Abstract
Objectives
To assess and compare the analgesic effects of orally administered glucose and sucrose and pacifiers. To determine the synergistic analgesic effect of sucrose and pacifiers.
Design
Randomised prospective study with validated behavioural acute pain rating scale.
Setting
Maternity ward.
Participants
150 term newborns undergoing venepuncture randomly assigned to one of six treatment groups: no treatment; placebo (2 ml sterile water); 2 ml 30% glucose; 2 ml 30% sucrose; a pacifier; and 2 ml 30% sucrose followed by a pacifier.
Results
Median (interquartile) pain scores during venepuncture were 7 (5-10) for no treatment; 7 (6-10) for placebo (sterile water); 5 (3-7) for 30% glucose; 5 (2-8) for 30% sucrose; 2 (1-4) for pacifier; and 1 (1-2) for 30% sucrose plus pacifier. Mann-Whitney U test P values for comparisons of 30% glucose, 30% sucrose, pacifier, and 30% sucrose plus pacifier versus placebo (sterile water) were 0.005, 0.01, <0.0001, and <0.0001, respectively. Differences between group median pain scores for these comparisons were 2 (95% confidence interval 1 to 4), 2 (0 to 4), 5 (4 to 7), and 6 (5 to 8), respectively. P values for comparisons of 30% glucose, 30% sucrose, and 30% sucrose plus pacifier versus pacifier were 0.0001, 0.001, and 0.06, respectively. Differences between group medians for these comparisons were 3 (2 to 5), 3 (1 to 5), and 1 (0 to 2), respectively.
Conclusion
The analgesic effects of concentrated sucrose and glucose and pacifiers are clinically apparent in newborns, pacifiers being more effective than sweet solutions. The association of sucrose and pacifier showed a trend towards lower scores compared with pacifiers alone. These simple and safe interventions should be widely used for minor procedures in neonates.
Key messages
The analgesic effects on newborn infants of sucrose, glucose, and pacifiers can be clearly detected by a behavioural pain rating scale
Pacifiers had a better analgesic effect than sweet solutions
A synergistic effect was found with a combination of sucrose and pacifiers
Sweet solutions and pacifiers constitute simple and safe interventions that can be used to provide analgesia in newborns during minor procedures
Introduction
Routine medical care of newborns includes blood sampling during the first days of life. These procedures are performed even in babies who are not sick, and, of course, they are more common in ill infants who need neonatal intensive care. The ability of neonates to perceive and react to pain has recently been acknowledged.1,2 Treating pain in the newborn is essential; firstly, for ethical reasons and, secondly, because pain can lead to decreased oxygenation, haemodynamic instability, or increased intracranial pressure.2 Recent research has shown that even short term pain can have lasting negative effects.3 This knowledge has led many neonatal teams to develop strategies to alleviate pain caused by diagnostic and therapeutic procedures undergone by newborns. For neonates receiving intensive care it is widely accepted that central analgesics, administered intravenously, should be used to relieve pain. Infants who are less sick or who are not in neonatal intensive care units, however, usually do not receive any analgesic for painful procedures. Obviously, central analgesics can not be used for occasional blood sampling performed in newborns who do not need intensive care; it is therefore essential to find simple, acceptable, and well tolerated methods to reduce pain in these infants.
Some recent studies have reported that simple and benign interventions—such as oral sugar solutions,4 milk,5 or sucking a pacifier (commonly called dummies in the United Kingdom)4,6—reduce pain in neonates during procedures. The analgesic effects of sucrose have been reported in term and preterm newborn infants.7–11 Glucose and non-sucrose sweet tasting solutions have also been found to have analgesic effects.12–14
Almost all of these previous studies regarding the analgesic effects of oral sugar, milk, or pacifiers have used crying as the principal tool to assess pain.15 But, although closely related, crying is not unique to pain. Hence, use of crying alone as an indication of pain has severe limitations.15,16 In fact evaluation of pain in newborns is difficult, and we have to rely on reactions such as changes in behaviour, modification in physiological variables, or release of stress hormones to infer pain. Assessments that rely on various behavioural changes seem more accurate for evaluation of pain in newborns.
