Abstract
The oral mucosa is relatively resistant to human immunodeficiency virus type 1 (HIV-1) transmission. The mechanisms contributing to this resistance remain incompletely understood, but may include HIV-induced synthesis of innate immune factors. We used fully differentiated oral epithelium as a surrogate for the oral mucosa in vivo, exposed it to X4- and R5-tropic HIV-1 in culture, and quantified mRNA expression of six innate immune factors. Neither virus increased expression of human beta defensin 2 (hBD-2) mRNA over supernatants from uninfected lymphoblast controls. HIV-1 also failed to induce mRNA of four additional innate immunity-related genes. Similar results were obtained with oral monolayer epithelial cells. Interestingly, the X4-tropic virus inhibited mRNA expression of hBD-2, and of three of the other factors, at higher dosages in the differentiated oral epithelium but not the monolayers. The failure of HIV-1 to induce innate immune factors in the differentiated epithelium was not due to a lack of tissue penetration, as we detected fluorescence-tagged virions up to 30 μm deep from the apical surface. HIV-1 does not trigger de novo innate immune factor synthesis in oral epithelium, pointing to the role of a constitutive innate immunity for protection against HIV-1 in the oral cavity.
Introduction
Epithelial cells that line the oral cavity are among the sites of first exposure to human immunodeficiency virus type 1 (HIV-1) through breast-feeding and oral–genital contact. However, compared to genital and anorectal tissues, oral mucosa appears to be naturally more resistant to infection with HIV-1.1 Antimicrobial peptides produced by oral epithelial cells may protect the oral mucosa against HIV-1 infection.2,3 However, some of these endogenous inhibitors of HIV-1 in saliva have also been found in the vagina and in semen, and yet these compartments are more susceptible to infection.4 Thus, it is not clear to what degree locally produced innate immune factors contribute to HIV-1 resistance of the oral mucosa.
Human beta defensins (hBDs) are small cationic antimicrobial proteins that are secreted by epithelial cells of several mucosae, including the mouth.5 Recombinant hBD-2 and -3 have both been shown to possess anti-HIV-1 activity.3 HBDs have a dual role in antiviral defense acting directly on the virion as well as on the target cell.6 The proposed mechanisms include interacting with viral glycoproteins such as gp120, disrupting viral envelopes by pore formation,6 and downmodulating the CXCR4 coreceptor on the host cell membrane.7 In the oral cavity, hBDs are concentrated as high as 100 μg/ml8 and may therefore aid in protecting against HIV invasion.9 This has also been suggested by the finding that in a monolayer cell culture of normal human oral keratinocytes both X4- and R5-tropic HIV-1 markedly increased hBD-2 and -3 mRNA expression.3 However, the relevance of this observation has not been confirmed in fully differentiated oral epithelium, which corresponds better to the conditions present in vivo. Thus, we sought to determine if the effects of HIV-1 on oral epithelial monolayer culture can indeed be extrapolated to differentiated multilayer oral epithelium. In these experiments, we employed rigorous control conditions to identify confounding effects by factors other than HIV-1.
Materials and Methods
Viral preparation
X4-tropic HIV-1Lai (NIH AIDS Reagent Program) or R5-tropic HIV-1386D (a primary HIV-1 strain) were grown in pooled phytohemagglutinin (PHA)-stimulated peripheral blood lymphoblasts (PBLB), cultured as previously described in RPMI 1640, supplemented with 20% fetal bovine serum (Gemini, Calabasas, CA) and 0.1% interleukin 2 (R-20).10 HIV-1 p24 antigen concentration and TCID50 (50% tissue culture infectious dose) were determined as previously described.11 HIV-1Lai and HIV-1386D stocks contained 2 × 106 pg/ml and 1 × 106 pg/ml Gag p24, which was equivalent to TCID50 titers of 15,800 U/ml and 6460 U/ml, respectively. For confocal microscopy, a molecular clone of R5-tropic HIV-1JR-CSF was fluorescent tagged by incorporation of a fusion protein of green fluorescent protein (GFP) and viral protein R (Vpr) into the viral core.12,13
Oral tissue model
Multilayered, organotypic epithelial tissue, cultured from oral keratinocytes derived from the gingival margin, was used (EpiOral, MatTek Corporation, Ashland, MA). Upon arrival, tissues were placed at the air–liquid interface in six-well plates with 5 ml serum-free medium provided by MatTek Corp (MatTek Corporation Media, MCM). Tissues were rested for 24 h in a 37°C incubator, and cultured either for 24 or 48 h with or without stimulants. Stimulants included HIV-1Lai or HIV-1386D (p24 antigen 1 × 103–6 × 106 pg/ml), added either on the apical or basal surfaces of the tissues. Apical viruses were added in a total volume of 20 μl, which completely covered the epithelial surface. For basal surfaces, the same virus concentration was added directly to the MCM. Tumor necrosis factor alpha (TNF-α) (Cell Sciences Inc., Norwood, MA) (100 ng/ml) served as a positive control for hBD-2 expression.7 Tissues left unstimulated, stimulated with R-20, stimulated with the supernatant of uninfected PBLB, or stimulated with R-20 plus supernatant of uninfected PBLB served as negative controls.
