Abstract
Background
Diabetic retinopathy is a microangiopathy of the retina from which nearly all persons with diabetes eventually suffer. Two of its complications threaten the patient’s vision: diabetic macular edema and proliferative diabetic retinopathy.
Methods
Selective literature review, based on national and international guidelines and a literature search from 1981 onward.
Results
Diabetic retinopathy is subdivided into non-proliferative and proliferative retinopathy. Macular edema can arise at any stage of the disease and threatens visual acuity. The main risk factors for the development and progression of diabetic retinopathy are long duration of diabetes and poor control of blood sugar and arterial blood pressure. Laser photocoagulation is an evidence-based treatment for proliferative retinopathy and macular edema. Vitreous surgery is indicated in cases of worsening vision due to a non-clearing vitreous hemorrhage or tractional retinal detachment. The current options for medical treatment involve the intravitreous injection of glucocorticosteroids or of a VEGF antagonist; both of these options are “off label” at present.
Conclusion
Diabetic retinopathy is the leading cause of blindness among persons of working age in the industrialized world. Regular ophthalmological examinations, timely laser therapy depending on the stage of the disease, and close interdisciplinary cooperation are essential to prevent loss of vision.
Medical advances in recent decades have paradoxically led to an increase in the incidence and prevalence of diabetes mellitus and its complications. Increased life expectancy in the industrialized world is one reason why diabetes is now more common; another is the increased prevalence of a sedentary life style and changed eating habits, resulting in overweight. The typical ocular complications range from impaired visual acuity due to diabetic retinopathy and premature cataracts all the way to blindness or loss of an eye. Even though diabetic retinopathy can be treated effectively, it nonetheless remains the most common cause of acquired blindness among persons of working age in the industrialized world. In Germany, about 15 000 people are blind as a complication of diabetes mellitus (1). In 2004, in the German state of Hesse, 2.2% of the diabetic patients covered by statutory health insurance were blind or severely visually impaired, according to the internal data of the insurance carriers. Visual acuity is lost when the site of sharpest vision on the retina is affected by pre- or intraretinal hemorrhage, macular edema, tractional retinal detachment, or loss of capillaries of the peripheral loop network. Blindness can, in fact, be prevented by regular preventive ophthalmological check-ups and timely treatment.
Epidemiology.
Even though it can be effectively treated, diabetic retinopathy remains the most common cause of acquired blindness among persons of working age in the industrialized world
The learning goals of this Continuing Medical Education article are the following:
The reader should appreciate the importance of preventive ophthalmological check-ups. Even when a patient can see well, both subjectively and objectively, diabetic retinopathy may already be present and urgently require treatment. The early detection and treatment of macular edema and proliferative retinopathy are essential for the maintenance of visual acuity.
The reader should become acquainted with the gold standard of ophthalmological treatment, i.e., timely, stage-appropriate laser therapy. Among surgical treatments, vitrectomy is of proven value for certain indications.
The possibilities and limitations of pharmacotherapy should be understood. The current pharmacotherapy of diabetic retinopathy is off label and involves the intravitreous injection of glucocorticoids or vascular endothelial growth factor (VEGF) antagonists.
Pathogenesis
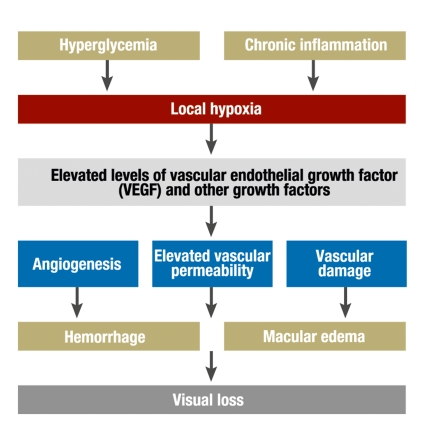
Diabetic retinopathy is a microangiopathy of the retina. It involves changes in the vascular wall and in the rheological properties of the blood. The combination of these factors leads to capillary occlusion and thereby to retinal ischemia and angiographically demonstrable leakage. The typical histopathological changes include loss of pericytes and endothelial cells and thickening of the basilar membrane. Microaneurysms, i.e., sites of outward ballooning of the capillary wall, are pathognomonic (figure 1).
Figure 1.
Schematic flowchart for the pathogenesis of diabetic retinopathy
Pathogenesis.
Diabetic microangiopathy leads to capillary occlusion and thereby to retinal ischemia and elevated vascular permeability, resulting in angiographically demonstrable leakage.
With respect to the rheological properties of the blood, the following factors lead to diminished fibrinolysis and elevated blood viscosity (2):
diminished deformability of erythrocytes,
elevated platelet aggregation,
elevated concentration of fibrinogen and α2-globulin,
reduced serum albumin concentration.