Recently, a behavioural acute pain rating scale for neonates, DAN (Douleur Aiguë du Nouveau-né), has been validated.17 This scale scores pain from 0 to 10, where 0 is no pain and 10 maximum pain. It evaluates three items: facial expression, limb movements, and vocal expression (table 1). In the validation study of this scale, two independent observers evaluated newborns during both painful and placebo or dummy procedures. The scale showed a good sensitivity and specificity because all possible scores were obtained; these were ⩾3 in 95% of painful procedures and ⩽2 in 88% of dummy procedures. High intercorrelation of items (internal consistency) was confirmed by a Cronbach's coefficient α of 0.88, and a good interrater agreement was shown by a Krippendorf's r of 91.2.
Table 1.
DAN*: A behavioural acute pain rating scale for neonates
| Measure | Score |
|---|---|
| Facial expressions | |
| Calm | 0 |
| Snivels and alternates gentle eye opening and closing | 1 |
| Determine intensity of one or more of: eye squeeze, brow bulge, nasolabial furrow: | |
| Mild, intermittent with return to calm | 2 |
| Moderate | 3 |
| Very pronounced, continuous | 4 |
| Limb movements | |
| Calm or gentle movements | 0 |
| Determine intensity of one or more of the following signs: pedals, toes spread, legs tensed and pulled up, agitation of arms, withdrawal reaction: | |
| Mild, intermittent with return to calm | 1 |
| Moderate | 2 |
| Very pronounced, continuous | 3 |
| Vocal expression | |
| No complaints | 0 |
| Moans briefly; for intubated child, looks anxious or uneasy | 1 |
| Intermittent crying; for intubated child, gesticulations of intermittent crying | 2 |
| Long lasting crying, continuous howl; for intubated child, gesticulations of continuous crying | 3 |
Douleur Aiguë du Nouveau-né.
The present study was undertaken to assess and compare the analgesic effects of orally administered glucose and sucrose and pacifiers with a validated behavioural acute pain rating scale and to determine the synergistic analgesic effect of sucrose and pacifiers during venepuncture in term neonates.
Methods
Protocol
This prospective, randomised clinical study was designed to include normal full term newborn infants treated in the maternity ward of the Poissy Hospital. The study protocol and the letter of permission addressed to parents were approved by the local committee for the protection of human subjects in medical research, according to current law in France. Written informed consent was obtained from a parent of each newborn before the infant participated in the study. The inclusion criteria were newborn aged ⩾24 hours who underwent venepuncture as part of routine medical care (the main reasons were screening for phenylketonuria and hypothyroidism and serum bilirubin sampling); no feeding for the previous 30 minutes; Apgar score ⩾7 at five minutes, and availability of one investigator (XC) who was present eight hours a day, at the time when most non-urgent blood samples were drawn, every day from Monday to Friday during the study period. Exclusion criteria were medical instability in the infant and naloxone administration during the previous 24 hours. All venepunctures were performed by two experienced nurses using the “broken needle” technique.
The primary outcome measure was the evaluation of pain induced by venepuncture in newborns with the DAN scale. Calculation of sample size with means and SD of 2.5 showed that to achieve 80% power and 5% significance to detect a 2 point difference in DAN scale among groups, 25 newborns were required in each one of the six groups planned. A subsequent power analysis adapted for the non-parametric Mann-Whitney U test showed that 25 newborns per group would give a 80% power and 1% significant level. The six groups were no treatment; placebo (2 ml sterile water); 2 ml 30% glucose; 2 ml 30% sucrose; sucking a pacifier; and 2 ml 30% sucrose followed by sucking a pacifier. Commercial vials of sterile water and 30% glucose were used; sterile 30% sucrose solutions were prepared in advance by a pharmacist.
Assignment
A hundred and fifty infants expected to be included in the study were randomly assigned to one of the six groups. Randomisation was performed in advance with a random number table by an assistant not involved in the study, and treatment allocations were inserted in opaque sealed envelopes numbered 1 to 150; investigators were blind to these allocations. Codes of allocation were kept secret by the assistant who performed randomisation, and they were broken only after the inclusion of the last neonate.
