3.7 | <0.05 | F(23,161) = 9.5 | <0.05 | F(23,161) = 8.6 | <0.05 |
| SWA | F(1,7) = 3.5 | <0.05 | F(11,77) = 5.2 | <0.05 | F(11,77) = 8.5 | <0.05 |
| Body temperature | F(1,7) = 40.3 | <0.05 | F(23,161) = 27.7 | <0.05 | F(23,161) = 71.3 | <0.05 |
| Activity | F(1,11) = 6.2 | <0.05 | F(23,253) = 10.9 | <0.05 | F(23,253) = 4.9 | <0.05 |
Individual tests compare baseline day and one of the three different time blocks of RF within the same genotype (RF 1–3, RF 4–6, and RF 8–12). SWA, slow wave activity; NS, not significant.
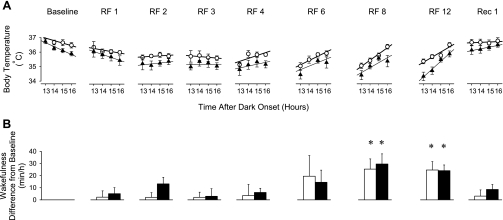
Fig. 2.
A: hourly body temperature values (±SE) with regression lines during the 4-h period before food access on RF days 1, 2, 3, 4, 6, 8, and 12 and during the same hours on the baseline and first recovery days. Open symbols: WT; filled symbols: KO. A significant positive correlation was apparent between time and temperature on RF days 8 and 12 in both genotypes, indicating food anticipation. On the recovery day, the correlation was absent. There was no significant difference between the parallelism (slopes) of the regression lines of WT and KO groups on any of the RF days, indicating that the anticipation was not statistically different in the two groups. B: amount of wakefulness in the 1-h period preceding food availability on the RF days or in the same hour on the recovery day. Values are expressed as change from baseline (±SE). *Significant change from baseline (P < 0.05, paired t-test).
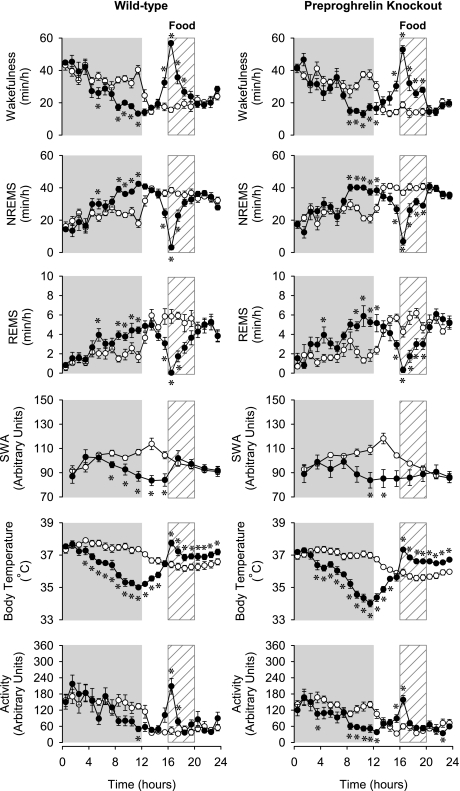
On RF days 4–6 (Fig. 3), changes in wakefulness showed a biphasic pattern during the night in control animals; it remained significantly elevated in the first half but was significantly reduced below baseline in the second half of the night. In KO animals, only the second response phase was present. During the feeding time, wakefulness increased in both genotypes. Changes in NREMS and REMS mirrored those in wakefulness.
Fig. 3.
The diurnal rhythm of wakefulness, NREMS, REMS, EEG SWA, body temperature, and locomotor activity of WT (left) and preproghrelin KO (right) mice on the baseline days (open symbols) and on the RF days 4–6. Data are averaged across RF days 4 and 6. *Significant difference between baseline days and treatment days (univariate tests of significance for planned comparisons, P < 0.05). See legend for Fig. 1 for details.
