Abstract
This study analyzes immunologic markers to predict and diagnose tuberculosis-associated immune reconstitution inflammatory syndrome (TB-IRIS) in HIV and TB coinfected adults who initiated antiretroviral therapy (ART) in Thailand. T helper 1 cytokines interleukin (IL)-2, IL-12, and interferon-gamma (IFN-γ) levels in response to PPD and RD1 antigens were assessed prior to ART, at weeks 6, 12, and 24 of treatment, and at time of TB-IRIS. Of 126 subjects, 22 (17.5%) developed TB-IRIS; 14 (64%) subjects received steroid treatment and 3 (14%) received NSAIDs; none of the subjects died. Median interval between ART initiation and TB-IRIS development was 14 days. IFN-γ, IL-2, and IL-12 responses did not differ between TB-IRIS and no TB-IRIS subjects (p > 0.05). More research into the immunopathogenesis of TB-IRIS and diagnostic potential of cytokine markers is warranted.
Introduction
Tuberculosis (TB) is one of the most common opportunistic infections afflicting HIV-infected patients worldwide.1,2 A critical issue is the difficulty of accurate and prompt diagnosis and management of TB-associated immune reconstitution inflammatory syndrome (TB-IRIS). TB-IRIS is a clinical syndrome characterized by a paradoxic worsening of preexisting, or unmasking of previously subclinical, TB infection in HIV-infected patients who have initiated combination antiretroviral therapy (ART).2–6 The former is often referred to as paradoxical TB-IRIS, while the latter is known as unmasking TB-IRIS or ART-associated TB.7 Because of its associated morbidity and, infrequently, mortality,8 and increased complexity of care, it is important to diagnose TB-IRIS in a timely manner to guide clinical management. Diagnosis of TB-IRIS is often complicated and factors such as drug toxicity, medication nonadherence, drug-resistant infection, drug interactions, and other opportunistic infections and neoplasms need to be excluded.3,5 This study was conducted to determine specific T helper 1 cytokine markers to predict and diagnose TB-IRIS in HIV and TB coinfected adults after initiating ART in Thailand, as well as to describe the incidence and clinical manifestations of TB-IRIS. The immunologic changes evaluated in the study included the following: (1) interferon gamma (IFN-γ) response to antigens encoded by the region of difference 1 (RD1) of the Mycobacterium tuberculosis genome (ESAT-6, CFP-10, TB 7.7) and purified protein derivative (PPD) at different intervals after ART commencement and at time of TB-IRIS using a whole blood IFN-γ release assay, (2) interleukin (IL)-2 and IL-12, and (3) tuberculin skin test (TST) response at the same time points.
Materials and Methods
The study was a substudy of a prospective randomized trial of 142 HIV and TB coinfected adults initiating ART at Bamrasnaradura Infectious Diseases Institute in Nonthaburi, Thailand from December 2006 to October 2007.9 The enrolled subjects were randomized to starting ART with either nevirapine (NVP) or efavirenz (EFV), with stavudine and lamivudine as the nucleoside reverse transcriptase inhibitor backbone. TB cases were treated with standard first-line therapy consisting of isoniazid, rifampicin, pyrazinamide, and ethambutol (HRZE) for 2 months followed by HR for at least 4 months. Antiretroviral medication adherence was assessed at each study visit with a questionnaire, while TB medications were administered under directly observed therapy. Subjects were included in the substudy if they met the following inclusion criteria: (1) have documented HIV-1 infection, (2) have documented TB disease by clinical features, such as having symptoms and radiographic evidence of TB, and/or positive acid fast bacilli (AFB) stain and/or positive culture from sputum or other body tissues, (3) have initiated rifampicin-based anti-TB treatment for at least 4 weeks but not more than 16 weeks, (4) between 18 and 60 years of age, (5) ART naive, and (6) CD4 < 350 cells/mm3 at baseline.10 Subjects were discontinued from the substudy if they had nontuberculous mycobacteria (NTM) isolated from cultures.
Subjects were defined as having definite TB-IRIS if they met two major criteria, or met criterion A of the major criteria and two minor criteria depicted in Table 1, adapted from a case definition previously described.11 An external reviewer independently confirmed TB-IRIS cases. Subjects who did not meet all of the criteria for definite TB-IRIS but who were considered by the external reviewer to have clinical manifestations consistent with the syndrome were classified as having possible TB-IRIS. The cases were later compared to the classification using the International Network for the Study of HIV-associated Immune Reconstitution Inflammatory Syndrome (INSHI) consensus case definition for TB-IRIS.7
Table 1.