Masking
Newborn infants were taken to a quiet nursery for venepunctures. As pain evaluation with the DAN scale needed observation of leg and foot movements these were uncovered. The observer started evaluations with an assessment of the arousal state by using Prechtl's observational rating system18: (1) eyes closed, regular respiration, no movements; (2) eyes closed, irregular respiration, gross movements; (3) eyes open, no gross movements; (4) eyes open, continual gross movements, no crying; (5) eyes open or closed, fussing, or crying. The observer then left the room and the infant was prepared for the procedure. A research assistant opened a consecutively numbered envelope that contained the treatment assigned for each infant. Two minutes before venepuncture the allocated solution was administered for 30 seconds by a sterile syringe into the infant's mouth. A pacifier (standard nipple stuffed with a gauze square for resistance) was also given two minutes before venepuncture and held gently in the infant's mouth by an assistant throughout the procedure. Pain was assessed during venepuncture and blood collection by the observer (XC). As pain evaluation was based on a behavioural scale blinding to the pacifier was not possible.
Statistical analysis was performed with simstat 3.5 software. Median scores of all groups were compared with the non-parametric Mann-Whitney U test. Because multiple pairwise comparisons were made P=0.01 was considered significant.
Results
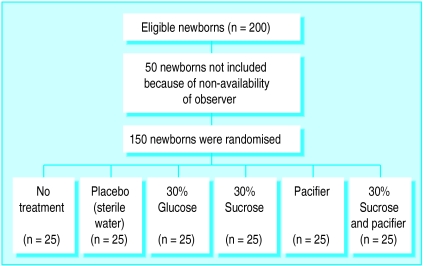
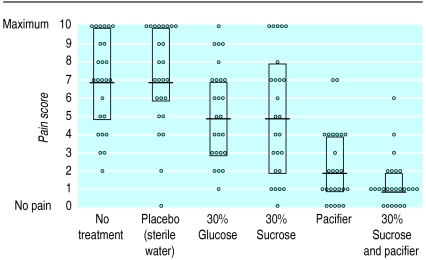
From April to the end of June 1997 we studied 150 newborn infants in six equal sized groups. All parents asked gave their consent for their infants to participate in the study. There were no withdrawals. Fifty neonates who underwent venepuncture and were potentially eligible were not included in the study because XC was not available; their perinatal characteristics were similar to those included in the study. Figure 1 shows a trial profile with participant flow. Birth weight, gestational age, Apgar scores, postnatal age, sex distribution, type of delivery, and arousal state for each group are shown in table 2. There were no substantial differences among the groups concerning these perinatal characteristics. Individual pain scores, median values, and interquartile ranges for each group during venepunctures are presented in figure 2. The median (interquartile) pain scores during venepuncture were 7 (5-10) for no treatment, 7 (6-10) for placebo sterile water, 5 (3-7) for 30% glucose, 5 (2-8) for 30% sucrose, 2 (1-4) for pacifier, and 1 (1-2) for 30% sucrose plus pacifier. Comparisons of median pain scores among groups are shown in table 3. No adverse effects were noted in any infant.
Figure 1.
Trial profile and participant flow; all randomised newborns completed trial
Table 2.