On RF days 8–12, changes in the sleep-wake pattern led to the partial reversal of the normal, nocturnal-type sleep-wake distribution. Nighttime wakefulness, mainly during the second half of the night, decreased below, and daytime wakefulness increased above, baseline in both KO and WT mice (Fig. 4 and Table 1). The daily, 24-h amounts of sleep and wakefulness remained at baseline levels. Both groups of mice exhibited FAA from RF day 8 as indicated by the significantly increased preprandial wakefulness (25.4 ± 8.5 min increase in WT, 29.6 ± 8.3 min increase in KO) 1 h before the scheduled feeding time, i.e., hour 16 (Fig. 2B). There was no significant difference in the anticipatory increases in wakefulness between control and KO animals. During the 4 h of the daily mealtime, wakefulness was significantly increased, and duration of NREMS and REMS was suppressed in both groups. Sleep-wake activity returned to baseline on recovery day 1 (data not shown), and the anticipatory increase in wakefulness was absent (Fig. 2B).
Episode number and average duration of episodes.
Under ad libitum feeding conditions, there was a tendency toward an increased number of wake and NREMS episodes and decreased average duration of these episodes in the KO animals compared with WT mice, although these differences were not significant (Figs. 5 and 6).
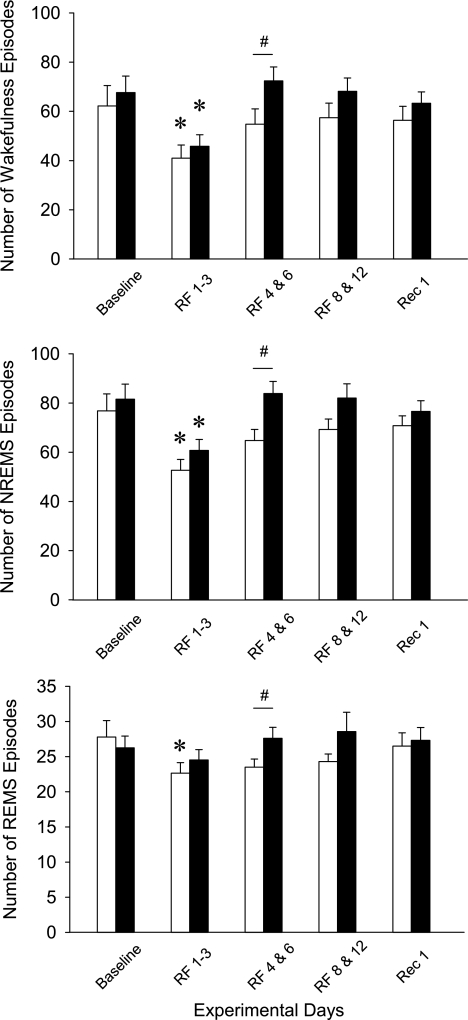
Fig. 5.
The average episode numbers of wakefulness, NREMS, and REMS episodes in WT (open bars) and preproghrelin KO (filled bars) mice during the experiment. Data are expressed as average durations during the 24-h periods. Bars represent the baseline day, the average of RF days 1–3, the average of RF days 4–6, the average of RF days 8–12, and recover day 1. Error bars, SE. *Significant difference between baseline days and treatment days for both genotypes (univariate tests of significance for planned comparison, P < 0.05). #Significant difference between WT and KO (univariate tests of significance for planned comparisons, P < 0.05).
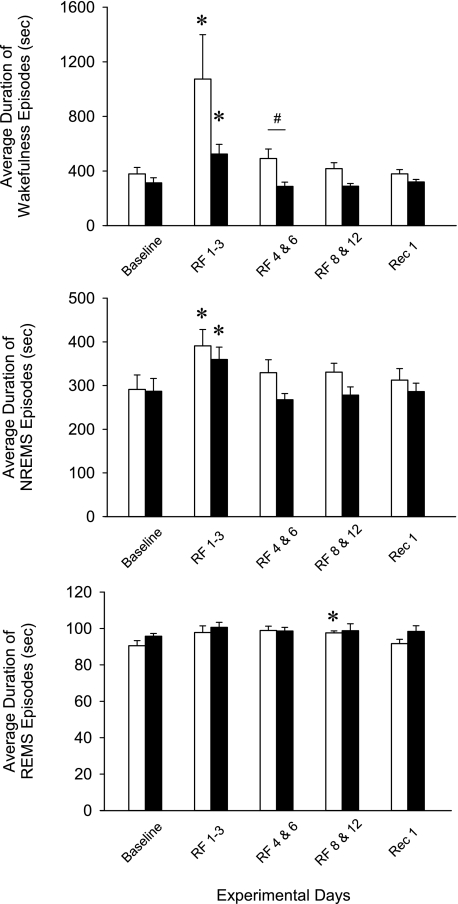
Fig. 6.