Multiple biochemical signal pathways are involved. The increased activity of protein kinase C and protein glycosylation lead to the formation of advanced glycation end products (AGE). These, in turn, lead to cell interactions involving vascular endothelial growth factor (VEGF) that cause neo-vascularization in the anterior and posterior segments of the eye, increased vascular permeability resulting in leakage, and collapse of the inner blood-retina barrier. AGE are taken exogenously in food and are also formed endogenously in greater quantities because of hypoglycemia; they seem to mediate nearly all complications of diabetes, including the vasoconstriction and inflammatory vessel wall changes that are associated with the formation of atheromatous plaques and influence the functioning of endothelial cells and macrophages. Inflammatory vessel wall changes are the target of the current therapeutic approach employing the intravitreous injection of glucocorticoids (3). Other growth factors that play a role in the pathogenesis of diabetic retinopathy include insulin-like growth factors I and II, transforming growth factor β, and pigment epithelium-derived growth factor.
The classification of diabetic retinopathy
Non-proliferative diabetic retinopathy
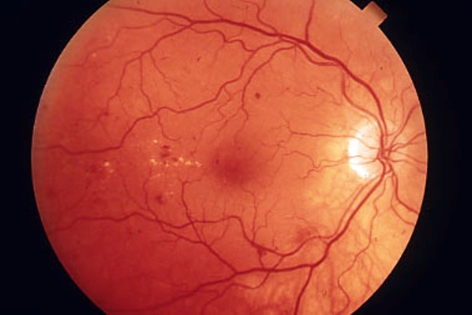
The earliest morphological sign of non-proliferative diabetic retinopathy is the formation of microaneurysms, i.e., outward balloonings of the capillary wall. These are usually initially located on the temporal side of the fovea, and they are usually asymptomatic when they first arise, though they may rupture and give rise to intraretinal punctuate hemorrhages. They are detectable only by ophthalmoscopy (figure 2). Fluorescein angiography reveals leakage, which is a cause of macular edema.
Figure 2.
Mild non-proliferative diabetic retinopathy with blot hemorrages, mainly on the temporal side of the macula, and clinically significant macular edema with hard exudates
The classification of diabetic retinopathy.
Diabetic retinopathy is classified as either proliferative or non-proliferative.
Further signs of non-proliferative diabetic retinopathy of increasing severity, from mild to moderate to severe, include flame shaped and blot hemorrhages, hard exudates, fluctuations of venous caliber (“venous beading”), and intraretinal microvascular anomalies. The latter are dilated telangiectatic capillaries in the area adjacent to capillary occlusions; they are visible under ophthalmoscopy as capillary widening. They are considered a classic sign of ischemia and a predictor of imminent progression to proliferative retinopathy (4). Microinfarcts of the nerve-fiber layer, known as “cotton wool spots,” may indicate poorly controlled arterial hypertension (2).
Proliferative diabetic retinopathy
The mild non-proliferative stage.
Clinically significant macular edema can arise as early as the mild non-proliferative stage and represents as great a threat to visual acuity as proliferative diabetic retinopathy.
As hypoperfusion in the retinal capillary bed becomes more severe and spreads across the retinal area, proliferative diabetic retinopathy develops. As a reaction to ischemia, neovascularization arises at the papilla (neovascularization of the disk, NVD) and on the retina outside the papilla (neovascularization elsewhere, NVE).
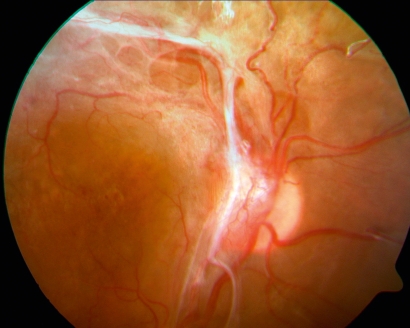
Retinal vascular proliferation can be thought of as a fruitless attempt to compensate for ischemia by forming new vessels at the papilla, on the retina (figure 3), and, finally, on the iris (neovascularization of the iris, NVI) (figure 4). Papillary and retinal neovascularization can lead to epiretinal and subhyaloid vitreous hemorrhages and can become organized in membranes and cords on the retinal surface (figure 5). Contraction of these pathological structures leads, later on, to tractional retinal detachment or tractional macular edema, either of which can cause blindness. The ultimate and most severe complication of diabetic retinopathy is neovascular glaucoma. The newly formed vessels grow from the pupil into the chamber angle, obstructing the outflow of the aqueous humor. Untreated neovascularization glaucoma can lead to painful blindness and shrinking of the eye.
Figure 3.
Severe proliferative diabetic retinopathy with neovascularization on the optic disk and vitreous homorrhages
Figure 4.
Neovascularization on the iridial surface extending from the edge of the pupil to the chamber angle
Figure 5.
Severe proliferative diabetic retinopathy with neovascularization on the optic disk and epiretinal membranes with connective-tissue organization, elevating the retina
Diabetic macular edema
Clinically significant macular edema (definition).
Retinal thickening and/or hard exudates within 500 µm of the fovea, or
a zone of edema that is larger than the optic disk at a distance of one optic disk diameter.