Perinatal characteristics of 150 newborns included in study of analgesic effects of sucrose, glucose, and pacifiers. Numbers are nedian (range) unless stated otherwise
| Detail | No treatment (n=25) | Placebo—sterile water (n=25) | 30% Glucose (n=25) | 30% Sucrose (n=25) | Pacifier (n=25) | 30% Sucrose plus pacifier (n=25) |
|---|---|---|---|---|---|---|
| Gestational age (weeks) | 39 (37-41) | 40 (37-41) | 40 (37-41) | 40 (37-42) | 40 (37-41) | 39 (37-41) |
| Birth weight (g) | 3320 (2320-4080) | 3280 (2460-4050) | 3340 (2400-3950) | 3420 (2260-4125) | 3370 (2460-4000) | 3320 (2630-3950) |
| No of boys/girls | 16/9 | 16/9 | 14/11 | 11/14 | 16/9 | 15/10 |
| No of vaginal/caesarean deliveries | 22/3 | 22/3 | 21/4 | 22/3 | 23/2 | 24/1 |
| Apgar score (5 min) | 10 (7-10) | 10 (7-10) | 10 (9-10) | 10 (9-10) | 10 (10-10) | 10 (8-10) |
| Postnatal age (interquartile range) (days) | 3 (3-4) | 4 (3-4) | 4 (3-4) | 4 (3-5) | 4 (3-4) | 3 (2-4) |
| Arousal state score | 3 (1-5) | 3 (1-5) | 3 (1-5) | 3 (1-5) | 3 (1-5) | 3 (1-3) |
Figure 2.
Pain evaluation with DAN scale (0 to 10) during venepuncture in 150 newborns randomised to six equal sized groups, with values for individual infants, median values, and interquartile ranges (for 30% sucrose and pacifier lower quartile coincides with median value)
Table 3.
Comparisons of median pain scores among groups
| Detail | 30% glucose (MPS=5) | 30% sucrose (MPS=5) | Pacifier (MPS=2) | 30% sucrose + pacifier (MPS=1) |
|---|---|---|---|---|
| Placebo (MPS=7): | ||||
| P value* | 0.005 | 0.01 | <0.0001 | <0.0001 |
| Median difference (95% CI) | 2 (1 to 4) | 2 (0 to 4) | 5 (4 to 7) | 6 (5 to 8) |
| Pacifier (MPS=2): | ||||
| P value* | 0.0001 | 0.001 | NA | 0,06 |
| Median difference (95% CI) | 3 (2 to 5) | 3 (1 to 5) | NA | 1 (0 to 2) |
MPS: median pain score. NA: not applicable.
For Mann-Whitney U test.
Discussion
This study has three main results. Firstly, during venepuncture in newborns the analgesic effects of 30% glucose, 30% sucrose, and non-nutritive sucking of pacifiers are large enough to be clinically significant and can thus be detected by a behavioural rating scale for acute pain. Secondly, 30% glucose, which is readily available in any hospital, showed at least the same analgesic effect as 30% sucrose, which is usually more difficult to obtain. Thirdly, the non-nutritive sucking of a pacifier was more effective than the oral administration of 30% glucose or 30% sucrose. It should be noted that although comparison of 30% sucrose versus placebo showed a P value of 0.01 and a median difference of 2 points, the 95% confidence interval of the latter included 0. We believe that this discrepancy was mainly due to the fact that the sample included was rather small; this sample had been calculated by using parametric measures—means and SD—whereas the analysis was performed with a non-parametric test. Although the administration of 2 ml 30% glucose and 30% sucrose reduced pain in neonates, the median pain score in each of these groups was 5.0, which is still relatively high. Therefore, we consider that although sweet solutions are effective in reducing pain in newborns they are not perfect analgesics.
We found that non-nutritive sucking provided a better analgesic effect than sweet solutions. Other authors have reported on the pacifying and comforting effects of non-nutritive sucking,19,20 but to our knowledge no comparison between pacifiers and sweet solutions had been done previously. It has also been reported that oral sucrose via a nipple is effective for pain relief in neonatal circumcision.11 We consider, however, that this analgesic effect may be essentially due to the pacifier more than to sucrose itself.