The average duration of wakefulness, NREMS, and REMS episodes in WT (open bars) and preproghrelin KO (filled bars) during the experiment. *Significant difference between baseline days and treatment days for both genotypes (univariate tests of significance for planned comparisons, P < 0.05). #Significant difference between WT and KO (univariate tests of significance for planned comparisons, P < 0.05). See legend for Fig. 5 for details.
During RF days 1–3, there was an increase in sleep consolidation as indicated by the reduced number and the increased average duration of wake and NREMS episodes in both groups. Duration of REMS episodes remained unaltered, and there was a slight but significant decrease in REMS episode number in WT mice. On RF days 4–6, the number and duration of the vigilance state episodes did not show any significant difference from baseline in either group of mice, but, when compared with control animals, KO mice had a significantly higher number of wake, NREMS, and REMS episodes. The average length of wake episodes was significantly shorter in KO animals. NREMS episode duration showed a similar trend, but the difference was not significant between the two genotypes.
On RF days 8–12, wake, NREMS, and REMS episode numbers and durations showed no significant difference from baseline in either group of mice. There was a general tendency toward increased episode numbers and decreased episode lengths in KO animals, but the differences were not significant. Similar tendencies were present on recovery day 1.
EEG SWA.
EEG delta power during NREMS showed a characteristic diurnal rhythm in both genotypes. SWA gradually increased during the dark, peaking at the beginning of the light, and then gradually decreased during the remaining daylight hours. RF significantly altered EEG SWA rhythms in both KO and control mice. During the dark phases of RF days 1–3, EEG SWA was significantly elevated above baseline in WT but not in KO animals (Fig. 1). During the following RF days, the nocturnal rise in SWA was attenuated in both genotypes, which led to the decreased diurnal amplitude of the EEG SWA rhythms (Fig. 3). On RF days 8–12, WT animals exhibited two peaks in EEG SWA, one at the beginning of the dark and one during the feeding period, in the middle of the light phase; the second peak was absent in preproghrelin KO mice (Fig. 4). On the recovery day, SWA returned to the baseline level (data not shown) (Figs. 1, 3, and 4).
Body temperature.
Baseline body temperature showed normal diurnal rhythms in both genotypes, with higher body temperatures during the dark period and lower during the daylight; there was no significant difference between the two genotypes. RF induced similar changes in body temperature in control and KO mice with three distinct daily response phases. The first phase was a hypothermic response during the dark period, and this was already apparent on the first RF day. The hypothermia became progressively deeper each day, leveling off at the end of dark on RF day 8, when body temperature was 2.4 ± 0.2°C and 3.0 ± 0.3°C below baseline in WT and KO mice, respectively (Figs. 1–4).
The hypothermia was followed by an increase in body temperature during the 4 h before feeding time, a characteristic sign of food anticipation. This response appeared on RF day 6 in both groups and became more pronounced during the following days (Fig. 2A). There was no significant difference in the slopes of the temperature increases between genotypes, indicating that this facet of FAA was similar in both strains.
A third response phase, hyperthermia during feeding and the following 4 h, was already present on RF days 1–3 and became progressively more pronounced over the course of the experiment. There was a consistent tendency toward accentuated hyperthermic responses in the KO animals compared with controls throughout the entire experiment; on RF day 12, the difference between WT and KO groups reached statistical significance (1.1 ± 0.1°C above baseline in KO vs. 0.8 ± 0.1°C in WT mice, P < 0.05, t-test). Body temperature returned to baseline on the recovery day (data not shown), and the anticipatory increases during the first 4 h of the light were absent (Fig. 2A).
