Abstract
The control of vascular tone during exercise is highly complex and integrated. Specifically, in regards to the contribution of nitric oxide (NO), the observed magnitude and muscle fiber-type dependency of the NO contribution to exercise hyperemia may differ depending on the timing of NO synthase (NOS) inhibition with respect to the exercise bout (i.e., administration prior to vs. during exercise). We tested the hypothesis that, in the presence of prior cyclooxygenase inhibition (indomethacin, 5 mg/kg-1), NOS inhibition (NG-nitro-l-arginine methyl ester, l-NAME; 10 mg/kg) administered during submaximal treadmill exercise would blunt blood flow and vascular conductance (VC) in the hindlimb muscle(s) of the rat with the greatest reductions in blood flow and VC occurring in the predominantly oxidative muscles. Adult female Wistar rats (n = 10, age: 3–4 mo) ran on a motor-driven treadmill (20 m/min, 10% grade). Total and regional hindlimb muscle blood flow and VC were determined via radiolabeled microspheres before (control) and after l-NAME administration during exercise. l-NAME reduced (P < 0.05) total hindlimb muscle blood flow (control: 123 ± 10, l-NAME: 103 ± 7 ml·min−1·100g−1) and VC (control: 0.95 ± 0.09, l-NAME: 0.63 ± 0.05 ml·min−1·100g−1·mmHg−1). There was a significant correlation (r = 0.51, P < 0.05) between the absolute reductions in VC after l-NAME and the percent sum of type I and IIa fibers in the individual muscles and muscle parts; however, there was no correlation (P = 0.62) when expressed as blood flow. Surprisingly, the highly oxidative muscles demonstrated a marked ability to maintain oxygen delivery, which differs substantially from previous reports of l-NAME infusion prior to exercise in these muscles. The demonstration that NO is an important regulator of blood flow and VC in the rat hindlimb during treadmill exercise, but that the fiber-type dependency of NO is altered markedly when NOS inhibition is performed during, vs. prior to, exercise, lends important insights into the integrated nature of vascular control during exercise.
Keywords: vasodilation, endothelium-derived relaxing factor, smooth muscle
the regulation of vascular smooth muscle tone during dynamic exercise is characterized by complex integration and redundancy, thus facilitating a tight coupling between metabolic rate and oxygen (O2) delivery. An extensive list of candidate vasoactive substances has been identified and investigated and among these, substantial interest has been focused on the potential contribution of nitric oxide (NO). Despite intensive research efforts, the role(s) of NO in skeletal muscle resistance vessel dilation during exercise has not been elucidated clearly (for excellent reviews, see Refs. 5, 16, 25, 29).
A common experimental method for examining the contribution of NO to active skeletal muscle hyperemia is to inhibit NO synthase (NOS) at rest and report measures of blood flow and/or vascular conductance (VC) during subsequent exercise. Using this method, researchers have found evidence for at least a moderate contribution of NO (∼10–35% reductions in blood flow and/or VC) (6, 12, 14, 22). However, the literature is equivocal on this issue, as studies have also demonstrated no effects of NOS inhibition (8, 9, 11, 24, 26, 30). Specifically, our laboratory has demonstrated that infusion of the NOS inhibitor NG-nitro-l-arginine methyl ester (l-NAME) prior to exercise onset in the presence of prior cyclooxygenase (COX) inhibition significantly reduced blood flow (∼25% reduction) (14) and VC (∼33% reduction) (22) compared with COX inhibition alone in rat hindlimb muscles in a fiber-type dependent manner (i.e., primarily in the highly oxidative muscles and muscle parts).
One putative confound to data interpretation when NOS blockade is performed prior to exercise onset is that reductions in blood flow and/or VC measured during subsequent exercise may simply parallel reductions in resting flow (9). It is possible that the observed magnitude or fiber-type dependency of the NO contribution to the exercise hyperemic response differs substantially depending on the timing of the NOS inhibition. Experimental protocols that administer NOS inhibition during contractions may more clearly characterize the NO contribution to exercise hyperemia per se. In this regard, Hussain et al. (15) reported that NOS inhibition administered during electrically induced contractions of the canine diaphragm partially reversed the contraction-induced vasodilation. In humans, NOS blockade administered independently (without COX inhibition) significantly attenuated forearm muscle blood flow and VC during handgrip exercise (7, 28) but not during isolated knee extensor exercise (26). Conversely, combined COX and NOS inhibition administered during knee extensor exercise reduced femoral artery blood flow (19), which suggests that substantial redundancy exists between the NOS and COX systems.
Despite these conclusions, a protocol administering NOS inhibition during exercise has yet to examine specifically whether NO contributes to the exercise hyperemia in the locomotor muscles during conscious whole-body dynamic exercise. Moreover, studies of bulk limb or isolated muscle flow cannot address whether the muscle fiber-type dependency of NO observed when NOS inhibition is administered prior to exercise exists when NOS inhibition is performed during exercise. Therefore, the purpose of the current study was to build on previous work and determine the effects of NOS blockade administered during conscious submaximal treadmill exercise on total and regional (intermuscular and intramuscular) skeletal muscle blood flow and VC in the rat hindlimb. We tested the hypotheses that l-NAME, infused during submaximal treadmill exercise in the presence of prior COX inhibition via indomethacin, would reduce total hindlimb muscle blood flow and VC compared with COX inhibition alone and that the reductions in blood flow in the individual muscles or muscle parts would be fiber-type dependent such that the highly oxidative muscles and muscle parts would exhibit the greatest reductions in flow and VC.