Oral monolayer model
Healthy gingiva was obtained from the area overlying the impacted third molar teeth of adult humans. Procedures were approved by the Institutional Review Board of the University of Washington. The gingival tissue samples were processed as previously described.14 Primary gingival epithelial cells were grown through two passages and frozen in 500,000-cell aliquots. For each experiment, these cells were thawed and grown to 80% confluence in serum-free keratinocyte growth medium (KGM) containing bronchial epithelial growth media supplements (Cambrex Corp., East Rutherford NJ) with 0.03 mM Ca2+, then shifted to 0.15 mM Ca2+ medium 24 h prior to stimulation. The epithelial monolayers were exposed to the same dosages of virus as the organotypic tissue model and cultured for either 24 or 48 h. TNF-α (100 ng/ml) was used as positive control. Unstimulated cells, as well as cells exposed to R-20, supernatant of uninfected PBLB, or R-20 plus supernatant of uninfected PBLB, served as negative controls.
cDNA preparation and real time PCR
Total RNA was extracted from oral tissues and keratinocytes using the RNeasy Mini Kit (Qiagen Inc., Valencia, CA). Reverse transcription was performed with 1 μg of total RNA using the RETROscript kit (Ambion, Inc., Austin TX). cDNA was analyzed with the iCycler system (Bio-Rad, Hercules, CA) for quantitative real-time PCR using the Brilliant SYBR Green QPCR Master Mix (Stratagene, La Jolla, CA). Reactions were set up in duplicate, with each reaction containing 25 μl of the SYBR green mixture, 5 μl of cDNA, and 250 nM of forward and reverse primers. The amplification conditions were denaturation at 95°C for 15 s, annealing at 65°C for 15 s, and elongation at 72°C for 30 s. Primers used were hBD-2 (5′) CCA GCC ATC AGC CAT GAG GGT and hBD-2 (3′) GGA GCC CTT TCT GAA TCC GCA; RPO (ribosomal phoshoprotein) (5′) CCA GCC ATC AGC CAT GAG GGT and RPO (3′) GCC TTG ACC TTT TCA GCA AG. Microarray analysis for interferon regulatory factor 1 (IRF1), interleukin 1 beta (IL-1β), chemokine (C-C motif ) ligand 5 (CCL5), and secretory leukocyte protease inhibitor (SLPI) was performed using the Oligo GEArray® Human HIV Infection & Host Response Microarray (OHS-051; Superarray, Frederick, MD). In initial experiments, amplification efficiency was determined for all primer pairs by quantitative real-time PCR. Results were normalized to the ribosomal phosphoprotein (RPO) gene (housekeeping gene). Results are expressed as the relative fold increase of the stimulated samples over the controls.15
Confocal microscopy
Organotypic oral tissues were rested for 24 h in a 37°C incubator after arrival and exposed to GFP-Vpr labeled HIV-1JR-CSF (20 μl, 1.22 μg total p24 antigen) on the apical surface for 24 h. The tissues were washed three times in PBS, harvested, cut into 3 × 5-mm pieces, and sequentially washed with 100 μl of washing solution (PBS, and PBS containing 0.1% Triton X-100), followed by a 20 min preincubation with PBS containing 1% bovine serum albumin (BSA). The tissues were stained with phalloidin Alexa Fluor 568 for actin filaments and TOPRO-3 (1 μM) for nuclei (Molecular Probes, Eugene, OR), embedded in Mowiol 40–88 containing 2.5% w/v DABCO (Aldrich, Milwaukee, WI), visualized with a Leica TCS SP spectral confocal microscope and analyzed using Imaris software (Bitplane AG, Zurich, Switzerland).
Statistical methods
Differences of hBD-2 mRNA fold increase in HIV-1 plus/minus TNF-α-treated tissues were evaluated by unpaired two-sided t testing between the lowest HIV dose and the two higher HIV doses.
