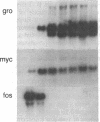
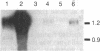
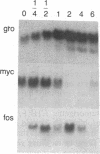
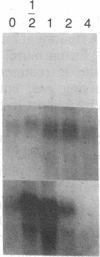
Abstract
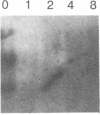
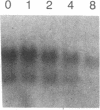
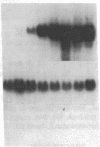
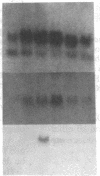
Previous studies of gro and related genes that are overexpressed in transformed fibroblasts suggest that gro may encode a specific growth regulator. However, DNA and protein sequence comparisons reveal relatedness to platelet factor 4 and other proteins involved in the inflammatory response. In this paper, both growth-related and cytokine-induced responses in gro gene expression are described. Human foreskin fibroblasts are shown to express approximately 10-fold elevated gro, myc, and fos mRNAs in response to serum and to phorbol 12-myristate 13-acetate stimulation, with early response kinetics indicative of growth regulation. In response to interleukin 1, however, in growing cells gro mRNA is elevated at least 100-fold but myc remains constant and fos is not expressed, suggesting a second regulatory pathway. In normal cultured mammary epithelial cells, gro is constitutively expressed, and elevated mRNA levels are induced by phorbol 12-myristate 13-acetate, but not by interleukin 1. However, most carcinoma cell lines examined do not express gro mRNA, suggesting a third function of gro as a negative growth regulator in epithelial cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almendral J. M., Sommer D., Macdonald-Bravo H., Burckhardt J., Perera J., Bravo R. Complexity of the early genetic response to growth factors in mouse fibroblasts. Mol Cell Biol. 1988 May;8(5):2140–2148. doi: 10.1128/mcb.8.5.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisowicz A., Bardwell L., Sager R. Constitutive overexpression of a growth-regulated gene in transformed Chinese hamster and human cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7188–7192. doi: 10.1073/pnas.84.20.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battey J., Moulding C., Taub R., Murphy W., Stewart T., Potter H., Lenoir G., Leder P. The human c-myc oncogene: structural consequences of translocation into the IgH locus in Burkitt lymphoma. Cell. 1983 Oct;34(3):779–787. doi: 10.1016/0092-8674(83)90534-2. [DOI] [PubMed] [Google Scholar]
- Bedard P. A., Alcorta D., Simmons D. L., Luk K. C., Erikson R. L. Constitutive expression of a gene encoding a polypeptide homologous to biologically active human platelet protein in Rous sarcoma virus-transformed fibroblasts. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6715–6719. doi: 10.1073/pnas.84.19.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg G. S., Pepper D. S., Chesterman C. N., Morgan F. J. Complete covalent structure of human beta-thromboglobulin. Biochemistry. 1978 May 2;17(9):1739–1744. doi: 10.1021/bi00602a024. [DOI] [PubMed] [Google Scholar]
- Castor C. W., Miller J. W., Walz D. A. Structural and biological characteristics of connective tissue activating peptide (CTAP-III), a major human platelet-derived growth factor. Proc Natl Acad Sci U S A. 1983 Feb;80(3):765–769. doi: 10.1073/pnas.80.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler M. E., Yunis J. J. A high resolution in situ hybridization technique for the direct visualization of labeled G-banded early metaphase and prophase chromosomes. Cytogenet Cell Genet. 1978;22(1-6):352–356. doi: 10.1159/000130970. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Cochran B. H., Reffel A. C., Stiles C. D. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell. 1983 Jul;33(3):939–947. doi: 10.1016/0092-8674(83)90037-5. [DOI] [PubMed] [Google Scholar]
- Curran T., Peters G., Van Beveren C., Teich N. M., Verma I. M. FBJ murine osteosarcoma virus: identification and molecular cloning of biologically active proviral DNA. J Virol. 1982 Nov;44(2):674–682. doi: 10.1128/jvi.44.2.674-682.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuel T. F., Keim P. S., Farmer M., Heinrikson R. L. Amino acid sequence of human platelet factor 4. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2256–2258. doi: 10.1073/pnas.74.6.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Biology of interleukin 1. FASEB J. 1988 Feb;2(2):108–115. [PubMed] [Google Scholar]