Baseline Demographic Characteristics
| Study case definition of TB-IRIS | INSHI case definition of TB-IRIS |
|---|---|
|
Definite TB-IRIS Two major criteria or criterion A of the major criteria and two minor criteria Major criteria A. Atypical presentation of tuberculosis in subjects responding to antiretroviral therapy (ART), manifested by any of the following: • Localized disease (e.g., lymph nodes, liver, spleen, central nervous system) • Exaggerated inflammatory reaction (e.g., marked fever) after excluding other causes • Atypical inflammatory response in affected tissues (e.g., granulomas, suppuration, necrosis) • Progression of organ dysfunction or enlargement of preexisting lesions after definite clinical improvement with appropriate anti-TB therapy B. Decrease in plasma HIV RNA level by 1 log10 copies/ml Minor criteria • Increase in CD4 count after ART of at least 25 cells/mm3 • Spontaneous resolution of disease with continuation of ART Possible TB-IRIS Met major criterion A and one minor criterion, and considered by external reviewer to have clinical manifestations possibly consistent with TB-IRIS |
(A) Antecedent requirements Both of the two following requirements must be met: • Diagnosis of tuberculosis: the tuberculosis diagnosis was made before starting ART and this should fulfill WHO criteria for diagnosis of smear-positive pulmonary tuberculosis, smear-negative pulmonary tuberculosis, or extrapulmonary tuberculosis • Initial response to tuberculosis treatment: the patient's condition should have stabilised or improved on appropriate tuberculosis treatment before ART initiation—e.g., cessation of night sweats, fevers, cough, weight loss (Note: this does not apply to patients starting ART within 2 weeks of starting tuberculosis treatment since insufficient time may have elapsed for a clinical response to be reported) (B) Clinical criteria The onset of tuberculosis-associated IRIS manifestations should be within 3 months of ART initiation, reinitiation, or regimen change because of treatment failure Of the following, at least one major criterion or two minor clinical criteria are required Major criteria • New or enlarging lymph nodes, cold abscesses, or other focal tissue involvement—e.g., tuberculous arthritis • New or worsening radiological features of tuberculosis (found by chest radiography, abdominal ultrasonography, CT, or MRI) • New or worsening CNS tuberculosis (meningitis or focal neurological deficit—e.g., caused by tuberculoma) • New or worsening serositis (pleural effusion, ascites, or pericardial effusion) Minor criteria • New or worsening constitutional symptoms such as fever, night sweats, or weight loss • New or worsening respiratory symptoms such as cough, dyspnea, or stridor • New or worsening abdominal pain accompanied by peritonitis, hepatomegaly, splenomegaly, or abdominal adenopathy (C) Alternative explanations for clinical deterioration must be excluded if possible* • Failure of tuberculosis treatment because of tuberculosis drug resistance • Poor adherence to tuberculosis treatment • Another opportunistic infection or neoplasm (it is particularly important to exclude an alternative diagnosis in patients with smear-negative pulmonary tuberculosis and extrapulmonary tuberculosis where the initial tuberculosis diagnosis has not been microbiologically confirmed) • Drug toxicity or reaction |
Cases were categorized as TB-IRIS based on criteria proposed by French et al.11 and were later compared to criteria proposed by INSHI (International Network for the Study of HIV-associated IRIS), which included antecedent requirements, clinical criteria, and exclusion of alternative explanations for clinical deterioration.
Subjects were prospectively followed after they initiated ART up to week 24 for the substudy. Baseline clinical history was collected at week 0 prior to starting ART, and clinical events during follow-up at weeks 6, 12, and 24 and at time of TB-IRIS were recorded. At week 0, TST was implanted on the skin of the subjects' forearms with reading by a study nurse 48–72 h later, and the procedure was repeated at week 24 and at time of TB-IRIS.
Whole blood samples were assessed for IFN-γ in response to RD1 peptide antigens (ESAT-6, CFP-10, TB 7.7) and PPD using QuantiFERON-TB Gold In-Tube (QFTGIT) assay (Cellestis, Inc, Melbourne, Australia) at weeks 0, 6, 12, and 24 and at time of TB-IRIS according to the manufacturer's instructions. IFN-γ levels in response to RD1 antigens and PPD were calculated by subtracting background IFN-γ levels detected in nil samples. Serum samples of the first 40 subjects enrolled in the substudy were analyzed for IL-2 and IL-12 using enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. IL-2 and IL-12 were repeated with stored plasma samples that had been incubated overnight with RD1 antigens and PPD, using the same procedures as described above.
The institutional review boards of the Thailand Ministry of Public Health, Bamrasnaradura Infectious Diseases Institute, and Columbia University approved the study.