Regarding the synergistic effect of sucrose and pacifiers, this was clearly established in the comparison with placebo as median scores in placebo, sucrose, and sucrose plus pacifier groups were 7, 5, and 1, respectively. A trend towards lower scores was observed in the group given 30% sucrose plus pacifier (median score 1) compared with the group given a pacifier alone (median score 2). This difference did not reach significance (P=0.06). It should be mentioned, however, that the study was designed to detect a 2 point difference between the groups and therefore it lacked enough power to detect a 1 point difference. It has also been suggested that the administration of sterile water may have some analgesic effect.21 We did not find any difference between the group given sterile water and the one given no intervention. One possible explanation is that the authors of that study used only measures of crying and not a behavioural pain scale.21
The rapid onset of the analgesic effect strongly suggests a mechanism activated by the presence of the solution in the mouth rather than any effect after ingestion. The pain relief elicited by sweet solutions is probably mediated by the activation of endogenous opioids; this view is supported by the fact that the effect can be blocked by the administration of an opioid antagonist.22
The precise mechanism by which pacifiers relieve pain remains to be identified. It has been suggested that two processes may play a part.19 The first is sensory dominance; as sucking is a powerful source of perceptual information for infants the sensations it elicits may have priority in deployment of attentional resources and thus effectively mute pain. The second hypothesis is that pacifiers reduce infant response to pain by facilitating self regulation. Provision of pacifiers enhances infants' ability to regulate their response to pain by giving the opportunity for sucking. Elicitation of sucking with a pacifier enables infants to control one source of incoming stimuli—oral stimulation—through their own activity. Pacifiers are accepted by most newborns, if not all, because they associate non-nutritive sucking with a pleasurable activity.
Limitations
Interpretation of the results of this study should acknowledge two limitations. Firstly, although the observer was blind to the type of solution administered he was not blind to the administration of a pacifier to newborns; it was impossible to avoid this potential bias because the study was based on a behavioural pain scale. The objectivity of the observer, however, can be underlined by the fact that the median pain scores of the two groups pacifier alone and pacifier plus sucrose tended to be different. Secondly, although the validation study of the DAN scale has shown that it discriminates pain in newborns, no study has proved yet that this scale can grade the degree of perception of pain. We assumed that the more pronounced the facial expressions, the limb movements, and the vocal expressions the greater the pain in the newborn.
Minor procedures
Minor procedures are common in newborns, and effective analgesia is seldom used in this setting. The non-nutritive sucking of a pacifier, the oral administration of concentrated glucose or sucrose, or, even better, the association of an oral sweet solution with the non-nutritive sucking of pacifier constitute simple, non-invasive, and benign manoeuvres that can relieve pain in newborns during minor procedures such as venepuncture, heel lancing, spinal tap, intramuscular vitamin K injection, or subcutaneous erythropoietin injection, and therefore we think that they should be routinely used. We insist on “minor procedures” as we consider that these simple interventions are not suitable for more aggressive procedures, when stronger analgesics, including central ones, should be administered.
Acknowledgments
We thank the nursing staff of the maternity ward of the Poissy Hospital for their help during the study and Dr Nicolas Simon, chief of the emergency department, Poissy Hospital, for reading the paper.
Editorial by Choonara
Footnotes
Funding: No external funding.
Competing interests: None declared.
References
- 1.Anand KJS, Carr DB. The neuroanatomy, neurophysiology and neurochemistry of pain, stress, and analgesia in newborns and children. Ped Clin N Am. 1989;36:795–822. doi: 10.1016/s0031-3955(16)36722-0. [DOI] [PubMed] [Google Scholar]