Clinically significant macular edema was precisely defined for the purposes of the Early Treatment of Diabetic Retinopathy (ETDR) Study. It is said to be present when there is retinal thickening and/or hard exudates within 500 µm of the fovea, or when there is a zone of edema that is larger than the papilla at a distance of one papillary diameter (4). In this stage, the patient can still see very well, yet vision is already acutely endangered by diabetic retinopathy. Because the inner blood retinal barrier is defective, fluids, proteins, and lipids leak into the sensory retina. This leakage is stereoscopically visible as retinal thickening and hard exudates (figure 2). The patient becomes aware of a deterioration of vision only when the fovea itself is affected. Fluorescein angiography localizes the site of the leak. The early detection and laser treatment of clinically significant macular edema are essential for the prevention of permanent loss of visual acuity (4).
Ischemic maculopathy is a further aspect of diabetic macular disease. This entity involves occlusion of the capillary network around the fovea. Ischemic maculopathy is not treatable and confers a poor prognosis for visual acuity. It is diagnosed with fluorescein angiography.
Risk factors for the occurrence and progression of diabetic retinopathy
The type of diabetes influences the occurrence and progression of diabetic retinopathy. In persons with inadequately controlled type 1 diabetes, proliferative retinopathy can arise as early as 10 years after diagnosis. In type 2 diabetes, the risk of macular edema is somewhat higher. In the prospective CALDIRET study, 635 persons with type 2 diabetes and mild, non-proliferative diabetic retinopathy were enrolled; after a follow-up interval of up to five years, proliferation was present in only three eyes (5), but clinically significant macular edema was present in 155.
The sex of the patient also plays a role. The overall risk of blindness is nearly twice as high in women, and women are also more likely than men to have diabetes. Thus, according to the statistics of the statutory health carriers in the German state of Hesse for the year 2004, 55% of all diabetics covered by statutory health insurance were women. A study carried out by the Marburg University Department of Ophthalmology revealed that, in 1997 and 1998, 446 women and 233 men with diabetes in the state of Hesse were blind or severely visually impaired (1). Pregnancy can lead to progression of diabetic retinopathy.
The duration of diabetes is the main factor for the development of diabetic retinopathy. In a study of 5596 persons with diabetes in Germany in the years 2002 to 2004, the prevalence of diabetic retinopathy was found to be 17% in persons with type 1 diabetes who had received their diagnosis before age 30 and had been ill for less than 5 years. Among patients who had had diabetes for 15 years or longer, the prevalence of retinopathy was 90% (6). The progression of diabetic retinopathy is also influenced by age-associated changes in Bruch’s membrane and the vitreous body. In contrast, children with diabetes do not develop proliferative retinopathy or any clinically significant degree of macular edema in the first 10 years after their diagnosis (7).
Sex-specific risk.
Women are twice as likely to go blind from diabetic retinopathy. Pregnancy can lead to progression of diabetic retinopathy.
The quality of glycemic control is very important. A randomized study (the Diabetes Control and Complications Trial, DCCT) involving 1441 patients followed up for six and a half years revealed that, for persons with type 1 diabetes, a low HbA1c of 7.2% under intensive insulin therapy or the use of an insulin pump lowered the incidence of diabetic retinopathy by 76%, and it also lowered the incidence of progression of diabetic retinopathy that was already present by 54%. The frequency of proliferative diabetic retinopathy or severe non-proliferative retinopathy was lowered by 47% in comparison to a rigid insulin schedule that yielded a mean HbA1c value of 9.1% (8). In another study (the United Kingdom Prospective Diabetes Study, UKPDS), a relative lowering of the HbA1c by 11% in patients with type 2 diabetes made laser coagulation unnecessary in one-third of patients (9). Both of these studies showed, however, that the rapid optimization of blood sugar values may lead at first to an accelerated progression of diabetic retinopathy. This “early worsening” is now known to reverse itself, and, indeed, the long-term prognosis is better if the blood sugar is immediately brought under good control. The use of insulin analogs has not been found to confer any disadvantage with regard to diabetic retinopathy (10).
Arterial hypertension is also an important factor for the occurrence and progression of diabetic retinopathy. The UKPD Study showed that reduction of arterial blood pressure to a target value of <150/85 mm Hg lowered the rate of progression of diabetic retinopathy by 34% and the necessity of laser coagulation by 35%, and was associated with 47% less loss of visual acuity, in comparison with a target value of <180/105 mm Hg. The mean difference in blood pressures between the two patient groups was only 10 mmHg (systolic) and 5 mmHg (diastolic). Arterial hypertension worsens diabetic macular edema and promotes vascular proliferation. Diabetic nephropathy can lead to a major worsening of macular edema, which is often ascribed to the associated arterial hypertension. In patients with diabetic retinopathy, uncontrolled arterial hypertension can impair the success of properly administered laser treatment. Improvement in this situation depends not only on the ophthalmologist, but also on the internist (2).