For the substudy, data were censored at last clinic visit or 24 weeks after ART initiation. The overall incidence of TB-IRIS at week 24 was reported. The association between potential predictor variables and TB-IRIS was evaluated. Student's t-test was used for analysis of continuous data with parametric distributions, while the Wilcoxon rank sum test was used for analysis of nonparametric data. Categorical data were analyzed using the chi-square test. Logistic regression was employed to identify independent predictors of TB-IRIS and control for confounding. A two-sided alpha ≤0.05 was considered significant. All statistical analyses were performed using SAS software, version 9.1 (SAS Institute, Cary, NC).
Results
Of the 142 adults who initiated ART during TB treatment as part of the main study, 12 subjects were discontinued from the substudy because their cultures later revealed NTM infection. One hundred and twenty-six subjects who met the substudy inclusion criteria and provided written informed consent were included in the analysis. Baseline demographic characteristics are summarized in Table 2. Of the 126 subjects, 83 (66%) were male. The median age was 35 years. Baseline median CD4 cell count prior to starting ART was 43 cells/mm3, and baseline median HIV-1 viral load was 5.9 log10 copies/ml. Fifty-three subjects (42%) had pulmonary TB, 28 (22%) had extrapulmonary TB, with cervical lymphadenitis as the most common extrapulmonary manifestation, and 45 (36%) had disseminated TB. A majority had TB confirmed either by AFB stain and/or culture, with 79 (63%) having a positive AFB stain and 66 (52%) having culture confirmation. The median interval between TB medications and ART commencement was 45 days.
Table 2.
Baseline Demographic Characteristics
| Characteristic | All (N = 126) | TB-IRIS (N = 22) | No TB-IRIS (N = 104) | p-value |
|---|---|---|---|---|
| Age (years), median (IQR) | 35 (31–42) | 32 (28–37) | 36 (32–43) | 0.04 |
| Male gender, n (%) | 83 (66) | 16 (73) | 67 (64) | 0.84 |
| Opportunistic and coinfections, n (%) | ||||
| Hepatitis B | 9 (7) | 1 (5) | 8 (8) | |
| Hepatitis C | 29 (23) | 4 (2) | 25 (24) | |
| Prior TB | 5 (4) | 2 (9) | 3 (3) | |
| Cryptococcosis | 3 (2) | 0 (0) | 3 (3) | |
| Pneumocystis jirovecii pneumonia | 7 (6) | 1 (5) | 6 (6) | |
| Oral/esophageal candidiasis | 49 (39) | 13 (59) | 36 (35) | 0.15 |
| Site of tuberculosis, n (%) | ||||
| Pulmonary TB (PTB) only | 53 (42) | 3 (14) | 50 (48) | |
| Extrapulmonary TB (EPTB) only | 28 (22) | 9 (41) | 19 (18) | |
| Both PTB and EPTB | 45 (36) | 10 (45) | 36 (35) | 0.51 |
| TB Diagnosis, n (%) | ||||
| AFB stain positive | 79 (63) | 16 (73) | 63 (61) | 0.51 |
| MTB culture positive | 66 (52) | 13 (59) | 53 (51) | 0.83 |
| ART regimen, n (%) | ||||
| Nevirapine-based | 64 (51) | 11 (50) | 53 (51) | |
| Efavirenz-based | 62 (49) | 11 (50) | 51 (49) | 0.94 |
| Baseline CD4 (cells/mm3), median (IQR) | 43 (23–92) | 35 (23–54) | 44 (23–104) | 0.18 |
| Baseline HIV-1 viral load (log10 copies/ml), median (IQR) | 5.9 (5.5–6.0) | 5.9 (5.6–6.0) | 5.8 (5.5–6.0) | 0.46 |
| CD4 increase at week 12 from baseline (cells/mm3), median (IQR) | 105 (55–164) | 144 (88–165) | 91 (54–158) | 0.12 |
| CD4 increase at week 24 from baseline (cells/mm3), median (IQR) | 119 (74–178) | 137 (76–187) | 118 (71–170) | 0.36 |