- 2.Anand KJS, Hickey PR. Pain and its effects in the human neonate and fetus. N Engl J Med. 1987;317:1321–1329. doi: 10.1056/NEJM198711193172105. [DOI] [PubMed] [Google Scholar]
- 3.Taddio A, Goldbach M, Ipp M, Stevens B, Koren G. Effect of neonatal circumcision on pain responses during vaccination in boys. Lancet. 1995;345:291–292. doi: 10.1016/s0140-6736(95)90278-3. [DOI] [PubMed] [Google Scholar]
- 4.Blass EM, Hoffmeyer LB. Sucrose as an analgesic for newborns infants. Pediatrics. 1991;87:215–218. [PubMed] [Google Scholar]
- 5.Blass EM. Milk-induced hypoalgesia in human newborns. Pediatrics. 1997;99:825–829. doi: 10.1542/peds.99.6.825. [DOI] [PubMed] [Google Scholar]
- 6.Gunnar M, Fisch RO, Malone S. The effects of pacifying stimulus on behavioral and adrenocortical responses to circumcision. J Am Acad Child Adolescs Psychiatry. 1984;23:34–38. doi: 10.1097/00004583-198401000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Ramenghi LA, Wood CM, Griffith GC, Levene MI. Reduction of pain response in premature infants using intraoral sucrose. Arch Dis Child. 1996;74:F126–F128. doi: 10.1136/fn.74.2.f126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rushforth JA, Levene MI. Effect of sucrose on crying in response to heel stab. Arch Dis Child. 1993;69:388–389. doi: 10.1136/adc.69.3.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haouari N, Wood C, Griffiths G, Levene M. The analgesic effect of sucrose in full term infants: a randomised controlled trial. BMJ. 1995;310:1498–1500. doi: 10.1136/bmj.310.6993.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bucher H, Moser T, Siebenthal KV, Keel M, Wolf M, Duc G. Sucrose reduces pain reaction to heel lancing in preterm infants: a placebo- controlled, randomized and masked study. Pediatr Res. 1995;38:332–335. doi: 10.1203/00006450-199509000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Herschel M, Khoshnood B, Ellman C, Maydew N, Mittendorf R. Neonatal circumcision. Arch Pediatr Adolesc Med. 1998;152:279–284. doi: 10.1001/archpedi.152.3.279. [DOI] [PubMed] [Google Scholar]
- 12.Blass EM, Smith BA. Differential effects of sucrose, fructose, glucose, and lactose on crying in 1- to 3-day-old human infants: qualitative and quantitative considerations. Dev Psychol. 1992;28:804–810. [Google Scholar]
- 13.Ramenghi LA, Griffith GC, Wood CM, Levene MI. Effect of non-sucrose sweet tasting solution on neonatal heel prick responses. Arch Dis Child. 1996;74:F129–F131. doi: 10.1136/fn.74.2.f129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skogsdal Y, Eriksson M, Schollin J. Analgesia in newborns given oral glucose. Acta Paediatr. 1997;86:217–220. doi: 10.1111/j.1651-2227.1997.tb08872.x. [DOI] [PubMed] [Google Scholar]
- 15.Stevens B, Taddio A, Ohlsson A, Einarson T. The efficacy of sucrose for relieving procedural pain in neonates—a systematic review and meta-analysis. Acta Paediatr. 1997;86:837–842. doi: 10.1111/j.1651-2227.1997.tb08607.x. [DOI] [PubMed] [Google Scholar]
- 16.Editorial. Pacifiers, passive behaviour, and pain. Lancet. 1992;339:275–276. [PubMed] [Google Scholar]
- 17.Carbajal R, Paupe A, Hoenn E, Lenclen R, Olivier-Martin M. DAN: une échelle comportementale d'évaluation de la douleur aiguë du nouveau-né. Arch Pédiatr. 1997;4:623–628. doi: 10.1016/s0929-693x(97)83360-x. [DOI] [PubMed] [Google Scholar]
- 18.Prechtl HFR, Beintema DJ. The neurological examination of the full term newborn infant. Clinics in developmental medicine. No 12. London: Heinemann; 1964. [Google Scholar]
- 19.Campos RG. Rocking and pacifiers: two comforting interventions for heelstick pain. Res Nursing Health. 1994;17:321–331. doi: 10.1002/nur.4770170503. [DOI] [PubMed] [Google Scholar]
- 20.Field T, Goldson E. Pacifying effects, of non-nutritive sucking on term and preterm neonates during heelstick procedures. Pediatrics. 1984;74:1002–1005. [PubMed] [Google Scholar]
- 21.Allen KD, White DD, Walburn JN. Sucrose as an analgesic agent for infants during immunization injections. Arch Pediatr Adolesc Med. 1996;150:270–274. doi: 10.1001/archpedi.1996.02170280040007. [DOI] [PubMed] [Google Scholar]
- 22.Blass EM, Fitzgerald E. Milk induced analgesia and comforting in 10-day-old rats: opioid mediation. Pharmacol Biochem Behav. 1988;29:9–13. doi: 10.1016/0091-3057(88)90266-3. [DOI] [PubMed] [Google Scholar]