Risk factors.
Long duration of diabetes
Poor glycemic control
Arterial hypertension
The regulation of blood flow in the retina and choroid seems to be under the influence of a local renin-angiotensin-aldosterone system (11). This is implied by the result of the prospective, randomized EUCLID study (Eurodiab Controlled Trial of Lisinopril-Dependent Diabetes), in which 530 normotensive persons with insulin-dependent diabetes were enrolled. Patients taking lisinopril had a lower rate of progression of diabetic retinopathy: Their retinopathy worsened by one level (out of a total of five) in 21 of 159 patients, as compared to 39 of 166 patients in the placebo group (12). On the other hand, the UKPD Study found no difference with respect to diabetic retinopathy between patients with type 2 diabetes öätaking atenolol and those taking captopril (13). The effect of an angiotensin II receptor blocker on the incidence of diabetic retinopathy in persons with type 1 diabetes, and on the occurrence and progression of diabetic retinopathy in persons with type 1 or type 2 diabetes, was investigated in the Diabetic Retinopathy Candesartan Trials (DIRECT) (14, 15). Here, too, only normotensive patients were enrolled in the study, in order to exclude the potential effect of a significant reduction of blood pressure. No significant protective effect could be demonstrated. Thus, it remains the case that no definitive medical recommendation can be made with respect to the choice of antihypertensive agent.
Hyperlipidemia is also under discussion as a possible risk factor for the development of diabetic retinopathy. The randomized FIELD (Fenofibrate Intervention and Event Lowering Diabetes) study included 995 persons with type 2 diabetes. A subgroup analysis revealed that the use of fenofibrate was associated with a significant lowering of the rate of progression of diabetic retinopathy and of the need for laser photocoagulation, independently of the blood lipid levels (16). On the other hand, the primary endpoint of this study—a significant reduction of cardiovascular events—was not reached. Thus, internists do not generally recommend fenofibrate, nor is there any clear ophthalmological recommendation for this type of treatment.
Cataract surgery as a risk factor.
A cataract operation that is not preceded by adequate laser treatment of macular edema and proliferative diabetic retinopathy is a major risk factor.
Surgery for premature cataracts in persons with diabetes is a risk factor for proliferative diabetic retinopathy and macular edema (17). Preoperative optimization of glycemic control and blood pressure, and the laser treatment of proliferative diabetic retinopathy and macular edema, are essential in all cases. Furthermore, glucocorticoids or VEGF analogs can be injected into the vitreous body at the end of the cataract operation.
Treatment
Laser photocoagulation
Laser photocoagulation is the evidence-based treatment of diabetic retinopathy and diabetic macular edema. The recommendation for this form of treatment is based on the prospective, randomized, controlled ETDR study, published in 1991, for which a total of 3711 patients were recruited (4). Accordingly, national guidelines have been published in Germany by the Initiative Group for the Early Detection of Diabetic Eye Diseases (Initiativgruppe zur Früherkennung diabetischer Augenerkrankungen, IFdA) and the Working Group on Diabetes and the Eye (Arbeitsgemeinschaft Diabetes und Auge, AGDA).
The laser wavelength of 532 nm is currently generated by a double-frequency neodymium:yttrium-aluminum-garnet (Nd:YAG) laser. The laser is coupled with a split-lamp microscope, and the treatment is delivered with the aid of a contact lens positioned on the cornea. Treatment with a Nd:YAG laser may not be possible in the presence of advanced opacification of the cornea or lens, because of poor visibility and scatter of the therapeutically applied light beam. In such cases, an 810 nm diode laser can be used, or else the cataract can be treated first and the laser treatment delivered within a few days of the cataract operation.
Laser photocoagulation based on the stage of disease.
Timely laser photocoagulation based on the stage of proliferative diabetic retinopathy and clinically significant macular edema is the cornerstone of treatment.
The goal of panretinal laser photocoagulation for proliferative diabetic retinopathy is regression of the newly formed vessels as a consequence of normalization of the partial pressure of oxygen in the peripheral avascular areas of the retina. This, in turn, lowers the risk of vitreous hemorrhage and membrane formation. As many as 2500 laser foci may be needed across the entire surface of the retina; the foci have a diameter of 500 µm and are distributed all over the periphery of the retina, with sparing of its center (figure 6). As early as 1976, the prospective, randomized Diabetic Retinopathy Study (DRS), involving a total of 1732 eyes, showed that this form of treatment lowers the risk of severe visual loss by more than 50%. Severe visual loss occurred in 129 of the treated eyes, but in only 56 of the untreated eyes (18).
Figure 6.