METHODS
Animal selection and care.
A total of 17 adult female Wistar rats (age: 3–4 mo, body weight: 257 ± 5 g) were used in this study. Rats were housed two per cage and maintained on a 12:12-h light-dark cycle with food and water available ad libitum. All rats were familiarized with running on a motor-driven treadmill over a ∼2-wk period, in which rats exercised for ∼5 min/day at a speed of 20 m/min up a 10% grade. All experiments were conducted under the guidelines established by the National Institutes of Health and approved by Kansas State University's Institutional Animal Care and Use Committee.
Surgical procedures and experimental protocol.
After it was established that all rats were proficient runners (i.e., able to run continuously for a minimum of 5 min), the animals were randomly assigned to either an experimental (n = 10) or time control (n = 7) group. All animals were initially anesthetized with 5% halothane gas. While being maintained on a 2% halothane-oxygen mixture, one catheter (PE-10 connected to PE-50; Clay Adams Brand, Sparks, MD) was placed in the ascending aorta via the right carotid artery and another was placed in the caudal (tail) artery, as described previously (23). Both catheters were tunneled subcutaneously to the dorsal aspect of the cervical region and exteriorized through a puncture wound in the skin. After closure of the incisions, anesthesia was terminated and the animal was given ≥ 2 h to recover.
Subsequent to the recovery period, the final protocol was initiated. A dose of 5 mg/kg body wt of indomethacin (Sigma Chemical, St. Louis, MO), a COX inhibitor, was administered to each rat via the tail artery catheter. Indomethacin was administered to limit the redundancy within the vasodilatory system, which may mask the physiological NO contribution (see discussion for details) and to facilitate the integration of the current results with our previous report of NOS inhibition prior to exercise (14). Each rat was then placed on the treadmill and, after a period of stabilization (∼2 h after instrumentation), 10 μg/kg of ACh (Sigma Chemical) was administered via the carotid artery catheter. The subsequent peak hypotensive response to ACh injection was measured and recorded from the tail artery catheter to determine the efficacy of endothelium-dependent vasodilation.
After ACh injection, the tail artery catheter was connected to a 1 ml plastic syringe, which connected to a Harvard infusion/withdrawal pump (model 907, Cambridge, MA). Exercise was initiated, and the speed of the treadmill was increased progressively during the next 30 s to a speed of 20 m/min (10% grade). This speed and grade have been demonstrated previously to elicit ∼55–65% of maximum oxygen uptake (V̇o2 max) (21) and is identical to the exercise protocol employed when l-NAME was administered prior to moderate-intensity exercise (14). The rat was then required to exercise steadily for another 5 min. After ∼5.5 min of total exercise time, blood withdrawal from the tail artery catheter was initiated at a rate of 0.25 ml/min. Simultaneously, heart rate (HR) and mean arterial blood pressure (MAP) were measured and recorded via the carotid artery catheter. The carotid artery catheter was then disconnected from the pressure transducer and 0.5–0.6 × 106 15-μm-diameter microspheres (46Sc or 85Sr in random order: Perkin Elmer Life and Analytical Sciences, Waltham, MA) were injected into the aortic arch of the exercising animal to determine hindlimb blood flow. Approximately 15–30 s after the injection (∼6 min of total exercise time), exercise was terminated, and each rat was allowed a minimum of 60 min to recover.
After recovery from the initial exercise bout, a second bout of exercise was initiated, and the speed of the treadmill was increased progressively during the next 30 s to a speed of 20 m/min (10% grade). The rat was then required to exercise steadily for another ∼2.5 min. Prior to the 3-min mark, 10 mg/kg of the l-arginine analog NG-nitro-l-arginine-methyl-ester (l-NAME; Sigma Chemical) was then infused into the tail artery catheter over a 10-s period, while HR and MAP were monitored continuously from the carotid artery catheter. After ∼5.5 min of total exercise time, a second microsphere injection (differently labeled from the first injection) was performed as described above for the first bout of exercise. Exercise was then terminated and each rat was given ∼5–10 min to recover. After this recovery period, a second ACh injection was performed, and the hypotensive response was measured. This response was then compared with that obtained before the control exercise bout to determine the degree of NOS inhibition attained by l-NAME.
Following the completion of the second ACh injection, each animal was euthanized via an overdose of pentobarbital (>50 mg/kg body wt) via the carotid artery catheter. The thorax was opened, and placement of the carotid artery catheter into the aortic arch was confirmed by anatomical dissection. The right and left kidneys and individual muscles and muscle parts of both hindlimbs, including the soleus; plantaris; red, white and mixed portions of the gastrocnemius; tibialis posterior; flexor digitorum longus; flexor halicus longus; red and white portions of the tibialis anterior; extensor digitorum longus; peroneals; vastus intermedius; vastus medialis; red, white, and mixed portions of the vastus lateralis; red and white portions of the rectus femoris; anterior and posterior biceps femoris; semitendinosus; red and white semimembranosus; adductor longus; adductor magnus and brevis; gracilis; and the pectineus were identified and removed. The tissues were blotted, weighed, and placed immediately into counting vials. The estimation of fiber type composition of each muscle or muscle part of the rat hindlimb as described by Delp and Duan (4) was used to examine the fiber-type dependency of the NO contribution.