Results
Expression of hBD-2 in response to HIV-1 in differentiated oral epithelium
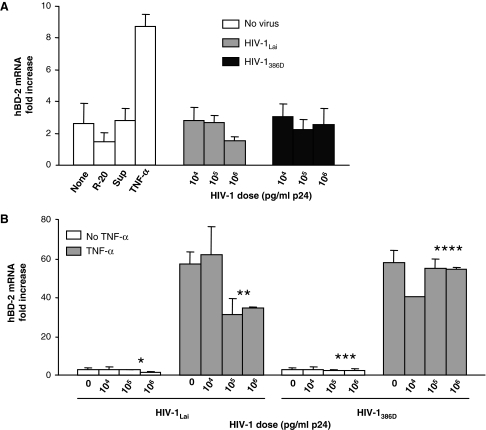
To determine if HIV-1 stimulates hBD-2 expression in oral epithelium, different concentrations of cell-free HIV-1 in culture media were added to the apical surface of the oral tissue (Fig. 1A). After 24 h exposure, neither X4- nor R5-tropic HIV-1 stimulated hBD-2 mRNA expression above the increase observed without stimulation, observed after treatment with supernatants from mock-infected PBLB, or observed after treatment with R-20. No dose dependency of HIV-1-induced hBD-2 mRNA expression was noted other than that the highest dose of HIV-1Lai decreased hBD-2 stimulation in comparison to the PBLB supernatant alone. TNF-α induced hBD-2 mRNA expression approximately 9-fold. Similar but overall weaker responses were observed after 48 h of culture (not shown).
FIG. 1.
Effect of HIV-1 on hBD-2 mRNA expression in an organotypic tissue culture of the oral mucosa. (A) Expression of hBD-2 mRNA in response to R5- and X4-tropic HIV-1. Viruses or stimulants were added to the apical side of the epithelial tissue. All tissue inserts were placed on culture media from MatTek Corporation. (B) Effect of HIV-1 on TNF-α-induced hBD-2 mRNA expression. TNF-α was added to the basal side at a concentration of 100 ng/ml. Viruses were added to the apical side of the tissue. Y axes: Fold-increase of hBD-2 mRNA expression at 24 h postexposure over baseline at 0 h (mean ± SEM, n = 2 or 3 separate cultures). None, no stimulant added on the apical surface; R-20, RPMI plus 20% fetal bovine serum; Sup, supernatant of mock-infected PHA-stimulated lymphoblasts; TNF-α, tumor necrosis factor alpha; HIV-1Lai, laboratory-adapted X4-tropic HIV-1 strain; HIV-1386D, primary R5-tropic HIV-1 strain. p values between the lowest HIV-1 dose and two higher doses were 0.49 (*), 0.05 (**), 0.57 (***), and 0.28 (****).
Because TNF-α was such a strong stimulator of hBD-2 expression, whereas HIV-1 had no or possibly an inhibitory effect, we were interested in determining whether HIV-1 mitigates the proinflammatory effect of TNF-α. To test this, we simultaneously added the virus to the apical surface and TNF-α to the basal side of the tissue. TNF-α was added to the basal side because preliminary experiments had shown that its effect was stronger when added to the basal than to the apical surface of the tissue (not shown). X4-tropic HIV-1Lai at the higher two doses inhibited the stimulatory effect of TNF-α, whereas the lowest dose had no such effect (Fig. 1B; p = 0.05). R5-tropic HIV-1386D did not significantly suppress the stimulatory effect of TNF-α (p = 0.28).
In summary, HIV-1 harvested from infected PBLB culture supernatants did not induce hBD-2 mRNA expression more than supernatants derived from mock-infected PBLB in our oral tissue model. In addition, higher doses of X4-tropic HIV-1Lai attenuate the stimulation of hBD-2 mRNA expression by TNF-α (Fig. 1B).
