Abstract
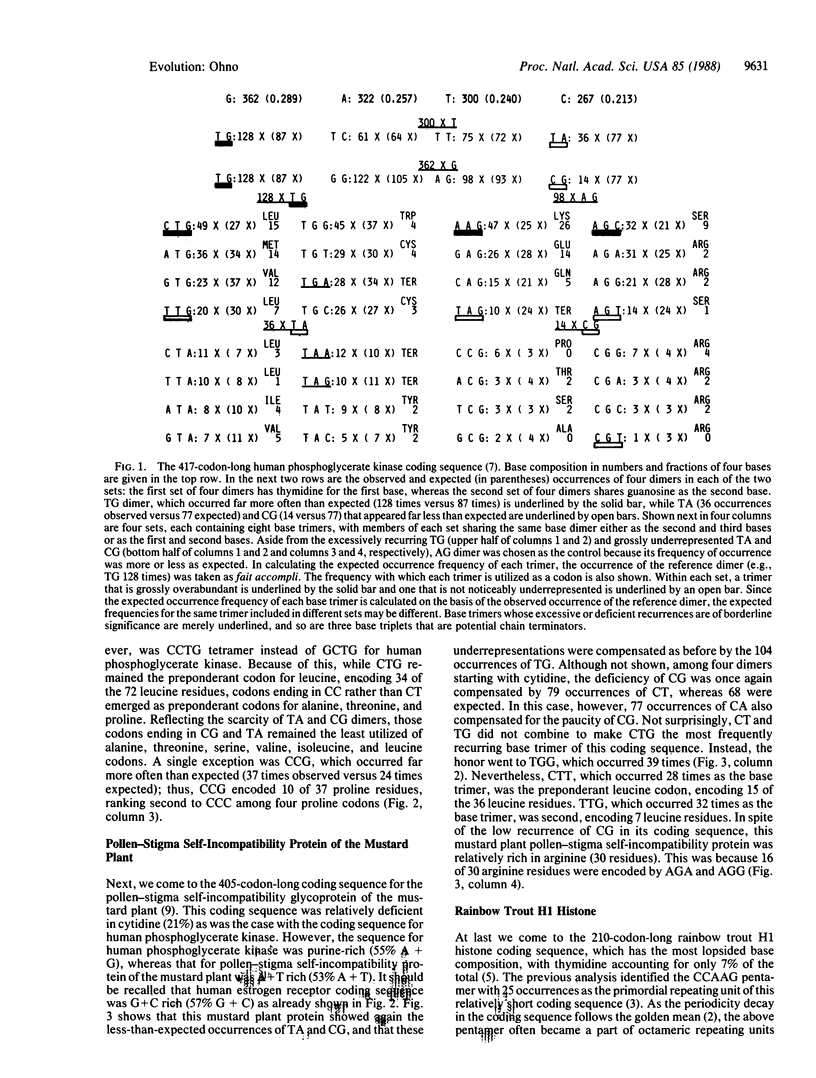
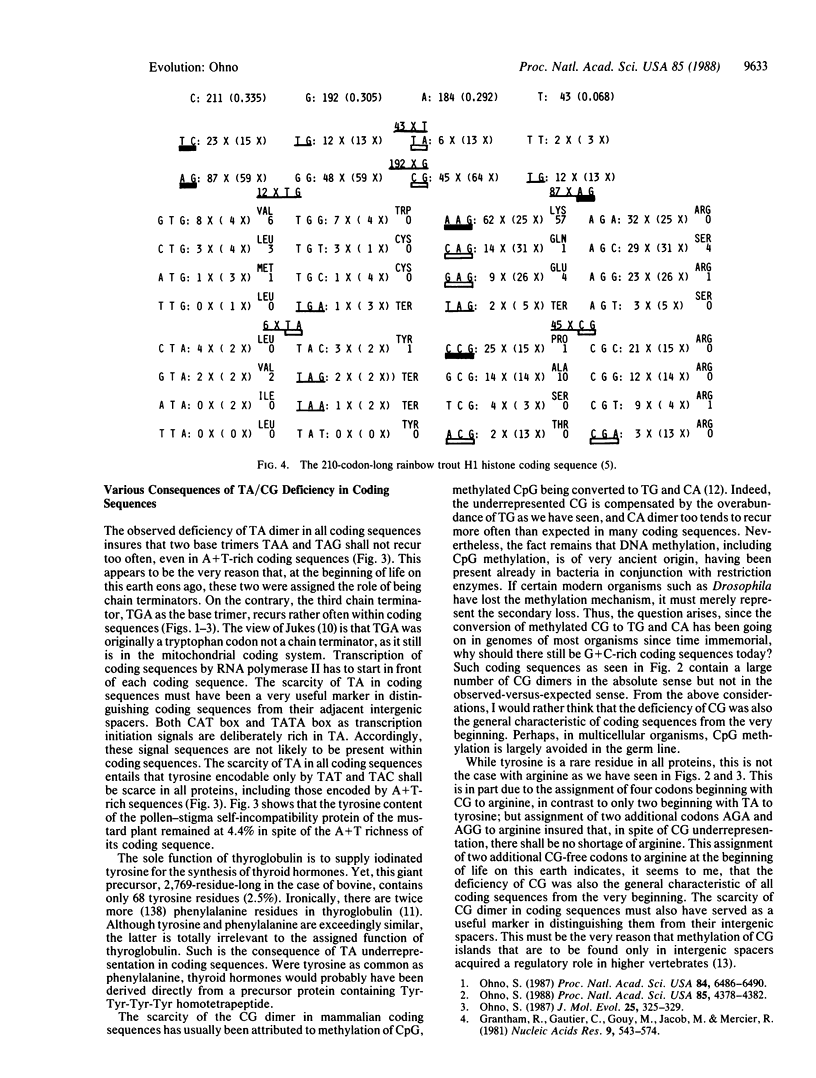
Each coding sequence is a finite resource as to the number and composition of four bases. Accordingly, the excessive recurrence of one base oligomer entails the noticeable underrepresentation by the other, so that if the former is the same in most, if not all, of the coding sequences, the latter too must necessarily be the same in all. Indeed, a previous series of studies on 20-odd divergent coding sequences established CTG as one of the most frequently recurring base trimers (if not the most frequent), and this excess was compensated by the underrepresentation by CG and TA dimer-containing base trimers. In this study, I have analyzed three additional coding sequences and reanalyzed one previously studied coding sequence. These four, derived from man, a plant, and a fish, were of variously lopsided base compositions that were not at all conducive to high recurrences of either CT dimer or CT and TG. Yet, the excess of CT and TG dimers accompanied by complementary deficiency of CG and TA dimers emerged as the common rule. Thus, I propose the above as the universal rule of coding sequence construction. The underrepresentation by CG and TA dimers within coding sequences explains why regulatory signals in intergenic spacers are of two kinds: one, TA dimer rich; and the other, CG dimer rich.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Greene G. L., Gilna P., Waterfield M., Baker A., Hort Y., Shine J. Sequence and expression of human estrogen receptor complementary DNA. Science. 1986 Mar 7;231(4742):1150–1154. doi: 10.1126/science.3753802. [DOI] [PubMed] [Google Scholar]
- Jukes T. H. Mitochondrial codes and evolution. Nature. 1983 Jan 6;301(5895):19–20. doi: 10.1038/301019a0. [DOI] [PubMed] [Google Scholar]