Proliferative diabetic retinopathy after panretinal laser coagulation
Clinically significant diabetic macular edema is treated by the targeted focal laser coagulation of leaking microaneurysms and capillaries in the area around the fovea, with laser foci ranging from 100 to 200 µm in size. The EDTR study, performed in 1985, involved 754 eyes that were treated with focal laser coagulation and 1490 eyes in an untreated control group. The risk of worsening of vision because of significant macular edema was found to be so massively reduced when the first interim evaluation of the study results was carried out at one year that further observation of the control group was held to be unethical, and laser coagulation was immediately offered to the patients in the control group. This treatment remains the gold standard today for patients with clinically significant macular edema that can be diagnosed and treated in timely fashion (4).
The gold standard of timely treatment.
The specific, focal laser coagulation of leaking microaneurysms and capillaries in the area around the fovea with laser foci of 100 to 200 µm is the gold standard for the treatment of clinically significant macular edema.
Laser treatment only rarely improves visual acuity. It is, therefore, very important that the impending loss of vision should be diagnosed by preventive examination, so that laser treatment can be performed to preserve acuity while the eye can still see well. Worsening of visual acuity can be irreversible.
Surgery
The indications for pars plana vitrectomy (PPV) include non-resorbing vitreous hemorrhage, subhyaloid hemorrhage, ghost-cell glaucoma, tractional retinal detachment, and tractional macular edema (19). Pars plana vitrectomy enables the removal of the turbid vitreous body and of scarred cords and membranes, the proper repositioning of the retina, and optimal laser photocoagulation treatment. The benefit of pars plana vitrectomy was confirmed, and its optimal time point determined, in a prospective, randomized, controlled study (the Diabetic Retinopathy Vitrectomy Study, DRVS). Patients undergoing early vitrectomy had significantly better vision than those who underwent the procedure a year later (20).
The main indication for vitrectomy.
The main indication for vitrectomy is a non-resorbing vitreous hemorrhage and tractional retinal detachment.
Thanks to contemporary microsurgical techniques, vitrectomy has become a routine procedure. In recent years, technical refinements have shortened the operative time and obviated the need for suturing. The diameter of the instruments that are currently introduced through trocars has been reduced from 1.0 to 0.6 mm. With vitrectomy, it is now possible to maintain at least rudimentary vision even in patients with advanced proliferative diabetic retinopathy. Painful neovascularization glaucoma is now a very rare event. In such cases, surgical removal of the blind eye may be necessary as a procedure of last resort to eliminate pain.
Pharmacotherapy
Intravitreous glucocorticoids are preferentially used for the treatment of diabetic macular edema. Their anti-angiogenic and anti-inflammatory effects lead to stabilization of the inner blood-retina barrier and are also beneficial against proliferative diabetic retinopathy (3). Their clinical effect against diabetic macular edema is so obvious that the off-label use of triamcinolone acetonide has rapidly become widespread, despite the lack of evidence from clinical trials. Doses ranging from 4 to 25 mg are used (3). One disadvantage is that effect is temporally limited, lasting only three months; thus, repeated injections are needed. Furthermore, secondary glaucoma can develop in one-third of patients. For this reason, dexamethasone is used as an alternative treatment. Secondary cataracts are also common.
VEGF is a mediator of vascular leakage and is thus partly responsible for the collapse of the inner blood retinal barrier. VEGF antagonists that have been found useful in the treatment of wet age-related macular degeneration (AMD) can also inhibit proliferation and leakage in diabetic macular edema (21). They have the disadvantage of a temporally limited effect, lasting only four to six weeks. At present, prospective, multicenter trials are being conducted to study the effect of bevacizumab (a humanized monoclonal antibody), ranibizumab (a recombinant, humanized monoclonal antibody fragment), and pegaptanib (an aptamer). In 2005, a randomized, double blind study already showed an effect on diabetic macular edema, consisting of a significant reduction of retinal thickness by 68 µm, while retinal thickness increased by 4 µm in the control group. The treated patients also had an improvement of visual acuity (22).
Similar results were obtained in the not yet published RESOLVE study, involving ranibizumab. The off-label nature of these treatments currently poses difficulties. Ranibizumab and pegaptanib have been approved to date only for the treatment of wet age-related macular degeneration. It is also worth mentioning that these medications are expensive: ranibizumab, for example, costs 1300 euros per injection. Pegaptinib and ranibizumab are expected to be approved for the treatment of diabetic macular edema by the end of 2011.
VEGF Trap Eye, a 115-kDa sized recombinant protein that blocks VEGF and has a longer half-life than ranibizumab, is currently under investigation (23). An injectable glucocorticoid (Ozurdex) with a prolonged effect lasting up to 12 months has already been approved in the USA for the treatment of central retinal vein occlusion and is being tested for use in diabetic macular edema.
Pharmacotherapy.
The pharmacotherapy of diabetic retinopathy is currently off label and involves the intravitreous injection of glucocorticoids or VEGF antagonists.
The spectrum of complications of intravitreous injections, also known as intravitreous operative medication (IVOM), corresponds to that of any intraocular surgical procedure and includes endophthalmitis, retinal detachment, and injury to the lens. The complication rate, however, is well under 1%. To keep the infection rate to a minimum, IVOM should be performed only in an aseptic operating room.