Expression of hBD-2 in response to HIV-1 in oral monolayer cell culture
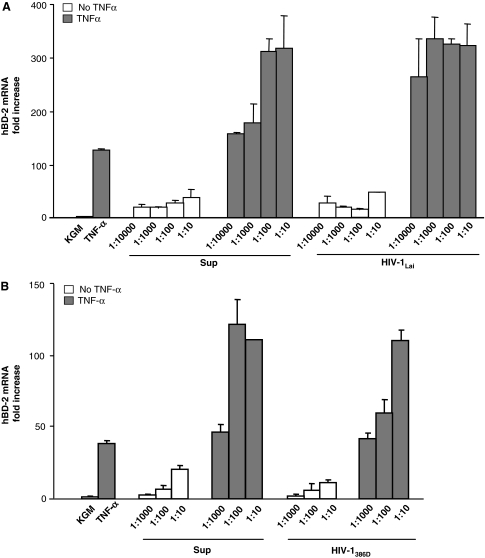
In light of a previous study that reported a stimulatory effect of HIV-1 on hBD-2 expression in monolayer cultures of oral epithelial cells,3 we were surprised by the failure of HIV-1 to upregulate hBD-2 in our organotypic tissue culture. To determine if epithelial monolayer cultures exhibit a different hBD-2 response to HIV-1 than differentiated squamous epithelium, we reevaluated the effect of HIV-1 on hBD-2 production in monolayers of gingival epithelial cells (Fig. 2). We exposed primary gingival epithelial cells from three different donors to either X4- or R5-tropic HIV-1. hBD-2 expression was not more strongly upregulated by X4-tropic HIV-1Lai (Fig. 2A) or R5-tropic HIV-1386D (Fig. 2B) than by supernatant of mock-infected PBLB. In contrast, upregulation of hBD-2 in response to TNF-α (Fig. 2) was much stronger, confirming the potential of gingival epithelial cells to express high levels of this innate immune factor. The stimulatory effects on hBD-2 expression of supernatants from mock-infected PBLB and of TNF-α were dose dependent (Fig. 2). We conclude that compared to mock controls, HIV-1 failed to stimulate increased hBD-2 mRNA expression in either organotypic oral tissue or primary oral epithelial monolayer cultures.
FIG. 2.
Effect of HIV-1 concentration on hBD-2 mRNA expression in a monolayer cell culture in the presence or absence of TNF-α (100 ng/ml). (A) Expression of hBD-2 mRNA in response to TNF-α and X4-tropic HIV-1Lai. (B) Expression of hBD-2 mRNA in response to TNF-α and R5-tropic HIV-1386D. Y axes: Fold-increase of hBD-2 mRNA expression at 24 h postexposure over baseline at 0 h (mean ± SEM, n = 3 independent experiments with cells from three donors). KGM, keratinocyte growth medium; Sup, supernatant of mock-infected PHA-stimulated lymphoblasts. The 1:10 dilution in (A) and (B) corresponded to 2 × 105 pg/ml p24 X4-tropic HIV-1Lai and 1 × 105 pg/ml p24 R5-tropic HIV-1386D, respectively.
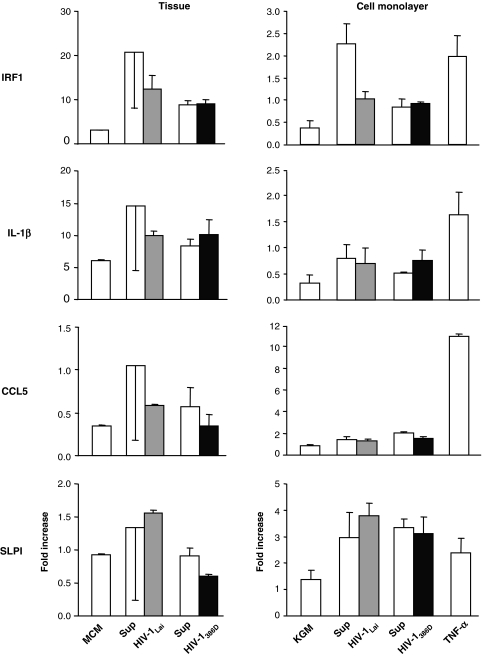
Effect of HIV-1 on a panel of five additional innate immune factors
Because we could not detect a specific stimulatory effect of HIV-1 on hBD-2 mRNA expression by oral epithelia, we wanted to know if HIV-1 triggered the production of other innate immune factors. We chose four factors based on their positive baseline expression in a preliminary microarray screening (not shown) and on their known involvement in innate immunity; IRF1, a member of the interferon regulatory transcription factor family;16 IL-1β, a central mediator of the early inflammatory response;17 CCL5, a chemotactic for T cells and other leukocytes;18 and SLPI, a secreted inhibitor that protects epithelial tissues from proteolytic enzymes and also has antimicrobial properties.19 We first investigated the effect of HIV-1 on these genes in our organotypic oral tissue model. Expression of the different genes was markedly upregulated by supernatants from mock-infected PBLB. However, no further increase was noted in response to the addition of either X4- or R5-tropic HIV-1 (Fig. 3, left panel). In fact, HIV-1Lai exhibited a trend to downregulate three of the four genes tested when compared to supernatants from mocked-infected PBLB (Fig. 3, left panel), although this was not statistically significant.