| HIV-1 viral load decrease at week 12 from baseline (log10 copies/ml), median (IQR) | 3.9 (3.5–4.3) | 4.1 (3.8–4.3) | 3.9 (3.4–4.2) | 0.08 |
| HIV-1 viral load decrease at week 24 from baseline (log10 copies/ml), median (IQR) | 4.0 (3.7–4.3) | 4.2 (3.9–4.3) | 4.0 (3.7–4.3) | 0.43 |
| Interval between TB medications and ART initiation (days), median (IQR) | 45 (35–70) | 42 (33–68) | 47 (35–74) | 0.38 |
Twenty-two (17.5%) subjects developed TB-IRIS, with 16 definite and 6 possible TB-IRIS cases. The classification of TB-IRIS was nearly consistent when the INSHI case definition was applied, with a concordance of 91.6%. Clinical manifestations of TB-IRIS were diverse: 16 (73%) developed lymph node enlargement, predominantly along the cervical chain, 13 (59%) had fever, 8 (36%) reported cough, and 4 (18%) had abdominal pain. A majority (18 subjects or 82%) had worsening of a preexisting site, while 4 (18%) had TB-IRIS development in a new location. Fourteen (64%) persons received steroid treatment (with a median duration of 64 days), 3 (14%) were treated with nonsteroidal antiinflammatory drugs (NSAIDs) and none died. The median (IQR) interval between ART initiation and TB-IRIS development was 14 (9–18) days. Among the TB-IRIS subjects, the median (IQR) CD4 increase was 144 (88–165) cells/mm3 and 137 (76–187) cells/mm3 at weeks 12 and 24, respectively, while the median (IQR) decrement of plasma HIV-1 viral load was 4.1 (3.8–4.3) log10 copies/ml and 4.2 (3.9–4.3) log10 copies/ml at weeks 12 and 24, respectively. Of TB-IRIS subjects 77% and 91% had viral suppression <50 copies/ml at weeks 12 and 24, respectively. No significant differences in CD4 increase, HIV-1 viral load decrease, and viral suppression <50 copies/ml at weeks 12 and 24 were noted between TB-IRIS and no TB-IRIS subjects. Results are summarized in Table 2. The only feature distinguishing TB-IRIS from no TB-IRIS subjects was age, with those who developed TB-IRIS being younger than those who did not develop the syndrome (median age 32 years vs. 36 years, p = 0.04). Using univariate logistic regression, factors significantly associated with TB-IRIS development were age <35 years (OR = 3.23, 95% CI 1.17–8.92), having extrapulmonary TB only (OR = 7.90, 95% CI 1.93–32.3) or disseminated TB (OR = 4.76, 95% CI 1.22–18.6), and having HIV-1 viral load decrease ≥4 log10 copies/ml at week 12 (OR = 2.56, 95% CI 1.00–6.59). However, using multivariate logistic regression, the only factors that remained significantly associated with TB-IRIS were having extrapulmonary TB only (OR = 8.63, 95% CI 1.99–37.50) or disseminated TB (OR = 4.17, 95% CI 1.03–16.86).
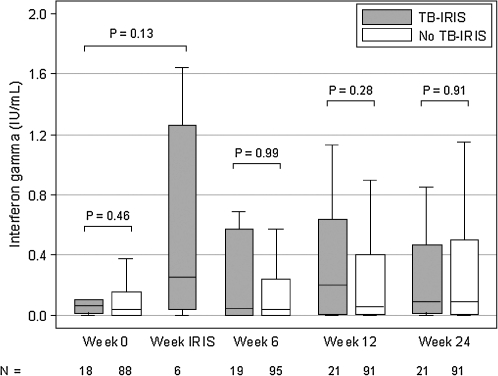
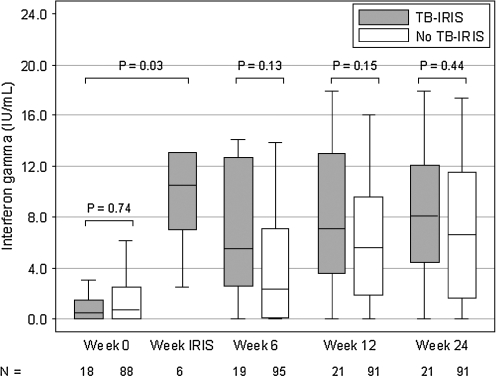
Using QFTGIT assays, there were no significant differences in IFN-γ responses to RD1 antigens or PPD pre-ART and at weeks 6, 12, and 24 post-ART (p > 0.05), although there was a trend toward higher IFN-γ response to PPD among subjects who developed TB-IRIS than among those who did not at weeks 6, 12, and 24. The IFN-γ responses to PPD at time of TB-IRIS in a subset of subjects, however, were significantly elevated from baseline levels (p = 0.03), with a median increase in IFN-γ level of 9.2 IU/ml. Results for the IFN-γ responses to RD1 antigens and PPD are depicted in Figs. 1 and 2.
FIG. 1.
IFN-γ response to RD1 antigens.
FIG. 2.
IFN-γ response to PPD.