Intravitreous glucocorticoids.
These are preferentially used to treat diabetic macular edema.
Totally different therapeutic approaches involving the oral or intramuscular administration of medications such as protein kinase C inhibitors (ruboxistaurin and protein kinase C 412) or somatostatin analogs (octreotide) have not yielded the desired results in prospective, controlled studies (24, 25). Calcium dobesilate has been administered orally for decades as part of the treatment of vascular disorders such as venous insufficiency; when taken by patients with type 2 diabetes, it did not prevent the occurrence of clinically significant macular edema, as shown by a randomized, controlled study (CALDIRET) in which 635 patients were initially enrolled (5). Among the patients taking calcium dobesilate, 86 developed clinically significant macular edema, as compared to 69 patients in the placebo group. A protective effect was revealed only by post-hoc subgroup analysis in women who had both an HbA1c greater than 9% and poorly controlled hypertension.
Recommendations for clinical practice
Glycemic control to near-normal values (HbA1c= 7.0%), the tight control of arterial hypertension to values not exceeding 130/80 mm Hg, and the treatment of hyperlipidemia can delay the appearance of diabetic retinopathy by many years. Weight loss, exercise, and good nutrition can lower the prevalence and incidence of diabetes mellitus, and thus of its complications as well. Diabetic retinopathy progresses for a long time before it becomes symptomatic; it follows that regular preventive check-ups by an ophthalmologist are essential if irreversible damage to the eye is to be avoided. According to the guidelines of the German Diabetes Society (Deutsche Diabetes Gesellschaft, DDG), persons with type 1 diabetes should undergo ophthalmoscopy with dilated pupils starting five years after their diagnosis, and children from the age of 11 years onward; the examinations should be performed annually as long as retinopathy has not yet developed, and otherwise at the ophthalmologist’s direction. Persons with type 2 diabetes should be referred to an ophthalmologist as soon as diabetes is diagnosed. Because the duration of diabetes in such persons cannot be precisely determined, they should undergo ophthalmological examination three months later. If diabetic retinopathy has not yet developed, annual reexamination suffices; once diabetic retinopathy is present, reexamination should be as directed by the ophthalmologist. Female patients desiring to have children should undergo ophthalmoscopy before they become pregnant and at three-month intervals during pregnancy, or at one-month intervals if diabetic retinopathy is already present; they should be reexamined immediately if they develop any new symptoms. From the ophthalmological point of view, there is no indication for caesarean section, rather than normal vaginal delivery.
Recommendation for clinical practice.
Any patient who receives the diagnosis of type 2 diabetes mellitus should be referred at once to an ophthalmologist and should have annual ophthalmological check-ups thereafter for as long as diabetic retinopathy does not develop.
The use of standard forms to document the ophthalmological findings is recommended, such as the ones that have been made available in Germany by the Initiative Group for the Early Detection of Diabetic Eye Diseases (Initiativgruppe zur Früherkennung diabetischer Augenerkrankungen, IFdA) and the Working Group on Diabetes and the Eye (Arbeitsgemeinschaft Diabetes und Auge, AGDA). Sending a copy of the completed forms to the other treating physicians improves the quality of interdisciplinary care, which is all-important in the treatment of the diabetic patient.
Further Information on CME.
This article has been certified by the North Rhine Academy for Postgraduate and Continuing Medical Education.
Deutsches Ärzteblatt provides certified continuing medical education (CME) in accordance with the requirements of the Medical Associations of the German federal states (Länder). CME points of the Medical Associations can be acquired only through the Internet, not by mail or fax, by the use of the German version of the CME questionnaire within 6 weeks of publication of the article. See the following website: cme.aerzteblatt.de
Participants in the CME program can manage their CME points with their 15-digit “uniform CME number” (einheitliche Fortbildungsnummer, EFN). The EFN must be entered in the appropriate field in the cme.aerzteblatt.de website under “meine Daten” (“my data”), or upon registration. The EFN appears on each participant’s CME certificate.
The solutions to the following questions will be published in issue 13/2010.
The CME unit “Drug-Resistant Tuberculosis” (issue 1–2/2010) can be accessed until 18 February 2010.
For issue 9/2010 we plan to offer the topic “Osteoarthritis of the Knee.”
Solutions to the CME questionnaire in issue 49/2009:
Hammerschmidt S, Wirtz H: “Lung Cancer: Current Diagnosis and Treatment.”
Solutions: 1c, 2d, 3e, 4b, 5c, 6c, 7b, 8a, 9d, 10a
Please answer the following questions to participate in our certified Continuing Medical Education program. Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
When should an adult who has just received the diagnosis of type 2 diabetes mellitus be referred to an ophthalmologist?
When visual symptoms arise
As soon as diabetes is diagnosed
When diabetic nephropathy arises
5 years after diagnosis
When marked hypertension develops
Question 2
How often should a diabetic patient who does not have diabetic retinopathy be checked by an ophthalmologist?