FIG. 3.
Effect of HIV-1 on the mRNA expression of different immune markers in organotypic tissue culture of the oral epithelium (left panel) and oral epithelial monolayer culture (right panel). Y axes: Fold increase of gene expression at 24 h postexposure over baseline at 0 h (mean ± SEM, n = 3). IRF1, interferon regulatory factor 1; IL-1β, interleukin-1beta; CCL5, chemokine (C-C motif ) ligand 5; SLPI, secretory leukocyte protease inhibitor; MCM, MatTek Corporation Media; Sup, supernatant of mock-infected PHA-stimulated lymphoblasts; HIV-1Lai, laboratory-adapted X4-tropic HIV-1 strain; HIV-1386D, primary R5-tropic HIV-1 strain; KGM, keratinocyte growth medium; TNF-α, tumor necrosis factor alpha. Tissues were exposed by adding the stimulants to the basal surface. Viral dosages were 2 × 105 pg/ml p24 for HIV-1Lai and 1 × 105 pg/ml p24 for HIV-1386D, and the TNF-α dosage was 100 ng/ml.
Next, we analyzed the effect of HIV-1 on the expression of these genes in primary oral epithelial monolayer cultures derived from three separate donors. As in the organotypic tissue, both X4- and R5-tropic HIV-1 failed to increase gene expression in comparison to supernatants from mocked-infected PBLB (Fig. 3, right panel). Thus, in both organotypic oral tissue and primary oral epithelial cells, HIV-1 did not upregulate these innate immune factors more than matched control supernatants.
Penetration of HIV-1 into the organotypic oral epithelium
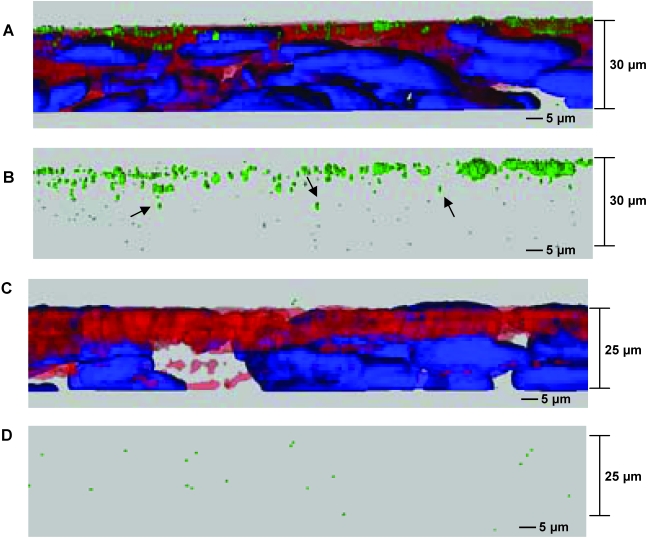
To evaluate how deeply HIV-1 penetrates the organotypic oral epithelium, we added GFP-Vpr tagged HIV-1JR-CSF to the apical surface of the tissue. By confocal microscopy, large amounts of green fluorescent virions attached to the outer surface of the epithelium and some virions clearly penetrated into the deeper tissue layers from the apical surface 24 h postexposure (Fig. 4).
FIG. 4.
Visualization of HIV-1 binding and penetration into oral epithelial tissue. Oral epithelial tissue was incubated with GFP-Vpr containing HIV-1JR-CSF for 24 h and then washed and fixed. HIV-1JR-CSF virions, green; actin, red; nuclei, blue. Arrows indicate representative virions that have penetrated beneath the epithelial surface. (A) A 30-μm cross-section showing virion binding to the apical surface and penetrating the oral epithelial tissue. Penetration of virions was detected several layers deep from the apical surface. (B) Red and blue are rendered transparent so that the virions within the tissue can be seen. The deeper the scanning, the fewer virions were present. (C, D) Unexposed tissue control. Images are representative of two binding experiments.
Discussion
Elucidating the mechanisms of natural protection against HIV-1 transmission across the oral mucosa may reveal clues about how HIV-1 infection could be prevented at other mucosal sites of exposure such as the vagina and the rectum. Human beta defensins are innate antimicrobial peptides with activity against HIV-13 and are produced in the oral cavity.5 An earlier study found that HIV-1 induced hBD-2 and hBD-3 mRNA expression in primary oral epithelial cells cultured as undifferentiated monolayers.3 It was thus concluded that the oral mucosa increases its production of beta defensins in response to HIV-1 and that this capacity constitutes a mechanism to prevent the virus from establishing infection. This notion is impossible to prove directly in vivo in humans, but as an alternative, the relevance of findings obtained with cultured monolayer cells could be confirmed in either animal models or by using organotypic tissue cultures that more closely mimic the complete oral mucosa found in vivo.