The radioactivity of each tissue was determined on a gamma scintillation counter (Packard Auto Gamma Spectrometer, model 5230; Packard, Downers Grove, IL). Taking into account cross-talk between isotopes, blood flows to each tissue were determined using the reference sample method (23) and expressed as milliliters per minute per 100 g of tissue (ml·min−1·100 g−1). Adequate mixing of the microspheres was evaluated and verified in all 17 animals for each injection by the demonstration of a <15% difference between blood flow to the right and left kidneys (mean difference exercise bout 1: 9.5 ± 2.2%, exercise bout 2: 8.7 ± 2.2%) and/or to the right and left hindquarter musculature (mean difference exercise bout 1: 7.9 ± 1.8%, exercise bout 2: 8.8 ± 1.3%). Blood flow results were also normalized to MAP and expressed as VC (ml·min−1·100 g−1·mmHg−1).
Time control experiments.
Time control studies (n = 7) were performed to determine the degree of reproducibility between the hindlimb blood flow and VC measured during the two bouts of exercise described in the present investigation. Control rats were required to perform the experimental protocol described above with the exception that l-NAME administration was excluded during the second bout of exercise.
Statistical analyses.
HR, MAP, muscle blood flow, and tissue VC measured during the first and second bouts of exercise were compared using two-tailed paired Student's t-tests. A one-way repeated-measures ANOVA was used to compare minute MAP values within exercise bouts to determine magnitude and time course of MAP increase after l-NAME administration during exercise. A z-test was used to determine whether the relative changes in blood flow between exercise bouts 1 and 2 for time control and experimental rats were different from zero. Pearson's product-moment correlations were performed to determine whether the absolute and relative reductions in blood flow (Δ blood flow) and VC (Δ VC) in the individual muscles or muscle parts produced by l-NAME administration during exercise were correlated with their estimated fiber-type composition. Values are presented as means ± SE. Significance was accepted at the P < 0.05 level.
RESULTS
Effects of l-NAME infusion during exercise on blood flow and VC.
Total hindquarter muscle blood flow (control: 123 ± 10, l-NAME: 103 ± 7 ml·min−1·100 g−1) and VC (control: 0.95 ± 0.09, l-NAME: 0.63 ± 0.05 ml·min−1·100 g−1·mmHg−1) were reduced (P < 0.05 for both) 16% and 34%, respectively, when l-NAME was administered in the exercising rat (Fig. 1). Blood flow was attenuated significantly (P < 0.05) in 11 of the 28 muscles or muscle parts in the rat hindlimb with the administration of l-NAME during exercise (Table 1). When the data were expressed as VC, significant reductions were observed after l-NAME in 20 of the 28 different muscles or muscle parts (Table 1).
Fig. 1.
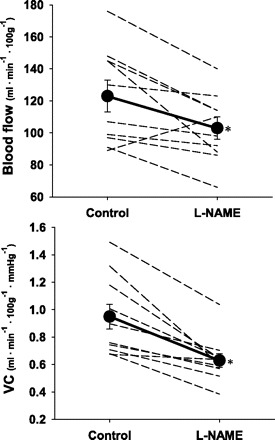
Individual responses and means (•) showing the effects of l-NAME administration during exercise on total hindlimb muscle blood flow and vascular conductance (VC). *P < 0.05 vs. control.
Table 1.
Effects of l-NAME administration during exercise on blood flow and vascular conductance in the individual muscles or muscle parts of the rat hindlimb
| Blood Flow |
Vascular Conductance |
|||
|---|---|---|---|---|
| Control | l-NAME | Control | l-NAME | |
| Ankle extensors | ||||
| Soleus | 281 ± 36 | 288 ± 32 | 2.19 ± 0.35 | 1.78 ± 0.23 |
| Plantaris | 146 ± 21 | 143 ± 18 | 1.13 ± 0.18 | 0.88 ± 0.12 |
| Gastrocnemius, red | 358 ± 41 | 376 ± 42 | 2.79 ± 0.40 | 2.32 ± 0.30 |
| Gastrocnemius, white | 34 ± 6 | 34 ± 6 | 0.26 ± 0.05 | 0.21 ± 0.04 |
| Gastrocnemius, mixed | 147 ± 14 | 142 ± 16 | 1.14 ± 0.13 | 0.88 ± 0.11* |
| Tibialis posterior | 108 ± 17 | 94 ± 33 | 0.82 ± 0.12 | 0.56 ± 0.19 |
| Flexor digitorum longus | 86 ± 22 | 100 ± 15 | 0.65 ± 0.15 | 0.61 ± 0.09 |
| Flexor halicus longus | 66 ± 11 | 63 ± 2 | 0.50 ± 0.07 | 0.39 ± 0.04 |
| Ankle flexors | ||||
| Tibialis anterior, red | 318 ± 45 | 216 ± 25* | 2.49 ± 0.40 | 1.34 ± 0.19* |