Overall, blunted serum IL-2 and IL-12 responses were noted in a sample of 40 subjects. Only two subjects, one with and one without TB-IRIS, had elevated serum IL-2 and IL-12, ranging from 20 to 180 pg/ml. There were no distinguishing features in these two subjects to explain the high levels. When IL-2 and IL-12 were retested using stored plasma that had been stimulated with RD1 antigens and PPD from the QFTGIT, blunted responses were similarly found.
Restricting the analysis to TB-IRIS subjects who received steroids or NSAIDs to those who did not, there were no significant differences in immunologic responses at all study time points. Similarly, no significant differences in IFN-γ, IL-2, and IL-12 were noted in TB-IRIS subjects who had extrapulmonary TB manifestations compared to those who had only pulmonary TB. Among those who had TB confirmed by culture, the immunologic responses were not dissimilar between the two groups.
There were no significant differences in TST response at week 0 and 24 between those who developed TB-IRIS and those who did not (p = 0.77 and p = 0.23, respectively). TB-IRIS subjects experienced a median increase of 9 (IQR 2–10) mm induration at time of TB-IRIS.
Of the 126 subjects in the substudy, 115 (91%) completed follow-up at 24 weeks. Six (5%) subjects died, all of whom did not have TB IRIS, while 5 (4%) were lost to follow-up. Among those subjects with TB-IRIS, 21 (96%) remained in the study at week 24; only one (5%) was lost to follow-up after week 18.
Discussion
In this analysis of 126 HIV and TB coinfected adults initiating ART in Thailand, TB-IRIS occurred in 22 (17.5%) subjects. No significant differences were noted in T helper 1 cytokines, specifically IFN-γ, IL-2, and IL-12, pre-ART and at weeks 6, 12, and 24 post-ART between those who developed TB-IRIS and those who did not. IFN-γ response to PPD, but not to the RD1 antigens, at time of TB-IRIS was found to be elevated from baseline levels in a subset of subjects with samples collected specifically at time of TB-IRIS.
TB-IRIS has been attributed to an aberrant host immune restoration response to mycobacterial antigens induced by ART. An imbalance of T effector and regulatory effects has been postulated to be the underlying pathogenesis of TB-IRIS.4,12–14 Recent studies have explored the role of IFN-γ to differentiate TB-IRIS, but the data are conflicting. Expansion of IFN-γ secreting CD4+ T cells to PPD has been described in HIV and TB coinfected adults with TB-IRIS.15 A more recent study reported a similarly dynamic T helper 1 response with proliferation of TB antigen-specific IFN-γ-secreting CD4+ T cells during TB-IRIS; however, this response was also observed in HIV and TB coinfected patients without TB-IRIS, suggesting the involvement of other immune regulatory factors.16
In a study of HIV-infected patients starting ART in Cambodia, QFTGIT and TST were utilized to explore the immunopathogenesis of the unmasking and paradoxic forms of TB-IRIS, as well as their diagnostic potential. Paradoxical TB-IRIS was predicted by elevated IFN-γ levels at baseline and was associated with a higher TST increase at time of TB-IRIS. PPD-stimulated IFN-γ levels, however, did not differentiate those who developed TB-IRIS and those who did not until month 6 of ART.17 The Cambodian cohort had a higher median baseline CD4 T cell count and fewer subjects who received antiinflammatory therapy for TB-IRIS than our study cohort. In our study, we did not find a distinguishing T helper 1 response between TB-IRIS and no TB-IRIS subjects at weeks 0, 6, 12, and 24, but we did observe an increase in IFN-γ levels to PPD at time of TB-IRIS from baseline in a subset of subjects who had blood samples drawn at this specific time point, albeit the limitation was that IFN-γ levels were not measured among those subjects without TB-IRIS at this time point and could not be directly compared. This dynamic T helper 1 response to PPD, but not to the RD1 antigens, at time of TB-IRIS has also been reported in several studies and has been attributed to paradoxic TB-IRIS representing an immunologic response to nonviable mycobacterial antigens rather than to RD1 antigens, which are released by living mycobacteria.15,18
Only one TB-IRIS subject in our study had a positive mycobacterial culture at TB-IRIS presentation. Steroid and NSAIDs administration in a majority of the TB-IRIS subjects in our study did not attenuate the immunologic response substantially, in contrast to previous reports showing decreased T helper 1 cytokine response after administration of steroids and other immunomodulatory medications.19–21 Finally, this study, along with most studies to date, focused on measuring immunologic markers in the blood compartment of persons experiencing TB-IRIS, rather than assessing the immunologic response from inflamed sites.16