For our study, we used a multilayered differentiated oral epithelium to determine if HIV-1 indeed induces the expression of hBD-2 mRNA. Treatment with TNF-α demonstrated that the oral epithelium was capable of strongly increasing hBD-2 mRNA expression in response to an inflammatory stimulus. However, induction of hBD-2 by both R5- and X-4-tropic HIV-1, derived from infected PHA-activated lymphoblasts, was significantly less and did not reach levels above those observed after treatment with control supernatant from mock-infected lymphoblasts. These results indicated that HIV per se was not a stimulant of hBD-2 de novo production. Because our results differed from what we had expected to find based on the observations of other authors with oral monolayer cells,3 we repeated our experiments using monolayer cell culture of oral epithelial cells. Our findings in the oral monolayer system were similar to those in the multilayered differentiated oral epithelium. The monolayer cells reacted strongly to TNF-α treatment, but stimulation of hBD-2 expression by HIV-1 was much weaker and did not exceed that obtained with supernatant of mock-infected PHA-activated lymphoblasts.
Finally, we tested the effect of HIV-1 on five additional markers of innate immunity. Similar to hBD-2, stimulation of these markers by HIV-1 did not exceed that observed by treatment with supernatant from mock-infected lymphoblasts in both the oral monolayers and the organotypic oral epithelium. Notably, in the organotypic epithelium, the X4-tropic HIV-1Lai showed a trend to reduce the expression of three of these markers in comparison to the mock supernatant (Fig. 3). At higher dosages of the X4-tropic virus, this inhibitory effect was also observed for hBD-2 expression in the tissue (Fig. 1). We conclude that HIV-1 not only failed to upregulate hBD-2 and other innate factors above levels observed after treatment with mock controls, but that the X4-tropic HIV-1Lai variant also had an inhibitory effect on the innate immune response in the oral mucosa.
The discrepancy between the findings by Quiñones-Mateu et al.3 and ours may have been due to differences in the experimental conditions. In the earlier study, monolayers of oral epithelial cells were exposed to various strains of HIV-1 at a multiplicity of infection (MOI) of 0.01 units/cell, while the highest MOIs used in our studies were equivalent to an estimated 0.003 units/cell for X4-tropic HIV-1 and 0.001 units/cell for R5-tropic HIV-1. Thus, viral dosage may have played a role in obtaining different findings, although our viral concentrations were still high compared to what is likely to be encountered in vivo. Importantly, Quiñones-Mateu et al.3 used supernatants from uninfected peripheral blood mononuclear cells (PBMCs) as mock controls, which did not induce either hBD-2 or hBD-3 mRNA expression. In our study, we used supernatants from mock-infected PHA-stimulated lymphoblasts as controls, rather than from uninfected PBMCs. Because HIV-1 virions are commonly produced from lymphocytes that are artificially activated in vitro, for example with PHA, the viral stock solutions are apt to contain a range of immune factors secreted into the cell culture supernatants along with the virus.20 We therefore believe that matched supernatants derived from uninfected lymphoblasts are the proper control to use for these experiments.
In our study, we observed greater upregulation of hBD-2 expression after adding TNF-α to the basal side rather than the apical side of the organotypic oral tissue. This suggests that basal cells, which are less differentiated, are more responsive to stimulants than fully differentiated apical cells. However, the response to apically applied TNF-α was still markedly stronger than to apical HIV-1. TNF-α may diffuse better through the tissue layers than HIV-1 virions and thus the weak response to HIV-1 could be due to poor penetration of the virus into the epithelium. However, by using confocal microscopy, we showed that HIV-1 penetrated into the oral tissue up to several layers deep from the apical surface. Penetration of HIV-1 virions into stratified mucosal epithelium has also been observed by other authors.21,22 Thus, the minimal innate immune response of the differentiated oral epithelium to HIV-1 in our study does not appear to be due to an inability of HIV-1 to penetrate the intact epithelial barrier.