| Tibialis anterior, white | 111 ± 13 | 78 ± 7* | 0.86 ± 0.11 | 0.48 ± 0.05* |
| Extensor digitorum longus | 71 ± 13 | 61 ± 8 | 0.55 ± 0.11 | 0.37 ± 0.04 |
| Peroneals | 155 ± 18 | 105 ± 13* | 1.20 ± 0.16 | 0.65 ± 0.09* |
| Knee extensors | ||||
| Vastus intermedius | 388 ± 30 | 392 ± 28 | 2.97 ± 0.26 | 2.39 ± 0.19* |
| Vastus medialis | 192 ± 21 | 164 ± 16 | 1.49 ± 0.19 | 0.99 ± 0.09* |
| Vastus lateralis, red | 392 ± 30 | 362 ± 27 | 3.02 ± 0.28 | 2.22 ± 0.21* |
| Vastus lateralis, white | 38 ± 4 | 32 ± 4 | 0.29 ± 0.04 | 0.19 ± 0.03* |
| Vastus lateralis, mixed | 167 ± 18 | 130 ± 12* | 1.29 ± 0.17 | 0.79 ± 0.08* |
| Rectus femoris, red | 208 ± 16 | 178 ± 13 | 1.61 ± 0.16 | 1.09 ± 0.09* |
| Rectus femoris, white | 89 ± 7 | 76 ± 7 | 0.69 ± 0.07 | 0.46 ± 0.04* |
| Knee flexors | ||||
| Biceps femoris, anterior | 56 ± 6 | 45 ± 5* | 0.43 ± 0.05 | 0.28 ± 0.03* |
| Biceps femoris, posterior | 84 ± 8 | 59 ± 6* | 0.65 ± 0.08 | 0.36 ± 0.04* |
| Semitendinosus | 54 ± 7 | 38 ± 4* | 0.41 ± 0.06 | 0.23 ± 0.03* |
| Semimembranosus, red | 152 ± 14 | 117 ± 7* | 1.17 ± 0.13 | 0.72 ± 0.06* |
| Semimembranosus, white | 43 ± 5 | 35 ± 4 | 0.33 ± 0.04 | 0.22 ± 0.03* |
| Thigh adductors | ||||
| Adductor longus | 348 ± 43 | 335 ± 34 | 2.69 ± 0.39 | 2.08 ± 0.28* |
| Adductor magnus & brevis | 123 ± 11 | 91 ± 7* | 0.95 ± 0.10 | 0.56 ± 0.06* |
| Gracilis | 57 ± 7 | 39 ± 4* | 0.44 ± 0.06 | 0.24 ± 0.03* |
| Pectineus | 60 ± 10 | 32 ± 6* | 0.46 ± 0.08 | 0.19 ± 0.03* |
Data are expressed as means ± SE
P < 0.05 vs. control. Values for blood flow are given in ml·min−1·100 g−1. Vascular conductance values are given in ml·min−1·100 g−1·mmHg−1.
Correlations with muscle fiber-type composition.
There was no correlation between the absolute Δ blood flow in the individual muscles or muscle parts after l-NAME and percent sum of type I and IIa fibers (Fig. 2). Indeed, there was a significant negative correlation (r = 0.40, P < 0.05) between the relative Δ blood flow and the percent sum of type I and IIa fibers, such that little or no effect of l-NAME was observed in the oxidative muscle regions (Fig. 2). The absolute Δ VC correlated significantly (r = 0.51, P < 0.01) with the percent sum of type I and IIa fibers found in the individual muscles or muscle parts of the rat hindlimb, however, when expressed in relative terms, the direction of the correlation with type I and IIa fibers (r = 0.40, P < 0.05) was reversed (Fig. 3).
Fig. 2.

Relationships between the percent sum of type I and IIa fibers in the individual muscles or muscle parts (n = 28) of the rat hindlimb and the absolute (top) and relative (bottom) reductions in blood flow (Δ blood flow) in the present investigation after l-NAME administration during exercise.
Fig. 3.
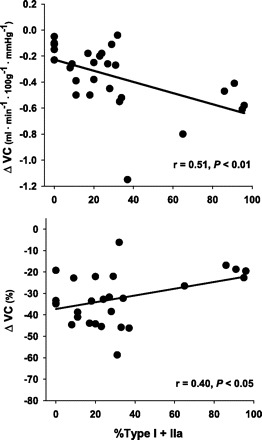
Relationships between the percent sum of type I and IIa fibers in the individual muscles or muscle parts (n = 28) of the rat hindlimb and the absolute (top) and relative (bottom) reductions in vascular conductance (Δ VC) in the present investigation after l-NAME administration during exercise.
Effects of l-NAME on HR, MAP, and hypotensive responses to ACh injections.
Preexercise HR and MAP were similar between the first and second bouts of exercise (Table 2). HR increased from resting values during the two exercise regimens and to a similar degree, however, the intra-arterial administration of l-NAME during the second bout of exercise, while the animal was running on the treadmill produced a significant (P < 0.05) increase in MAP and decrease in HR (Fig. 4, Table 2). Importantly, the increase in MAP was evident within <10 s of l-NAME infusion, and the entirety of the increase was complete within ∼2.5 min (i.e., by the time the microsphere injection was performed, Fig. 4). The hypotensive response (absolute and relative change in MAP) produced by the injection of ACh was blunted significantly following the administration of l-NAME (Fig. 5), demonstrating that significant NOS inhibition had been achieved in the exercising rats.
Table 2.