The TB-IRIS incidence of 17.5% falls within the 8–43% range described in the medical literature.2,5,22–25 The most common clinical manifestation of TB-IRIS in this study was lymph node enlargement, predominantly along the cervical chain, followed by fever, respiratory symptoms, and abdominal pain with hepatomegaly. A majority of the subjects developed TB-IRIS at the same location as their preexisting TB infection. Most subjects presented with TB-IRIS within 30 days of starting ART, with a median of 14 days. Over 75% received prolonged treatment for TB-IRIS with steroids or NSAIDs. It is important to note that the decision making for treatment with steroids or NSAIDs was not standardized in the study and was based on the clinician's judgment. There was no mortality reported among the TB-IRIS cases in our study, consistent with previously published investigations whereby mortality from TB-IRIS is uncommon, typically <1%.4
The study found having extrapulmonary TB only or disseminated TB were the only factors significantly associated with TB-IRIS development on multivariate analysis. Having extrapulmonary or disseminated TB as a risk factor for TB-IRIS has been reported in the medical literature.24,26–28 This may be explained by a greater mycobacterial burden present in those with extrapulmonary or disseminated TB and a greater immunologic response to the dead or dying mycobacteria with ART during TB-IRIS.2,14 Even though our study showed an increased risk for TB-IRIS with increased rate of virologic suppression on univariate analysis, it was not a significant factor on multivariate analysis and on reanalysis with restriction to only those subjects who attained virologic suppression by week 24. The rapid decline in HIV-1 viral load as a risk factor for TB-IRIS has been reported in some, but not all, studies.23,29 Interestingly, baseline CD4 count and HIV-1 viral load prior to starting ART were not associated with TB-IRIS. Moreover, a shorter interval between ART initiation and TB diagnosis was not found to be an important risk factor in this cohort. This is likely caused by the constraints in the main study's inclusion criteria, which limits enrollment of subjects who have been diagnosed and treated with TB between 4 and 16 weeks.
The strengths of this study included its prospective design, high retention rates during the 6-month follow-up period, use of an external reviewer to classify TB-IRIS cases, and utilization of two case definitions to corroborate TB-IRIS identification. Several case definitions of TB-IRIS have been proposed,7 including one that was modified for TB-IRIS and utilized in our study.11 This case definition incorporated both clinical and laboratory parameters, specifically CD4 count and HIV-1 viral load change. When the TB-IRIS cases identified by this study case definition were subsequently compared to the case definition for TB-IRIS proposed by INSHI,7 a high concordance level was observed. The INSHI case definition is a consensus definition for use in resource-limited settings that lack CD4 and HIV-1 viral load laboratory capabilities. The high concordance in TB-IRIS cases using the two definitions substantiates the use of the INSHI case definition to diagnose TB-IRIS cases in resource-limited countries.
There are several limitations to this study. First, we were unable to collect blood samples of every subject at specific study time points. Missing data were censored from the analyses and may have affected the results. However, this effect is likely minimal since the clinical characteristics did not differ between TB-IRIS subjects with missing blood samples at time of TB-IRIS and those with complete data, except for the longer interval between anti-TB therapy and ART initiation in those with missing data. Another limitation of the study is the long intervals of the regularly scheduled follow-up visits. Some cases of TB-IRIS might have been missed, even though the subjects were instructed by the study investigators to present to the hospital for any atypical symptoms that arise in between scheduled visits. Finally, since most of the TB-IRIS cases occurred within the first 2–3 weeks of starting ART and those without TB-IRIS, as a result of the study design, did not have blood samples collected during this interval, any direct comparison of cytokine markers between TB-IRIS and no TB-IRIS subjects during this period was prohibited.
In summary, this study showed that TB-IRIS is a common manifestation among HIV and TB coinfected adults starting ART in Thailand. The T helper 1 immunologic response did not differentiate those with TB-IRIS from those without TB-IRIS. Concordance between our study TB-IRIS case definition and the INSHI general consensus definition suggests that the lack of HIV viral load and CD4 monitoring does not hinder the ability to diagnose TB-IRIS. More research into the immunopathogenesis of TB-IRIS and diagnostic potential of cytokine markers is warranted.
Acknowledgments
This study was funded by the Bristol-Myers Squibb Virology Fellows Research Program. H.V.T. was supported by the Center for Infectious Disease Epidemiologic Research Training Grant (T32 AIO49821-07) at Columbia University Mailman School of Public Health. We thank Simon Tsiouris, MD, MPH for his assistance as an external reviewer for the study.
Disclosure Statement
No competing financial interests exist.