Although our study does not implicate HIV-induced production of innate immune factors, in particular hBD-2, as a major mechanism of protection against oral transmission, this by no means rules out constitutive expression of these factors as a contributor to the oral defense against HIV. Moreover, intraepithelial Langerhans cells or infiltrating lymphocytes are lacking in our tissue model but are usually present in vivo, and thus the participation of these cells in the innate immune response could not be determined in our studies. Defensins have been found as constituents of oral secretions5,8 and they clearly possess anti-HIV properties at doses that are compatible with or below those measured in the oral cavity.3,23 Based on our finding that supernatants of PHA-activated but HIV-uninfected lymphoblasts induced hBD-2 expression, we speculate that any potential trigger of lymphocyte activation will also indirectly increase innate epithelial cell defenses. These triggers could be delivered by oral bacteria, contact with food or other environmental antigens, and exposure to semen or vaginal secretions. Moreover, innate defenses in oral epithelial cells could be directly activated by interaction of pathogen-associated molecular patterns in bacteria and viruses with extracellular and intracellular pathogen recognition receptor (PRR) proteins, for example, toll-like receptors.24 Because the oral mucosa endures a constant exposure to commensal and environmental microbes,5 this may result in what appears to be “constitutive” expression of innate immune factors.
Our finding that X4-tropic HIV-1 at higher doses diminished the induction of hBD-2 and other innate immune factor expression underscores the notion that the constitutive presence of these factors provides a potential barrier for HIV-1 that the virus seeks to counteract. However, not every virus variant seems to be capable of this, as our R5-tropic strain even at higher concentrations did not counteract hBD-2 stimulation. Thus, R5-tropic viruses may encounter constitutive innate immune responses in the oral cavity that they cannot effectively overcome, thus lowering the chance for oral transmission. In contrast, X4-tropic variants may be able to better counteract defensin production in the oral cavity. However, during early HIV-1 infection in vivo X4-tropic viruses, for still unclear reasons, are not able to gain a systemic foothold as easily as R5-tropic HIV-1 variants.25
In conclusion, although HIV-1 virions clearly penetrated the differentiated oral tissue in our model, the virus did not stimulate hBD-2 expression beyond that observed after treatment with supernatant from uninfected PHA-activated lymphoblasts. Yet, general hBD-2 expression levels were relatively high in all control setups, indicating constitutive expression of innate immune factors in the oral mucosa. Thus, defensins and other innate factors may contribute to protection against HIV-1 infection in the oral mucosa, but are not particularly inducible by HIV-1. On the contrary, it appears that some virus variants, such as in this study X4-tropic HIV-1Lai, may counteract hBD-2 production, but how this is achieved and whether it increases HIV-1 transmissibility in vivo remain to be determined.
Acknowledgments
This study was supported by NIH Grants R01-DE-14827, R01-DE-13573, R01-HD-51455, P01 HD-40540, K08-AI-51980, and R44-DE-013277, and the University of Washington Center for AIDS Research (CFAR) AI-27757. The oral tissue model was provided by MatTek Corporation (Ashland, MA). W.N. was a scholar in the International AIDS Research and Training Program (IARTP) at the University of Washington, which is supported by the Fogarty International Center (NIH Grant D43-TW00007). We thank Janet Kimball, Beth Hacker, Polachai Sakchalathorn, Lamar Ballweber, and Joan Dragavon for technical assistance.
Disclosure Statement
Mitchell Klausner is associated with MatTek Corporation, which provided the oral tissues for this study free of charge.
References
- 1.Cohen MS. Shugars DC. Fiscus SA. Limits on oral transmission of HIV-1. Lancet. 2000;356(9226):272. doi: 10.1016/S0140-6736(00)02500-9. [DOI] [PubMed] [Google Scholar]
- 2.Shugars DC. Sweet SP. Malamud D. Kazmi SH. Page-Shafer K. Challacombe SJ. Saliva and inhibition of HIV infection: Molecular mechanisms. Oral Dis. 2002;8(Suppl. 2):169–175. doi: 10.1034/j.1601-0825.8.s2.7.x. [DOI] [PubMed] [Google Scholar]
- 3.Quiñones-Mateu ME. Lederman MM. Feng Z, et al. Human epithelial β-defensins 2 and 3 inhibit HIV-1 replication. AIDS. 2003;17(16):F39–F48. doi: 10.1097/00002030-200311070-00001. [DOI] [PubMed] [Google Scholar]