Once per year
Four times a year
Every two years
The answer depends on the blood pressure
The answer depends on the long-term blood sugar value
Question 3
What is the evidence-based primary treatment of proliferative diabetic retinopathy?
Operative treatment with pars plana vitrectomy
Intravitreous glucocorticoid injection
Intramuscular injection of somatostatin analogs
Panretinal laser photocoagulation
Intravitreous administration of VGEF antagonists
Question 4
Which of the following is an indication for pars plana vitrectomy?
The presence of ischemic maculopathy
The presence of severe non-proliferative diabetic retinopathy
A non-resorbing vitreous hemorrhage
The presence of neovascularization glaucoma
The presence of proliferative diabetic retinopathy
Question 5
What is the definition of clinically significant macular edema?
Thickening of the retinal periphery
The presence of hemorrhages in the macular area
Retinal thickening and/or exudates within 500 µm of the fovea
The development of neovascularization in the area of the optic disk
The presence of microaneurysms in the area of the macula
Question 6
What is the gold standard of treatment for diabetic maculopathy?
The intravitreous administration of glucocorticoids
The intravitreous administration of VEGF antagonists
Focal laser coagulation
The intravitreous administration of antihypertensive agents
Pars plana vitrectomy
Question 7
What is the optimal value of HbA1c with respect to the development and progression of diabetic retinopathy?
>11%
10–11%
9–10%
8–9%
<7%
Question 8
Which of the following medications or classes of medications are approved for the treatment of diabetic retinopathy?
Calcium dobesilate
ACE inhibitors
Somatostatin analogs
None
Insulin analogs
Question 9
You have just diagnosed diabetes mellitus in a 7-year-old child. When should the child be referred for a first consultation with an ophthalmologist to check for diabetic retinopathy?
At once
When the child reaches puberty
After 5 years of illness
At age 11
When the child’s mother requests it
Question 10
A young woman with diabetes who wants to have a child comes to your office to ask about the possible visual complications of pregnancy. What advice do you give her?
There is no cause for concern as long as the blood sugar is well controlled.
There is no cause for concern, because diabetic retinopathy only affects the elderly.
Visual complications can arise during pregnancy, and you will therefore refer the patient to an ophthalmologist.
You advise the patient not to get pregnant.
You advise the patient to have a caesarean section rather than a vaginal delivery.
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
Professor Ulbig has received lecture honoraria and reimbursement of travel expenses from the Novartis, Pfizer, Lilly, Bausch & Lomb, Takeda, and Sanofi companies. The CALDIRET study was performed under the direction of the clinical department where Prof. Ulbig works and was financed by Sanofi-Synthelabo. Dr. Kollias declares that he has no conflict of interest as defined by the guidelines of the International Committee of Medical Journal Editors.
References
- 1.Hörle S, Grüner F, Kroll P. Epidemiologie diabetischer Erblindungen - eine Übersicht. Klin Monatsbl Augenheilkd. 2002;219(11):777–784. doi: 10.1055/s-2002-36318. [DOI] [PubMed] [Google Scholar]
- 2.Hamilton AMP, Ulbig MW, Polkinghome P. Management of diabetic retinopathy. London: BMJ Publishing Group; 1996. [Google Scholar]
- 3.Jonas JB. Intravitreal Triamcinolone acetonide for diabetic retinopathy. Dev Ophthalmol. 2007;39:96–110. doi: 10.1159/000098502. [DOI] [PubMed] [Google Scholar]
- 4.Early Treatment Diabetic Retinopathy Study Research Group. Early treatment diabetic retinopathy study. Ophthalmology. 1991;98(Suppl 5):739–840. [PubMed] [Google Scholar]
- 5.Haritoglou C, Gerss J, Sauerland C, Kampik A, Ulbig MW. Effect of calcium dobesilate on occurrence of diabetic macular oedema (CALDIRET study): randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2009;373:1364–1371. doi: 10.1016/S0140-6736(09)60218-X. [DOI] [PubMed] [Google Scholar]
- 6.Blum M, Kloos C, Muller N, et al. Prävalenz der diabetischen Retinopathie. Studie bei Versicherten der Deutschen Betriebskrankenkasse 2002-2004. Ophthalmologe. 2007;104(6):499–504. doi: 10.1007/s00347-007-1522-0. [DOI] [PubMed] [Google Scholar]
- 7.Ulbig MW, Kampik A, Hamilton AM. Diabetische Retinopathie. Epidemiologie, Risikofaktoren und Stadieneinteilung. Ophthalmologe. 1993;90(2):197–209. [PubMed] [Google Scholar]