Activity.
In general, changes in activity paralleled changes in wakefulness in both genotypes. In the 1st h of feeding, activity was increased on all days in both groups. Locomotor activity was significantly elevated in WT animals during the first 8 h of the night on RF day 1–3 and RF day 4–6. In KO mice, this response was absent. Starting from RF days 4–6, motor activity became suppressed during the latter part of the dark in both groups. On RF day 8–12, anticipatory increases in locomotor activity were evident in both groups in hour 16. On the recovery day, activity returned to baseline (data not shown) (Figs. 1, 3, and 4).
Body weight and food intake.
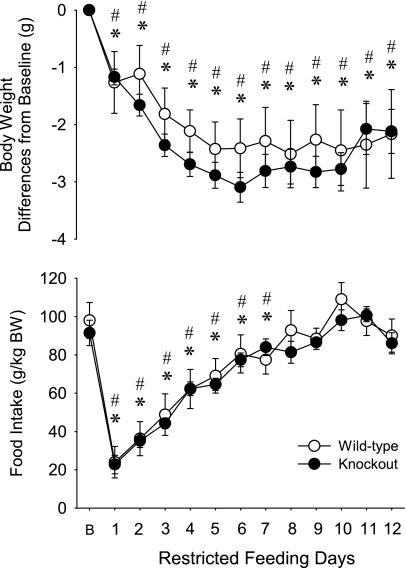
There was no significant difference in the baseline body weight (WT mice: 30.8 ± 1.3 g, KO mice: 32.2 ± 0.5 g) and food intake (Fig. 7 and Table 3) between the two genotypes. In response to RF, both groups of mice showed similar changes in body weight and food intake. Body weight in both genotypes gradually decreased from RF day 1–6 when it reached 92.2% of baseline in WT mice and 90.4% in KO mice. On RF day 1, mice ate ∼20–25% of their normal daily food intake; feeding increased during the following days, reaching ∼90% of baseline on RF day 8. By this day, both KO and WT mice adapted to the RF regimen and were able to consume their normal daily food intake during the 4-h access period.
Fig. 7.
Daily body weight and food intake of WT (open symbols) and KO (filled symbols) mice on the baseline day (B) and during the course of RF. Error bars, SE. *Significant difference between baseline and RF days for WT group. #Significant difference between baseline and RF days for KO (paired t-test, P < 0.05). There was no significant difference in body weight and food intake between the control and KO mice on any day.
Table 3.
Results of statistical analyses (2-way mixed ANOVA, repeated measure: day, independent measure: genotype) performed on daily body weight and food intake data
| Body Weight |
Food Intake |
|||
|---|---|---|---|---|
| F value | P | F value | P | |
| Genotype | F(1,18) = 1.72 | NS | F(1,18) = 0.2 | NS |
| Day | F(12,216) = 25.1 | <0.05 | F(12,216) = 60.4 | <0.05 |
| Genotype × day interaction | F(12,216) = 0.99 | NS | F(12,216) = 0.6 | NS |
DISCUSSION
The current findings indicate that RF alters the diurnal distribution of sleep and wakefulness, EEG SWA, locomotor activity, and body temperature in preproghrelin KO and WT mice. Changes during the first 3 days of RF were different between the two genotypes, but, by RF day 8, both groups of mice developed food anticipatory behavior. This suggests that intact ghrelin signaling is not required for the normal function of the FEO or for afferent signaling or efferent mechanisms arising from it, but ghrelin likely plays a role to arousal responses to fasting.
During the first 3 days of RF, mice of both genotypes were partially food deprived since they consumed <50% of their normal daily food intake. Short-term food deprivation induces wakefulness, and our results with WT mice confirm this finding. We hypothesized that increases in wakefulness and feeding at night in nocturnal rodents (“dark onset syndrome”) are the result of the activation of the hypothalamic ghrelin-orexin-NPY neuronal circuit by metabolic and circadian signals (37, 38). The finding that the arousal responses to partial food deprivation were significantly attenuated in preproghrelin KO mice is in line with this hypothesis. In a separate pilot study, we found a similar attenuated wakefulness response to 48 h of fasting in preproghrelin KO mice (n = 12, unpublished observation). It is unlikely that the drive for feeding is attenuated in the KO animals, since we found no significant difference in food intake of the two genotypes, which also confirms previous observations (32, 36). Similar to our findings in preproghrelin KO mice, orexin neuron-ablated mice show no appreciable increase in arousal during fasting (44).