Hemodynamic variables measured in experimental rats during exercise bouts 1 and 2 at rest and during exercise before and after l-NAME administration during the second exercise bout
| HR, bpm | MAP, mmHg | |
|---|---|---|
| Exercise Bout 1 (Control) | ||
| Rest | 470 ± 9 | 129 ± 4 |
| Exercise | 536 ± 8* | 132 ± 4 |
| Exercise Bout 2 (l-NAME During Exercise) | ||
| Rest (pre l-NAME) | 464 ± 16 | 134 ± 4 |
| Exercise (pre l-NAME) | 542 ± 7* | 140 ± 3 |
| Exercise (post l-NAME) | 494 ± 11†‡ | 163 ± 5*†‡ |
Data are expressed as means ± SE
P < 0.05 vs. rest.
P < 0.05 vs. exercise bout 1.
P < 0.05 vs. exercise (pre l-NAME).
Fig. 4.
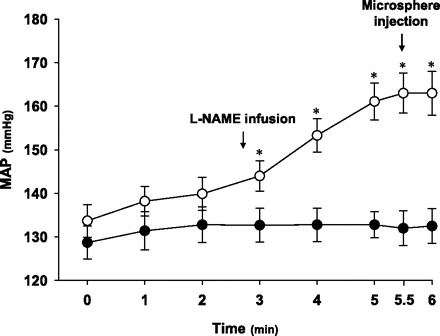
Mean arterial pressure (MAP) at rest (time 0) and during exercise for the first (•) and second (○) exercise bouts in the experimental group (n = 10). During the second run l-NAME was infused (see arrow) over an ∼10-s period immediately prior to the 3-min MAP measurement. Radioactive microspheres were injected (see arrow) at ∼5.5 min of total exercise time. *P < 0.05 vs. rest and minutes 1 and 2 of exercise.
Fig. 5.
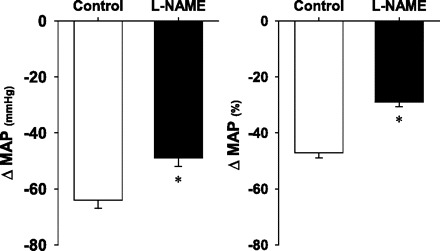
Hypotensive responses to acetylcholine injection expressed as absolute (left) and relative (right) changes in MAP before (control) and after l-NAME administration during exercise. *P < 0.05 vs. control.
Time controls.
Results from the time control animals (n = 7) demonstrated that HR, MAP, and the hypotensive response to ACh injections were not different between exercise bout 1 and 2. Total hindlimb musculature blood flow (exercise bout 1: 109 ± 9 and exercise bout 2: 110 ± 11 ml·min−1·100 g−1, P > 0.05) and VC (exercise bout 1: 0.83 ± 0.08 and exercise bout 2: 0.81 ± 0.09 ml·min−1·100 g−1·mmHg−1, P > 0.05) were not different between the first and second exercise bouts (Fig. 6). Moreover, blood flow to all 28 individual skeletal muscles or muscle parts examined in this study was not different (P > 0.05) between the first and second exercise bouts. This demonstration that both the hemodynamic and the skeletal muscle blood flow responses were reproducible between the repeated bouts of exercise in the time control animals allows for the direct comparison of these variables in the rats that were subjected to the administration of l-NAME during the second exercise bout.
Fig. 6.
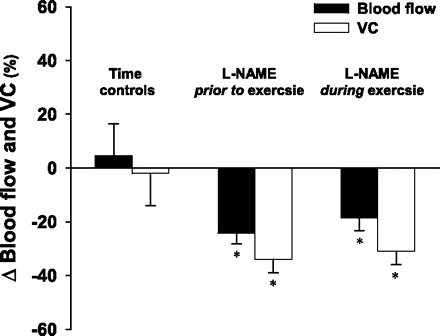
Comparison of the relative changes in blood flow (Δ blood flow) and vascular conductance (Δ VC) between first and second bouts of exercise for time controls (present study, n = 7), and when l-NAME was administered prior to (ref. 14, n = 6) and during (present study, n = 10) the second exercise bout. *Significantly different from zero.
DISCUSSION
The vasoregulatory pathways controlling vessel tone and blood flow (and therefore O2 delivery) at rest, during dynamic blood flow adjustments, and during the exercising steady-state may vary markedly (3, 10, 13). As a result, attenuations in skeletal muscle blood flow and VC measured during exercise subsequent to NOS inhibition administered prior to the exercise bout, may only identify NO as a modulator of resting vasomotor control. Accordingly, there are two novel findings of the present investigation: 1) consistent with our hypothesis, in the presence of prior COX inhibition, the potent NOS inhibitor l-NAME administered during submaximal whole-body exercise significantly reduced total rat hindlimb muscle blood flow and VC compared with that observed with COX inhibition alone, and 2) there was a positive correlation between the relative reductions in VC after l-NAME and the percent sum of type I and IIa muscle fibers but, surprisingly, the fiber-type dependency of muscle blood flow reductions observed with NOS inhibition administered prior to submaximal treadmill exercise (14) was completely abolished when NOS inhibition was administered during exercise.
Comparison with previous research.