- 8.Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on development and progression of long-term complications in insulin dependent diabetes mellitus. New Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 9.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes. Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 10.Rosenstock J, Fonseca V, McGill JB, et al. Similar progression of diabetic retinopathy with insulin glargine and neutral protamine Hagedorn (NPH) insulin in patients with type 2 diabetes: a long-term, randomized, open-label study. Diabetologia. 2009 Jun 13; doi: 10.1007/s00125-009-1415-7. [EPUB ahead of press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmieder RE, Martin S, Lang GE, Bramlage P, Böhm M. Angiotensin blockade to reduce microvascular damage in diabetes mellitus [Angiotensinblockade zur Reduktion mikrovaskulärer Schäden bei Diabetes] Dtsch Arztebl Int. 2009;106(34-35):556–562. doi: 10.3238/arztebl.2009.0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaturvedi N, Sjolie AK, Stephenson JM, et al. Effect of lisinopril on progression of retinopathy in normotensive people with type 1 diabetes. The EUCLID Study Group. EURODIAB Controlled Trial of Lisinopril in Insulin-Dependent Diabetes Mellitus. Lancet. 1998 351;(9095):28–31. doi: 10.1016/s0140-6736(97)06209-0. [DOI] [PubMed] [Google Scholar]
- 13.UKPDS 39. UK Prospective Diabetes Study Group. Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes. BMJ. 1998;317:713–720. [PMC free article] [PubMed] [Google Scholar]
- 14.Chaturvedi N, Porta M, Klein R, et al. Effect of candesartan on prevention (DIRECT-Prevent 1) and progression (DIRECT-Protect 1) of retinopathy in type 1 diabetes: randomised, placebo-controlled trials. Lancet. 2008;372:1394–1402. doi: 10.1016/S0140-6736(08)61412-9. [DOI] [PubMed] [Google Scholar]
- 15.Sjolie AK, Klein R, Porta M, et al. Effect of candesartan on progression and regression of retinopathy in type 2 diabetes (DIRECT-Protect 2): a randomised placebo-controlled trial. Lancet. 2008 372;(9647):1385–1393. doi: 10.1016/S0140-6736(08)61411-7. [DOI] [PubMed] [Google Scholar]
- 16.Keech AC, Mitchell P, Summanen PA, et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. 2007;370(9600):1687–1697. doi: 10.1016/S0140-6736(07)61607-9. [DOI] [PubMed] [Google Scholar]
- 17.Lanzagorta-Aresti A, Palacios-Pozo E, Menezo Rozalen JL, Navea-Tejerina A. Prevention of vision loss after cataract surgery in diabetic macular edema with intravitreal bevacizumab: a pilot study. Retina. 2009;29(4):530–535. doi: 10.1097/IAE.0b013e31819c6302. [DOI] [PubMed] [Google Scholar]
- 18.The Diabetic Retinopathy Study Research Group. Preliminary report on the effects of photocoagulation therapy. Am J Ophthalmol. 1976;81:383–396. doi: 10.1016/0002-9394(76)90292-0. [DOI] [PubMed] [Google Scholar]
- 19.Gandorfer A, Kampik A. Pars-plana-Vitrektomie bei diabetischer Retinopathie. Vom pathogenetischen Prinzip zur operativen Strategie. Ophthalmologe. 2000;97(5):325–330. doi: 10.1007/s003470050531. [DOI] [PubMed] [Google Scholar]
- 20.The Diabetic Retinopathy Vitrectomy Study Research Group. Early vitrectomy for severe proliferative diabetic retinopathy in eyes with useful vision. Clinical application of results of a randomized trial. Diabetic Retinopathy Vitrectomy Study Report 4. Ophthalmology. 1988;95(10):1321–1334. doi: 10.1016/s0161-6420(88)33014-9. [DOI] [PubMed] [Google Scholar]
- 21.Beck RW, Edwards AR, Aiello LP, et al. Three-year follow-up of a randomized trial comparing focal/grid photocoagulation and intravitreal triamcinolone for diabetic macular edema. Arch Ophthalmol. 2009;127(3):245–251. doi: 10.1001/archophthalmol.2008.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cunnigham ET, Adamis AP, Altaweel M, et al. Macugen Diabetic Retinopathy Study Group: A phase II randomized double-masked trial of pegaptanib, anti-vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology. 2005;112(10):1747–1757. doi: 10.1016/j.ophtha.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 23.Do DV, Nguyen QD, Shah SM, et al. An exploratory study of the safety, tolerability and bioactivity of a single intravitreal injection of vascular endothelial growth factor Trap-Eye in patients with diabetic macular oedema. Br J Ophthalmol. 2009;93(2):144–149. doi: 10.1136/bjo.2008.138271. [DOI] [PubMed] [Google Scholar]
- 24.Effect of ruboxistaurin in patients with diabetic macular edema: thirty-month results of the randomized PKC-DMES clinical trial. Arch Ophthalmol. 2007;125(3):318–324. doi: 10.1001/archopht.125.3.318. [DOI] [PubMed] [Google Scholar]
- 25.Campochiaro PA. Reduction of diabetic macular edema by oral administration of the kinase inhibitor PKC412. Invest Ophthalmol Vis Sci. 2004;45(3):922–931. doi: 10.1167/iovs.03-0955. [DOI] [PubMed] [Google Scholar]