During the first 3 days of RF, there was a net sleep loss in both genotypes that was significantly more robust in the WT animals. The sleep loss was accompanied by increased EEG SWA in WT mice. EEG SWA is, in part, a function of prior wakefulness and considered an indicator of NREMS intensity and sleep pressure (4). It is likely that increased EEG SWA in the second half of the dark period in WT mice is due to the prior sleep loss because sleep loss induces elevated EEG SWA during the rebound NREMS. In KO mice, the moderate sleep loss did not lead to increases in EEG SWA, although these mice have a normal capacity to enhance EEG SWA after sleep loss (39).
Long-term (4–11 days) food deprivation induces sleep fragmentation in rats, as indicated by the higher number and decreased duration of vigilance state episodes (3, 16). In the present experiment, however, during the first 3 days of RF, wakefulness and NREMS became more consolidated than under ad libitum feeding conditions in both groups of mice. Consistent with these observations, we previously found that normal mice respond to short-term food deprivation in a cold environment with more consolidated sleep architecture (40). It is possible that increased sleep consolidation is an adaptive, energy-saving mechanism when food availability is transiently decreased. Longer-term food deprivation is accompanied by more severe metabolic changes, stress responses, and more desperate food-seeking behavior, which together could lead to increased sleep fragmentation.
During the RF day 4–6, the daily amount of sleep and wakefulness gradually returned to baseline in both genotypes, but profound changes in the diurnal distribution of sleep/wakefulness remained in both groups. When food availability is restricted to the 12-h light period, the diurnal distribution of REMS is reversed, and the amount of NREMS becomes equally distributed between the light and dark phases in rats (29, 31). We found that 4 h of RF during the light period also alters the normal diurnal distribution of sleep and wakefulness, and the amount of wakefulness, NREMS, and REMS became equally (∼50%) distributed between the dark and light phases. This is consistent with a previous finding in which 4 h RF increased daytime wakefulness from 31 to 52.1% in rats (13). Another study reported a more robust, complete reversal of sleep-wake rhythms in normal and orexin\ataxin mice kept under 4 h RF conditions (21). Importantly, the daily amounts of sleep and wakefulness in preproghrelin KO and WT mice did not change during the course of RF, confirming findings of previous studies (13, 21, 31).
Twelve hours of fasting during the light phase do not alter the diurnal distribution of EEG SWA during NREMS in rats (31). We found that, in response to 4 h of RF, WT animals exhibited two peaks in EEG SWA. In contrast, preproghrelin KO mice had only a single peak in EEG SWA despite the comparable sleep loss between KO and WT during the light period. This may indicate a subtle deficiency in homeostatic sleep regulation in the KO animals. Stimulation of homeostatic sleep mechanisms by a more robust challenge, 6 h total sleep deprivation, however, induces a similar increase in EEG SWA in both genotypes (39).
The relationship between body temperature, food intake, and metabolic rate is well characterized. Our finding that hypothermia develops during the dark under RF conditions is consistent with the general notion that body temperature and metabolic rate decline during fasting periods and also confirms previous findings showing premeal reduction in body temperature during RF. It is possible that increased NREMS during the hypothermic period contributes to energy conservation. When subjected to a more severe metabolic challenge, food deprivation in a cold environment, WT mice show similar NREMS increases while preproghrelin KO animals remain awake and enter a precipitous hypothermia, indicating a sleep and thermoregulatory impairment. In the present study, the metabolic challenge was much milder, intermittent 20-h fasting at thermoneutral ambient temperature; under these conditions, KO mice enhanced their sleep although their hypothermic responses were augmented.