Recently, it has been suggested that the search for a single key vasodilating substance is futile and the nature of the vasoregulatory system is one of redundancy and synergy (3). While NOS inhibition has identified a role for NO in regulating active muscle hyperemia in animal models with some consistency, whether NO contributes importantly to the hyperemic response in human locomotor muscle during dynamic exercise has been a point of major contention in the literature. Possible reasons for this discrepancy have been discussed in detail elsewhere (see relevant discussions in Refs. 7, 25, 28, 29). Two major considerations are pertinent to the current study. First, it is uncertain whether, or to what degree, NOS inhibitors administered at rest actually reach the specific vessels within the muscles recruited during a subsequent exercise bout (28). This issue is circumvented by performing NOS inhibition during exercise when a substantial proportion of the cardiac output is directed to the working muscles (27). While it may be argued that performing NOS inhibition prior to exercise is a more appropriate model of exercise conditions in disease states in which reduced NO bioavailability is implicated in the underlying pathology, performing NOS inhibition during exercise is warranted given that different vascular control mechanisms may be active at rest, during the onset of contractions and during the contracting steady state (3, 10). Thus, the current protocol lends direct mechanistic insight into integrated NO-mediated vascular control during exercise in healthy subjects. Second, experimental approaches using combined NOS and COX inhibition (as in the current study) and independent NOS inhibition have been used to examine the physiological role(s) of NO, and it is important to recognize that these dissimilar approaches answer very specific and different research questions. For example, there is strong evidence that supports substantial redundancy between the NOS and COX systems (2, 11, 18, 19, 26), although this redundancy is not evident in all experimental models (28). When NOS inhibition is administered independently, the COX system may compensate and mask the physiological NO contribution, thus presenting ambiguity regarding NO's role in the physiological hyperemia. In these cases, the conclusion is only that the physiological NO signal (as reported in the present study) is not an obligatory phenomenon (9, 26). Notwithstanding this consideration, it is important to note that both experimental designs (i.e., independent NOS inhibition vs. combined COX and NOS inhibition) provide valuable insight into the NO-mediated regulation.
Studies administering either NOS inhibition independently or combined NOS and COX inhibition during muscle contractions have identified NO as a mediator of active muscle bulk hyperemia in the contracting human forearm (7, 28) and leg (2, 19) and isolated canine diaphragm (15). Consistent with those studies, we currently found 16% and 34% reductions in blood flow and VC, respectively, within the rat hindlimb muscles and defined the regional distribution of that flow within and among locomotory muscles during whole-body dynamic exercise. Moreover, our current findings expand on previous reports from our laboratory in which l-NAME was administered prior to the initiation of submaximal (14) and maximal (22) exercise. Specifically, current results establish clearly that NO is an integral mediator of exercise hyperemia and VC rather than reflecting simply that NO regulates solely basal peripheral vascular tone (Fig. 6).
NO and muscle fiber-type composition.
A significant limitation of previous investigations that have administered NOS inhibition during contractions is that it has been possible to evaluate only the NO contribution to bulk limb (7, 26, 28) or an individual muscle (15) blood flow. Analysis of solely bulk or one individual muscle flow cannot elucidate possible varying inter- and/or intramuscular effects of NOS inhibition. For example, enhanced endothelium-dependent vasodilatory potential in vessels supplying oxidative muscles vs. those supplying glycolytic muscles has been reported in various experimental models, including isolated arterioles (20), the isolated perfused rat hindlimb (17), and intact exercising animals (14, 22). Presently, we report a correlation between VC and the percent sum of type I and IIa muscle fibers, which is consistent with those reports. However, the relationship between oxidative fibers and VC found herein is qualitatively and quantitatively (P < 0.01) different (i.e, less robust fiber-type dependency) vs. that found with NOS inhibition prior to exercise (Fig. 7). Moreover, we found no correlation between muscle-fiber type and the absolute reductions in blood flow induced by l-NAME administration during exercise. This finding is inconsistent with NOS inhibition performed prior to exercise (Fig. 7) and surprising considering that the submaximal treadmill speed utilized is expected to recruit primarily oxidative motor units (1, 4). Interestingly, the divergence from our previous work (14) appears to result principally from consistent differences occurring within the most oxidative muscles or muscle parts (i.e., all muscles or muscle parts containing greater than ∼80% oxidative fibers: the soleus, red portion of the gastrocnemius, vastus intermedius, and adductor longus). Of particular interest is that these oxidative regions sustain the largest hyperemic responses and, presumably, a significant portion of l-NAME was directed to these muscles. The present finding identifies that the potential for redundancy of blood flow control within these highly oxidative fibers is enhanced when NOS blockade is performed during vs. prior to exercise.
Fig. 7.
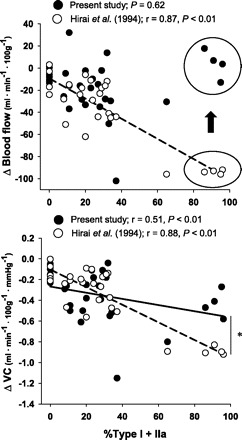
Correlations between the absolute changes in blood flow (Δ blood flow; top) and VC (Δ VC, bottom) and the percent sum of type I and IIa fibers found in the individual muscles and muscle parts of the rat hindlimb after l-NAME administration during (•, solid line; present investigation) vs. prior to (○, dashed line; ref. 17) exercise. Note the consistently lower (or absent) attenuations in blood flow and VC after l-NAME administration during vs. prior to exercise in the muscles comprising greater than ∼80% type I and IIa fibers (i.e., circled data in top panel; see text for discussion). *Significantly different relationship with fiber-type (i.e., less robust fiber-type dependency) when l-NAME is administered during as opposed to before exercise.