References
- 1.El-Sadr WM. Tsiouris SJ. HIV-associated tuberculosis: Diagnostic and treatment challenges. Semin Respir Crit Care Med. 2008;29:525–531. doi: 10.1055/s-0028-1085703. [DOI] [PubMed] [Google Scholar]
- 2.Lawn SD. Wilkinson RJ. Lipman MC. Wood R. Immune reconstitution and “unmasking” of tuberculosis during antiretroviral therapy. Am J Respir Crit Care Med. 2008;177:680–685. doi: 10.1164/rccm.200709-1311PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dhasmana DJ. Dheda K. Ravn P. Wilkinson RJ. Meintjes G. Immune reconstitution inflammatory syndrome in HIV-infected patients receiving antiretroviral therapy: Pathogenesis, clinical manifestations and management. Drugs. 2008;68:191–208. doi: 10.2165/00003495-200868020-00004. [DOI] [PubMed] [Google Scholar]
- 4.French MA. HIV/AIDS: Immune reconstitution inflammatory syndrome: A reappraisal. Clin Infect Dis. 2009;48:101–107. doi: 10.1086/595006. [DOI] [PubMed] [Google Scholar]
- 5.McIlleron H. Meintjes G. Burman WJ. Maartens G. Complications of antiretroviral therapy in patients with tuberculosis: Drug interactions, toxicity, and immune reconstitution inflammatory syndrome. J Infect Dis. 2007;196(Suppl. 1):S63–75. doi: 10.1086/518655. [DOI] [PubMed] [Google Scholar]
- 6.Manabe YC. Breen R. Perti T. Girardi E. Sterling TR. Unmasked tuberculosis and tuberculosis immune reconstitution inflammatory disease: A disease spectrum after initiation of antiretroviral therapy. J Infect Dis. 2009;199:437–444. doi: 10.1086/595985. [DOI] [PubMed] [Google Scholar]
- 7.Meintjes G. Lawn SD. Scano F, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: Case definitions for use in resource-limited settings. Lancet Infect Dis. 2008;8:516–523. doi: 10.1016/S1473-3099(08)70184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawn SD. Myer L. Bekker LG. Wood R. Tuberculosis-associated immune reconstitution disease: Incidence, risk factors and impact in an antiretroviral treatment service in South Africa. AIDS. 2007;21:335–341. doi: 10.1097/QAD.0b013e328011efac. [DOI] [PubMed] [Google Scholar]
- 9.Manosuthi W. Sungkanuparph S. Tantanathip P, et al. A randomized trial comparing plasma drug concentrations and efficacies between 2 nonnucleoside reverse-transcriptase inhibitor-based regimens in HIV-infected patients receiving rifampicin: The N2R Study. Clin Infect Dis. 2009;48:1752–1759. doi: 10.1086/599114. [DOI] [PubMed] [Google Scholar]
- 10.Manosuthi W. Sungkanuparph S. Lueangniyomkul A. Mankatitham W. Tansuphaswadikul S. Prasithsirikul W. Ruxrungtham K the N2R Study Team. A randomized control trial of two non-nucleoside reverse transcriptase inhibitor-based regimens in HIV-infected patients receiving rifampicin; 48th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy/Infectious Diseases Society of America 46th Annual Meeting; Washington, DC. October 25–28; 2008. [Abstract H-1237]. [Google Scholar]
- 11.French MA. Price P. Stone SF. Immune restoration disease after antiretroviral therapy. AIDS. 2004;18:1615–1627. doi: 10.1097/01.aids.0000131375.21070.06. [DOI] [PubMed] [Google Scholar]
- 12.Lim A. D'Orsogna L. Price P. French MA. Imbalanced effector and regulatory cytokine responses may underlie mycobacterial immune restoration disease. AIDS Res Ther. 2008;5:9. doi: 10.1186/1742-6405-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.French MA. Disorders of immune reconstitution in patients with HIV infection responding to antiretroviral therapy. Curr HIV/AIDS Rep. 2007;4:16–21. doi: 10.1007/s11904-007-0003-z. [DOI] [PubMed] [Google Scholar]
- 14.Lawn SD. French MA. Immune reconstitution disease: Recent developments and implications for antiretroviral treatment in resource-limited settings. Curr Opin HIV AIDS. 2007;2:339–345. doi: 10.1097/COH.0b013e3281a3c0a6. [DOI] [PubMed] [Google Scholar]