The rising body temperature during the premeal hours and elevated body temperature during the feeding period and the following 4 h are, at least in part, because of increased behavioral activity. In the last 4 h of the light period, wakefulness and motor activity returned to baseline levels, but temperature remained elevated, likely because of diet-induced/postprandial thermogenesis. This temperature response was more robust in preproghrelin KO mice, which cannot be explained by higher energy intake, since we found no difference in food consumption and body weight between KO and WT animals. Ghrelin decreases energy expenditure and metabolic heat production (42, 45). In humans, high ghrelin levels are associated with suppressed postprandial thermogenesis (35). The lack of these influences may explain the increased diet-induced thermogenesis in the KO animals.
Ghrelin has orexigenic effects in several species, including mice. Despite the profound effects of ghrelin on feeding, preproghrelin and ghrelin receptor KO mice have normal food intake and body weight (36). We found that both preproghrelin KO and WT mice are able to adapt to scheduled feeding; both genotypes increased food intake over the course of the experiment and reached 90% of their normal daily food intake by RF day 6. This indicates that the homeostatic drive to feed is intact in preproghrelin KO mice. These findings confirm a previous observation (32), and they are also similar to those that were reported in orexin KO (17) and orexin/ataxin KO (21) mice on the RF paradigm.
Targeted gene disruption is widely used to study biological functions of genes; negative findings, however, have to be viewed with caution. When the immediate consequences of gene disruption are studied (e.g., transcription of its message), the lack of a functional gene is usually apparent. Complex biological phenomena, such as feeding behavior or vigilance, are far removed from the function of a single gene. The consequence of single gene disruption on such phenomena is often ambiguous because of developmental compensation or the activation of redundant mechanisms. Negative findings simply suggest that either the gene product has no significant role in the given function or, if it has, its lack can be compensated for.
Perspective and Significance
Our results show that intact ghrelin signaling is not required for the normal function of the FEO or for its input or output mechanisms. Ghrelinergic mechanisms, however, play a role in food deprivation-induced arousal. This supports the hypothesis that ghrelinergic neurons are part of those hypothalamic circuits that are responsible for triggering behavioral activation at the beginning of the active phase of the day. In rats and mice, the first part of the dark period is characterized by increased arousal and enhanced feeding, a set of concerted responses named dark-onset syndrome. In humans, similar responses occur at the beginning of the light period. Night-eating syndrome is possibly related to the inadequate timing of the hypothalamic ghrelinergic activating mechanisms.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS-27250.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank Kenneth Carias, Jacob Pellinen, and Sean Woodward for help analyzing the data.
REFERENCES
- 1.Akiyama M, Yuasa T, Hayasaka N, Horikawa K, Sakurai T, Shibata S. Reduced food anticipatory activity in genetically orexin (hypocretin) neuron-ablated mice. Eur J Neurosci 20: 3054–3062, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Bodosi B, Gardi J, Hajdu I, Szentirmai E, Obal F, Jr, Krueger JM. Rhythms of ghrelin, leptin, and sleep in rats: effects of the normal diurnal cycle, restricted feeding, and sleep deprivation. Am J Physiol Regul Integr Comp Physiol 287: R1071–R1079, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Borbély AA. Sleep in the rat during food deprivation and subsequent restitution of food. Brain Res 124: 457–471, 1977 [DOI] [PubMed] [Google Scholar]
- 4.Borbély AA. A two process model of sleep regulation. Hum Neurobiol 1: 195–204, 1982 [PubMed] [Google Scholar]
- 5.Cowley MA, Smith RG, Diano S, Tschöp M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD, Horvath TL. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 37: 649–661, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 50: 1714–1719, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Danguir J. Cafeteria diet promotes sleep in rats. Appetite 8: 49–53, 1987 [DOI] [PubMed] [Google Scholar]