The systemic infusion of l-NAME resulted in significant increases in MAP. Therefore, it was important to analyze the fiber-type correlations expressed as both blood flow and VC. Although VC is the most appropriate indicator of vessel tone when experimental paradigms result in increases in systemic driving pressure, it is interesting to note that blood flow in the highly oxidative muscle(s) was essentially unchanged after l-NAME infusion (Fig. 2 and 7). Tschakovsky and Joyner (29) have suggested that when calculated VC is reduced in the face of unchanged blood flow, it may represent a normal metabolic autoregulatory response that effectively matches oxygen delivery to oxygen demand. Thus, our data may be interpreted to suggest that a greater ability to match O2 delivery to O2 demand is retained in the highly oxidative muscles when NOS is inhibited during vs. prior to exercise.
Experimental considerations.
As discussed above, in the present investigation we used a unique experimental protocol in which the COX inhibitor indomethacin was administered prior to the control exercise bout to limit the potential for vasodilating prostaglandins to mask the physiological NO contribution with l-NAME administration. Therefore, NOS inhibition during the second exercise bout was performed in the presence of previously administered COX inhibition. Our results suggest that NO does have an important role in blood flow regulation under normal physiological conditions. While it is possible that our results may differ if NOS inhibition is administered independently, we believe the current model addresses an important question regarding the physiological NO contribution.
Systemic infusion of l-NAME in the current investigation produced significant increases in MAP (occurring within <10 s of infusion, Fig. 3) and decreases in HR. As evident in Fig. 4, the entire effect of l-NAME administration during the second exercise bout on MAP was complete by the time the microsphere injection was performed. Furthermore, Hirai et al. (14) systematically administered doses of l-NAME higher than 10 mg/kg and observed no further effect on either blood flow or the hypotensive response to ACh injection. Although the experimental model used presently precludes the determination of the precise degree of NOS inhibition, these observations suggest strongly that the ability to inhibit NOS in vivo was maximized.
Analysis of the hindlimb musculature blood flow and VC during the two exercise bouts from the experimental and time control rats via repeated-measures two-way ANOVA with an a priori comparison produced the same statistical interpretation as performing paired Student's t-tests. We chose to present the results from the t-test comparisons to facilitate clarity of data presentation.
Perspectives and Significance
Varying the timing of NOS inhibition with respect to an exercise bout can lend important mechanistic insights into the integrated nature of vascular and blood flow regulation. On the basis of NOS inhibition initiated prior to the onset of exercise, it was unclear whether or not subsequent reductions in exercising muscle blood flow resulted from a carry-over of decreased resting conductance. By specifically choosing to initiate NOS inhibition during exercise, the present investigation presents clear and unique evidence that NO is a quantitatively important regulator of exercise hyperemia in locomotory muscles during submaximal whole-body exercise. l-NAME administered during exercise reduced blood flow in 11 of the 28 individual muscles or muscle parts of the rat hindlimb and VC in 20 of the 28 muscles or muscle parts. We found a significant correlation between the change in absolute VC after l-NAME and the percent sum of type I and IIa muscle fibers, which is consistent with the observation of fiber-type dependency of the physiological NO signal; however, the strength of this relationship was reduced substantially compared with our previous investigation in which l-NAME was administered prior to exercise. Moreover, there was no correlation between the absolute reduction in blood flow after l-NAME during exercise and muscle fiber-type composition, which differs substantially from the robust fiber-type dependency of NO contribution to blood flow observed when l-NAME is administered prior to exercise (14). Specifically, the current experimental model unveiled, for the first time, that highly oxidative muscles (i.e., >80% oxidative fibers) demonstrate a marked ability to maintain perfusion levels after l-NAME administration during exercise.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
These experiments were supported by Public Health Service (PHS) grants 1RO1-HL50306 to D. C. Poole and -1RO1-AG11535 to T. I. Musch.