- Goldring M. B., Krane S. M. Modulation by recombinant interleukin 1 of synthesis of types I and III collagens and associated procollagen mRNA levels in cultured human cells. J Biol Chem. 1987 Dec 5;262(34):16724–16729. [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Griffin C. A., Emanuel B. S., LaRocco P., Schwartz E., Poncz M. Human platelet factor 4 gene is mapped to 4q12----q21. Cytogenet Cell Genet. 1987;45(2):67–69. doi: 10.1159/000132431. [DOI] [PubMed] [Google Scholar]
- Harper M. E., Saunders G. F. Localization of single copy DNA sequences of G-banded human chromosomes by in situ hybridization. Chromosoma. 1981;83(3):431–439. doi: 10.1007/BF00327364. [DOI] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Knudson A. G., Jr Hereditary cancer, oncogenes, and antioncogenes. Cancer Res. 1985 Apr;45(4):1437–1443. [PubMed] [Google Scholar]
- Kozma S. C., Bogaard M. E., Buser K., Saurer S. M., Bos J. L., Groner B., Hynes N. E. The human c-Kirsten ras gene is activated by a novel mutation in codon 13 in the breast carcinoma cell line MDA-MB231. Nucleic Acids Res. 1987 Aug 11;15(15):5963–5971. doi: 10.1093/nar/15.15.5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus M. H., Yuasa Y., Aaronson S. A. A position 12-activated H-ras oncogene in all HS578T mammary carcinosarcoma cells but not normal mammary cells of the same patient. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5384–5388. doi: 10.1073/pnas.81.17.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. X., Vilcek J. Tumor necrosis factor and interleukin-1 cause a rapid and transient stimulation of c-fos and c-myc mRNA levels in human fibroblasts. J Biol Chem. 1987 Sep 5;262(25):11908–11911. [PubMed] [Google Scholar]
- Luster A. D., Unkeless J. C., Ravetch J. V. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature. 1985 Jun 20;315(6021):672–676. doi: 10.1038/315672a0. [DOI] [PubMed] [Google Scholar]
- Poncz M., Surrey S., LaRocco P., Weiss M. J., Rappaport E. F., Conway T. M., Schwartz E. Cloning and characterization of platelet factor 4 cDNA derived from a human erythroleukemic cell line. Blood. 1987 Jan;69(1):219–223. [PubMed] [Google Scholar]
- Richmond A., Balentien E., Thomas H. G., Flaggs G., Barton D. E., Spiess J., Bordoni R., Francke U., Derynck R. Molecular characterization and chromosomal mapping of melanoma growth stimulatory activity, a growth factor structurally related to beta-thromboglobulin. EMBO J. 1988 Jul;7(7):2025–2033. doi: 10.1002/j.1460-2075.1988.tb03042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager R. Genetic suppression of tumor formation: a new frontier in cancer research. Cancer Res. 1986 Apr;46(4 Pt 1):1573–1580. [PubMed] [Google Scholar]
- Schmid J., Weissmann C. Induction of mRNA for a serine protease and a beta-thromboglobulin-like protein in mitogen-stimulated human leukocytes. J Immunol. 1987 Jul 1;139(1):250–256. [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Wakefield L. M., de Crombrugghe B. Some recent advances in the chemistry and biology of transforming growth factor-beta. J Cell Biol. 1987 Sep;105(3):1039–1045. doi: 10.1083/jcb.105.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman G., Sager R. Genetic analysis of tumorigenesis: a conserved region in the human and Chinese hamster genomes contains genetically identified tumor-suppressor genes. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9099–9102. doi: 10.1073/pnas.84.24.9099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson M. L., Goldring M. B., Birkhead J. R., Krane S. M., Rahmsdorf H. J., Angel P. Stimulation of procollagenase synthesis parallels increases in cellular procollagenase mRNA in human articular chondrocytes exposed to recombinant interleukin 1 beta or phorbol ester. Biochem Biophys Res Commun. 1987 Apr 29;144(2):583–590. doi: 10.1016/s0006-291x(87)80006-2. [DOI] [PubMed] [Google Scholar]
- Sugano S., Stoeckle M. Y., Hanafusa H. Transformation by Rous sarcoma virus induces a novel gene with homology to a mitogenic platelet protein. Cell. 1987 May 8;49(3):321–328. doi: 10.1016/0092-8674(87)90284-4. [DOI] [PubMed] [Google Scholar]
- Yoshimura T., Matsushima K., Tanaka S., Robinson E. A., Appella E., Oppenheim J. J., Leonard E. J. Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9233–9237. doi: 10.1073/pnas.84.24.9233. [DOI] [PMC free article] [PubMed] [Google Scholar]