- 8.Danguir J, Nicolaidis S. Dependence of sleep on nutrients' availability. Physiol Behav 22: 735–740, 1979 [DOI] [PubMed] [Google Scholar]
- 9.Danguir J, Nicolaidis S. Intravenous infusions of nutrients and sleep in the rat: an ischymetric sleep regulation hypothesis. Am J Physiol Endocrinol Metab 238: E307–E312, 1980 [DOI] [PubMed] [Google Scholar]
- 10.Dewasmes G, Duchamp C, Minaire Y. Sleep changes in fasting rats. Physiol Behav 46: 179–184, 1989 [DOI] [PubMed] [Google Scholar]
- 11.Drazen DL, Vahl TP, D'Alessio DA, Seeley RJ, Woods SC. Effects of a fixed meal pattern on ghrelin secretion: evidence for a learned response independent of nutrient status. Endocrinology 147: 23–30, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Fuller PM, Lu J, Saper CB. Differential rescue of light- and food-entrainable circadian rhythms. Science 320: 1074–1077, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gooley JJ, Schomer A, Saper CB. The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat Neurosci 9: 398–407, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ, Smith RG, Van der Ploeg LH, Howard AD. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res 48: 23–29, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Hansen MK, Kapás L, Fang J, Krueger JM. Cafeteria diet-induced sleep is blocked by subdiaphragmatic vagotomy in rats. Am J Physiol Regul Integr Comp Physiol 274: R168–R174, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Jacobs BL, McGinthy DJ. Effects of food deprivation on sleep and wakefulness in the rat. Exp Neurol 30: 212–222, 1971 [DOI] [PubMed] [Google Scholar]
- 17.Kaur S, Thankachan S, Begum S, Blanco-Centurion C, Sakurai T, Yanagisawa M, Shiromani PJ. Entrainment of temperature and activity rhythms to restricted feeding in orexin knock out mice. Brain Res 1205: 47–54, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landry GJ, Simon MM, Webb IC, Mistlberger RE. Persistence of a behavioral food-anticipatory circadian rhythm following dorsomedial hypothalamic ablation in rats. Am J Physiol Regul Integr Comp Physiol 290: R1527–R1534, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Landry GJ, Yamakawa GR, Mistlberger RE. Robust food anticipatory circadian rhythms in rats with complete ablation of the thalamic paraventricular nucleus. Brain Res 1141: 108–118, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Landry GJ, Yamakawa GR, Webb IC, Mear RJ, Mistlberger RE. The dorsomedial hypothalamic nucleus is not necessary for the expression of circadian food-anticipatory activity in rats. J Biol Rhythms 22: 467–478, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Mieda M, Williams SC, Sinton CM, Richardson JA, Sakurai T, Yanagisawa M. Orexin neurons function in an efferent pathway of a food-entrainable circadian oscillator in eliciting food-anticipatory activity and wakefulness. J Neurosci 24: 10493–10501, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mistlberger RE, Rechtschaffen A. Recovery of anticipatory activity to restricted feeding in rats with ventromedial hypothalamic lesions. Physiol Behav 33: 227–235, 1984 [DOI] [PubMed] [Google Scholar]
- 23.Mistlberger RE, Mumby DG. The limbic system and food-anticipatory circadian rhythms in the rat: ablation and dopamine blocking studies. Behav Brain Res 47: 159–168, 1992 [DOI] [PubMed] [Google Scholar]
- 24.Mistlberger RE, Antle MC. Neonatal monosodium glutamate alters circadian organization of feeding, food anticipatory activity and photic masking in the rat. Brain Res 842: 73–83, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Mistlberger RE, Antle MC, Kilduff TS, Jones M. Food- and light-entrained circadian rhythms in rats with hypocretin-2-saporin ablations of the lateral hypothalamus. Brain Res 980: 161–168, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Mistlberger RE, Buijs RM, Challet E, Escobar C, Landry GJ, Kalsbeek A, Pevet P, Shibata S. Standards of evidence in chronobiology: critical review of a report that restoration of Bmal1 expression in the dorsomedial hypothalamus is sufficient to restore circadian food anticipatory rhythms in Bmal1−/− mice. J Circadian Rhythms 26: 73, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitchell V, Bouret S, Beauvillain JC, Schilling A, Perret M, Kordon C, Epelbaum J. Comparative distribution of mRNA encoding the growth hormone secretagogue-receptor (GHS-R) in Microcebus murinus (Primate, lemurian) and rat forebrain and pituitary. J Comp Neurol 429: 469–489, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Moreira AC, Krieger DT. The effects of subdiaphragmatic vagotomy on circadian corticosterone rhythmicity in rats with continuous or restricted food access. Physiol Behav 28: 787–790, 1982 [DOI] [PubMed] [Google Scholar]