REFERENCES
- 1. Armstrong RB, Laughlin MH. Metabolic indicators of fibre recruitment in mammalian muscles during locomotion. J Exp Biol 115: 201–213, 1985 [DOI] [PubMed] [Google Scholar]
- 2. Boushel R, Langberg H, Gemmer C, Olesen J, Crameri R, Scheede C, Sander M, Kjaer M. Combined inhibition of nitric oxide and prostaglandins reduces human skeletal muscle blood flow during exercise. J Physiol 543: 691–698, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clifford PS, Hellsten Y. Vasodilatory mechanisms in contracting skeletal muscle. J Appl Physiol 97: 393–403, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol 80: 261–270, 1996 [DOI] [PubMed] [Google Scholar]
- 5. Delp MD, Laughlin MH. Regulation of skeletal muscle perfusion during exercise. Acta Physiol Scand 162: 411–419, 1998 [DOI] [PubMed] [Google Scholar]
- 6. Dietz NM, Engelke KA, Samuel TT, Fix RT, Joyner MJ. Evidence for nitric oxide-mediated sympathetic forearm vasodiolatation in humans. J Physiol 498: 531–540, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dyke CK, Proctor DN, Dietz NM, Joyner MJ. Role of nitric oxide in exercise hyperaemia during prolonged rhythmic handgripping in humans. J Physiol 488: 259–265, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ekelund U, Bjornberg J, Grande PO, Albert U, Mellander S. Myogenic vascular regulation in skeletal muscle in vivo is not dependent of endothelium-derived nitric oxide. Acta Physiol Scand 144: 199–207, 1992 [DOI] [PubMed] [Google Scholar]
- 9. Endo T, Imaizumi T, Tagawa T, Shiramoto M, Ando S, Takeshita A. Role of nitric oxide in exercise-induced vasodilation of the forearm. Circulation 90: 2886–2890, 1994 [DOI] [PubMed] [Google Scholar]
- 10. Ferreira LF, Padilla DJ, Musch TI, Poole DC. Temporal profile of rat skeletal muscle capillary haemodynamics during recovery from contractions. J Physiol 573: 787–797, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Frandsen U, Bangsbo J, Sander M, Hoffner L, Betak A, Saltin B, Hellsten Y. Exercise-induced hyperaemia and leg oxygen uptake are not altered during effective inhibition of nitric oxide synthase with N(G)-nitro-l-arginine methyl ester in humans. J Physiol 531: 257–264, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gilligan DM, Panza JA, Kilcoyne CM, Waclawiw MA, Casino PR, Quyyumi AA. Contribution of endothelium-derived nitric oxide to exercise-induced vasodilation. Circulation 90: 2853–2858, 1994 [DOI] [PubMed] [Google Scholar]
- 13. Hamann JJ, Valic Z, Buckwalter JB, Clifford PS. Muscle pump does not enhance blood flow in exercising skeletal muscle. J Appl Physiol 94: 6–10, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Hirai T, Visneski MD, Kearns KJ, Zelis R, Musch TI. Effects of NO synthase inhibition on the muscular blood flow response to treadmill exercise in rats. J Appl Physiol 77: 1288–1293, 1994 [DOI] [PubMed] [Google Scholar]
- 15. Hussain SN, Stewart DJ, Ludemann JP, Magder S. Role of endothelium-derived relaxing factor in active hyperemia of the canine diaphragm. J Appl Physiol 72: 2393–2401, 1992 [DOI] [PubMed] [Google Scholar]
- 16. Joyner MJ, Dietz NM. Nitric oxide and vasodilation in human limbs. J Appl Physiol 83: 1785–1796, 1997 [DOI] [PubMed] [Google Scholar]
- 17. McAllister RM. Endothelium-dependent vasodilation in different rat hindlimb skeletal muscles. J Appl Physiol 94: 1777–1784, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Mortensen SP, Gonzalez-Alonso J, Bune LT, Saltin B, Pilegaard H, Hellsten Y. ATP-induced vasodilation and purinergic receptors in the human leg: roles of nitric oxide, prostaglandins, and adenosine. Am J Physiol Regul Integr Comp Physiol 296: R1140–R1148, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Mortensen SP, Gonzalez-Alonso J, Damsgaard R, Saltin B, Hellsten Y. Inhibition of nitric oxide and prostaglandins, but not endothelial-derived hyperpolarizing factors, reduces blood flow and aerobic energy turnover in the exercising human leg. J Physiol 581: 853–861, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Muller-Delp JM, Spier SA, Ramsey MW, Delp MD. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol 283: H1662–H1672, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Musch TI, Bruno A, Bradford GE, Vayonis A, Moore RL. Measurements of metabolic rate in rats: a comparison of techniques. J Appl Physiol 65: 964–970, 1988 [DOI] [PubMed] [Google Scholar]
- 22. Musch TI, McAllister RM, Symons JD, Stebbins CL, Hirai T, Hageman KS, Poole DC. Effects of nitric oxide synthase inhibition on vascular conductance during high speed treadmill exercise in rats. Exp Physiol 86: 749–757, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Musch TI, Terrell JA. Skeletal muscle blood flow abnormalities in rats with a chronic myocardial infarction: rest and exercise. Am J Physiol Heart Circ Physiol 262: H411–H419, 1992 [DOI] [PubMed] [Google Scholar]
- 24. Persson MG, Gustafsson LE, Wiklund NP, Hedqvist P, Moncada S. Endogenous nitric oxide as a modulator of rabbit skeletal muscle microcirculation in vivo. Br J Pharmacol 100: 463–466, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Radegran G, Hellsten Y. Adenosine and nitric oxide in exercise-induced human skeletal muscle vasodilatation. Acta Physiol Scand 168: 575–591, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Radegran G, Saltin B. Nitric oxide in the regulation of vasomotor tone in human skeletal muscle. Am J Physiol Heart Circ Physiol 276: H1951–H1960, 1999 [DOI] [PubMed] [Google Scholar]
- 27. Rowell L. Human Cardiovascular Control. New York, NY: Oxford University, 1993 [Google Scholar]
- 28. Schrage WG, Joyner MJ, Dinenno FA. Local inhibition of nitric oxide and prostaglandins independently reduces forearm exercise hyperaemia in humans. J Physiol 557: 599–611, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tschakovsky ME, Joyner MJ. Nitric oxide and muscle blood flow in exercise. Appl Physiol Nutr Metab 33: 151–161, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Wilson JR, Kapoor S. Contribution of endothelium-derived relaxing factor to exercise-induced vasodilation in humans. J Appl Physiol 75: 2740–2744, 1993 [DOI] [PubMed] [Google Scholar]