- Liskay R. M., Evans R. J. Inactive X chromosome DNA does not function in DNA-mediated cell transformation for the hypoxanthine phosphoribosyltransferase gene. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4895–4898. doi: 10.1073/pnas.77.8.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercken L., Simons M. J., Swillens S., Massaer M., Vassart G. Primary structure of bovine thyroglobulin deduced from the sequence of its 8,431-base complementary DNA. Nature. 1985 Aug 15;316(6029):647–651. doi: 10.1038/316647a0. [DOI] [PubMed] [Google Scholar]
- Mezquita J., Connor W., Winkfein R. J., Dixon G. H. An H1 histone gene from rainbow trout (Salmo gairdnerii). J Mol Evol. 1984;21(3):209–219. doi: 10.1007/BF02102355. [DOI] [PubMed] [Google Scholar]
- Michelson A. M., Markham A. F., Orkin S. H. Isolation and DNA sequence of a full-length cDNA clone for human X chromosome-encoded phosphoglycerate kinase. Proc Natl Acad Sci U S A. 1983 Jan;80(2):472–476. doi: 10.1073/pnas.80.2.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S. Codon preference is but an illusion created by the construction principle of coding sequences. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4378–4382. doi: 10.1073/pnas.85.12.4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S. Early genes that were oligomeric repeats generated a number of divergent domains on their own. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6486–6490. doi: 10.1073/pnas.84.18.6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S. Evolution from primordial oligomeric repeats to modern coding sequences. J Mol Evol. 1987;25(4):325–329. doi: 10.1007/BF02603117. [DOI] [PubMed] [Google Scholar]
- Riggs A. D. X inactivation, differentiation, and DNA methylation. Cytogenet Cell Genet. 1975;14(1):9–25. doi: 10.1159/000130315. [DOI] [PubMed] [Google Scholar]
- Watson H. C., Walker N. P., Shaw P. J., Bryant T. N., Wendell P. L., Fothergill L. A., Perkins R. E., Conroy S. C., Dobson M. J., Tuite M. F. Sequence and structure of yeast phosphoglycerate kinase. EMBO J. 1982;1(12):1635–1640. doi: 10.1002/j.1460-2075.1982.tb01366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