- 29.Mouret JR, Bobillier P. Diurnal rhythms of sleep in the rat: augmentation of paradoxical sleep following alterations of the feeding schedule. Int J Neurosci 2: 265–269, 1971 [DOI] [PubMed] [Google Scholar]
- 30.Rechtschaffen A, Bergmann BM, Everson CA, Kushida CA, Gilliland MA. Sleep deprivation in the rat. X. Integration and discussion of the findings. Sleep 12: 68–87, 1989 [PubMed] [Google Scholar]
- 31.Roky R, Kapás L, Taishi TP, Fang J, Krueger JM. Food restriction alters the diurnal distribution of sleep in rats. Physiol Behav 67: 697–703, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Sato T, Kurokawa M, Nakashima Y, Ida T, Takahashi T, Fukue Y, Ikawa M, Okabe M, Kangawa K, Kojima M. Ghrelin deficiency does not influence feeding performance. Regul Pept 145: 7–11, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Shemyakin A, Kapás L. L-364,718, a cholecystokinin-A receptor antagonist, suppresses feeding-induced sleep in rats. Am J Physiol Regul Integr Comp Physiol 280: R1420–R1426, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 141: 846–850, 2004 [DOI] [PubMed] [Google Scholar]
- 35.St-Pierre DH, Karelis AD, Cianflone K, Conus F, Mignault D, Rabasa-Lhoret R, St-Onge M, Tremblay-Lebeau A, Poehlman ET. Relationship between ghrelin and energy expenditure in healthy young women. J Clin Endocrinol Metab 89: 5993–5997, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Sun Y, Ahmed S, Smith RG. Deletion of ghrelin impairs neither growth nor appetite. Mol Cell Biol 23: 7973–7981, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szentirmai E, Hajdu I, Obal F, Jr, Krueger JM. Ghrelin-induced sleep responses in ad libitum fed and food-restricted rats. Brain Res 1088: 131–140, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Szentirmai É, Kapás L, Krueger JM. Ghrelin microinjection into forebrain sites induces wakefulness and feeding in rats. Am J Physiol Regul Integr Comp Physiol 292: R575–R585, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Szentirmai E, Kapás L, Sun Y, Smith RG, Krueger JM. Spontaneous sleep and homeostatic sleep regulation in ghrelin knockout mice. Am J Physiol Regul Integr Comp Physiol 293: R510–R517, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Szentirmai É, Kapás L, Sun Y, Smith RG, Krueger JM. The preproghrelin gene is required for the normal integration of thermoregulation and sleep in mice. Proc Natl Acad Sci USA 106: 14069–14074, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med 1: e62, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Theander-Carrillo C, Wiedmer P, Cettour-Rose P, Nogueiras R, Perez-Tilve D, Pfluger P, Castaneda TR, Muzzin P, Schurmann A, Szanto I, Tschöp MH, Rohner-Jeanrenaud F. Ghrelin action in the brain controls adipocyte metabolism. J Clin Invest 116: 1983–1993, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tschöp M, Wawarta R, Riepl RL, Friedrich S, Bidlingmaier M, Landgraf R, Folwaczny C. Post-prandial decrease of circulating human ghrelin levels. J Endocrinol Invest 24: RC19–RC21, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, Tominaga M, Yagami K, Sugiyama F, Goto K, Yanagisawa M, Sakurai T. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron 38: 701–713, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Yasuda T, Masaki T, Kakuma T, Yoshimatsu H. Centrally administered ghrelin suppresses sympathetic nerve activity in brown adipose tissue of rats. Neurosci Lett 349: 75–78, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Zhou D, Shen Z, Strack AM, Marsh DJ, Shearman LP. Enhanced running wheel activity of both Mch1r- and Pmch-deficient mice. Regul Pept 124: 53–63, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol 494: 528–548, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]