- 15.Bourgarit A. Carcelain G. Martinez V, et al. Explosion of tuberculin-specific Th1-responses induces immune restoration syndrome in tuberculosis and HIV co-infected patients. AIDS. 2006;20:F1–7. doi: 10.1097/01.aids.0000202648.18526.bf. [DOI] [PubMed] [Google Scholar]
- 16.Meintjes G. Wilkinson KA. Rangaka MX, et al. Type 1 helper T cells and FoxP3-positive T cells in HIV-tuberculosis-associated immune reconstitution inflammatory syndrome. Am J Respir Crit Care Med. 2008;178:1083–1089. doi: 10.1164/rccm.200806-858OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elliott J. Khol V. Sarun S, et al. Investigation of the pathogenesis and diagnosis of tuberculosis-associated immune reconstitution inflammatory syndrome and antiretroviral therapy-associated tuberculosis using skin testing and a whole blood interferon-gamma release assay; XVII International AIDS Conference; Mexico City, Mexico. 03–08 August; 2008. [Google Scholar]
- 18.Tan DB. Yong YK. Tan HY, et al. Immunological profiles of immune restoration disease presenting as mycobacterial lymphadenitis and cryptococcal meningitis. HIV Med. 2008;9:307–316. doi: 10.1111/j.1468-1293.2008.00565.x. [DOI] [PubMed] [Google Scholar]
- 19.Almerighi C. Sinistro A. Cavazza A. Ciaprini C. Rocchi G. Bergamini A. 1alpha,25-Dihydroxyvitamin D3 inhibits CD40L-induced pro-inflammatory and immunomodulatory activity in human monocytes. Cytokine. 2009;45(3):190–197. doi: 10.1016/j.cyto.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Vidyarani M. Selvaraj P. Jawahar MS. Narayanan PR. 1,25-Dihydroxyvitamin D3 modulated cytokine response in pulmonary tuberculosis. Cytokine. 2007;40:128–134. doi: 10.1016/j.cyto.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Jirapongsananuruk O. Melamed I. Leung DY. Additive immunosuppressive effects of 1,25-dihydroxyvitamin D3 and corticosteroids on TH1, but not TH2, responses. J Allergy Clin Immunol. 2000;106:981–985. doi: 10.1067/mai.2000.110101. [DOI] [PubMed] [Google Scholar]
- 22.Kumarasamy N. Chaguturu S. Mayer KH, et al. Incidence of immune reconstitution syndrome in HIV/tuberculosis-coinfected patients after initiation of generic antiretroviral therapy in India. J Acquir Immune Defic Syndr. 2004;37:1574–1576. doi: 10.1097/00126334-200412150-00007. [DOI] [PubMed] [Google Scholar]
- 23.Shelburne SA. Visnegarwala F. Darcourt J, et al. Incidence and risk factors for immune reconstitution inflammatory syndrome during highly active antiretroviral therapy. AIDS. 2005;19:399–406. doi: 10.1097/01.aids.0000161769.06158.8a. [DOI] [PubMed] [Google Scholar]
- 24.Breton G. Duval X. Estellat C, et al. Determinants of immune reconstitution inflammatory syndrome in HIV type 1-infected patients with tuberculosis after initiation of antiretroviral therapy. Clin Infect Dis. 2004;39:1709–1712. doi: 10.1086/425742. [DOI] [PubMed] [Google Scholar]
- 25.Narita M. Ashkin D. Hollender ES. Pitchenik AE. Paradoxical worsening of tuberculosis following antiretroviral therapy in patients with AIDS. Am J Respir Crit Care Med. 1998;158:157–161. doi: 10.1164/ajrccm.158.1.9712001. [DOI] [PubMed] [Google Scholar]
- 26.Michailidis C. Pozniak AL. Mandalia S. Basnayake S. Nelson MR. Gazzard BG. Clinical characteristics of IRIS syndrome in patients with HIV and tuberculosis. Antiviral Ther. 2005;10:417–422. doi: 10.1177/135965350501000303. [DOI] [PubMed] [Google Scholar]
- 27.Burman W. Weis S. Vernon A, et al. Frequency, severity and duration of immune reconstitution events in HIV-related tuberculosis. Int J Tuberc Lung Dis. 2007;11:1282–1289. [PubMed] [Google Scholar]
- 28.Manosuthi W. Kiertiburanakul S. Phoorisri T. Sungkanuparph S. Immune reconstitution inflammatory syndrome of tuberculosis among HIV-infected patients receiving antituberculous and antiretroviral therapy. J Infect. 2006;53:357–363. doi: 10.1016/j.jinf.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Olalla J. Pulido F. Rubio R, et al. Paradoxical responses in a cohort of HIV-1-infected patients with mycobacterial disease. Int J Tuberc Lung Dis. 2002;6:71–75. [PubMed] [Google Scholar]