- 4.Shugars DC. Endogenous mucosal antiviral factors of the oral cavity. J Infect Dis. 1999;179(Suppl. 3):S431–S435. doi: 10.1086/314799. [DOI] [PubMed] [Google Scholar]
- 5.Dale BA. Fredericks LP. Antimicrobial peptides in the oral environment: Expression and function in health and disease. Curr Issues Mol Biol. 2005;7(2):119–133. doi: 10.1093/jac/dki103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klotman ME. Chang TL. Defensins in innate antiviral immunity. Nat Rev Immunol. 2006;6(6):447–456. doi: 10.1038/nri1860. [DOI] [PubMed] [Google Scholar]
- 7.Feng Z. Dubyak GR. Lederman MM. Weinberg A. Cutting edge: Human beta defensin 3–a novel antagonist of the HIV-1 coreceptor CXCR4. J Immunol. 2006;177(2):782–786. doi: 10.4049/jimmunol.177.2.782. [DOI] [PubMed] [Google Scholar]
- 8.Shi J. Zhang G. Wu H. Ross C. Blecha F. Ganz T. Porcine epithelial β-defensin 1 is expressed in the dorsal tongue at antimicrobial concentrations. Infect Immun. 1999;67(6):3121–3127. doi: 10.1128/iai.67.6.3121-3127.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kimball JR. Nittayananta W. Klausner M. Chung WO. Dale BA. Antimicrobial barrier of an in vitro oral epithelial model. Arch Oral Biol. 2006;51(9):775–783. doi: 10.1016/j.archoralbio.2006.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coombs RW. Collier AC. Allain JP, et al. Plasma viremia in human immunodeficiency virus infection. N Engl J Med. 1989;321(24):1626–1631. doi: 10.1056/NEJM198912143212402. [DOI] [PubMed] [Google Scholar]
- 11.Grandadam M. Cesbron JY. Candotti D, et al. Dose-dependent systemic human immunodeficiency virus infection of SCID-hu mice after intraperitoneal virus injection. Res Virol. 1995;146(2):101–112. doi: 10.1016/0923-2516(96)81079-x. [DOI] [PubMed] [Google Scholar]
- 12.Hladik F. Sakchalathorn P. Ballweber L, et al. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity. 2007;26(2):257–270. doi: 10.1016/j.immuni.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDonald D. Vodicka MA. Lucero G, et al. Visualization of the intracellular behavior of HIV in living cells. J Cell Biol. 2002;159(3):441–452. doi: 10.1083/jcb.200203150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krisanaprakornkit S. Weinberg A. Perez CN. Dale BA. Expression of the peptide antibiotic human beta-defensin 1 in cultured gingival epithelial cells and gingival tissue. Infect Immun. 1998;66(9):4222–4228. doi: 10.1128/iai.66.9.4222-4228.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyamoto M. Fujita T. Kimura Y, et al. Regulated expression of a gene encoding a nuclear factor, IRF-1, that specifically binds to IFN-beta gene regulatory elements. Cell. 1988;54(6):903–913. doi: 10.1016/s0092-8674(88)91307-4. [DOI] [PubMed] [Google Scholar]
- 17.Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 2002;13(4–5):323–340. doi: 10.1016/s1359-6101(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 18.Ward SG. Bacon K. Westwick J. Chemokines and T lymphocytes: More than an attraction. Immunity. 1998;9(1):1–11. doi: 10.1016/s1074-7613(00)80583-x. [DOI] [PubMed] [Google Scholar]
- 19.Abe T. Kobayashi N. Yoshimura K, et al. Expression of the secretory leukoprotease inhibitor gene in epithelial cells. J Clin Invest. 1991;87(6):2207–2215. doi: 10.1172/JCI115255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ganz T. Defensins: Antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3(9):710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 21.Maher D. Wu X. Schacker T. Horbul J. Southern P. HIV binding, penetration, and primary infection in human cervicovaginal tissue. Proc Natl Acad Sci USA. 2005;102(32):11504–11509. doi: 10.1073/pnas.0500848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCoombe S. Trull A. McRaven M, et al. Early interactions of fluorescently labeled HIV with the female genital tract. 15th Conference on Retroviruses and Opportunistic Infection; February 8–11; Boston, MA. 2008. [Abstract no. 97]. [Google Scholar]
- 23.Sun L. Finnegan CM. Kish-Catalone T, et al. Human β-defensins suppress human immunodeficiency virus infection: Potential role in mucosal protection. J Virol. 2005;79(22):14318–14329. doi: 10.1128/JVI.79.22.14318-14329.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uehara A. Takada H. Synergism between TLRs and NOD1/2 in oral epithelial cells. J Dent Res. 2008;87(7):682–686. doi: 10.1177/154405910808700709. [DOI] [PubMed] [Google Scholar]
- 25.Margolis L. Shattock R. Selective transmission of CCR5-utilizing HIV-1: The 'gatekeeper' problem resolved? Nat Rev Microbiol. 2006;4(4):312–317. doi: 10.1038/nrmicro1387. [DOI] [PubMed] [Google Scholar]