Editor: Single-dose nevirapine (SD-NVP) involving a single 200 mg dose to the mother during labor and a single dose to the infant within 72 h of birth was shown to be effective in reducing perinatal HIV transmission in a clinical trial in Uganda.1,2 The simplicity of the intervention has facilitated rapid scale up of programs to prevent mother-to-child HIV transmission (PMTCT) in many low resource settings.3 Nevirapine, a nonnucleoside reverse transcriptase inhibitor (NNRTI), is rapidly absorbed after oral administration and readily crosses the placenta. The drug also has a long half-life in pregnant women (median 61–66 h) and infants (45–54 h). These properties make it an effective agent to reduce transmission but are also unfortunately associated with selection of drug resistance mutations that reduce the efficacy of NNRTI-based therapeutic regimens when started within a year of delivery.4,5 Despite these constraints, SD-NVP remains one of the most widely used PMTCT interventions because of its simplicity and low cost.
CD4+ and CD8+ T cell responses are important in the control of HIV-1 infection. A number of studies have evaluated the impact of highly active antiretroviral therapy (HAART) on these responses, with the majority of these reporting decreases in CD4+ 6,7 and CD8+ 7–12,13 T cell responses in individuals treated with antiretroviral therapy. It was generally concluded that it is suppression by HAART of viral replication that results in reduced T cell responses to HIV-1. However, there are reports that show effects of short-course antiretroviral interventions given for PMTCT (short course zidovudine/lamivudine and SD-NVP) on HIV-stimulated T-helper cell reactivity [measured in vitro using an interleukin (IL)-2-dependent proliferation assay]14,15 and plasma immune activation markers16 in cord blood of infants born to HIV-1-seropositive mothers. Given that the majority of infants were uninfected, these findings suggest that antiretroviral drugs may have immunomodulatory potential aside from their antiviral effects.
We therefore questioned whether SD-NVP would affect detection of HIV-specific CD4+ and CD8+ T cell responses among 76 HIV-1-infected women recruited soon after delivery, and tested within 6 weeks. Results were not different if we excluded all samples that were not taken within 48 h of onset of labor (time of NVP dosing), therefore data were analyzed as the total group of 76 women. Of these 56 women were from Chris Hani Baragwanath Hospital in Soweto and 20 women were from Coronation Hospital in Johannesburg, South Africa. Fifty-one of the women, whose HIV status was known prior to delivery, were given standard SD-NVP (200 mg orally at the onset of labor) to prevent mother-to-child HIV transmission. The infants were additionally given 0.6 ml nevirapine as part of the standard protocol.1,2 The HIV status of the other 25 women was determined only postdelivery and hence none of these women had consumed any antiretroviral drugs prior to testing. For the infants of these women, one dose of nevirapine was given as “postexposure prophylaxis.” The median maternal viral load was 13,400 copies/ml (range 399–491,000), and the median CD4+ T cell count was 439 cells/μl (range 40–1655 cells/μl) for the cohort. There was no significant difference in the CD4+ T cell count or viral loads between the women who had been given SD-NVP and those who had not (p > 0.05) (Table 1).
Table 1.
Clinical Characteristics of Individuals within the Study Groups
| |
Study groupa |
|
|---|---|---|
| No NVP | NVP | |
| Number of subjects | 25 | 51 |
| Age (years) | 28 (18–39) | 28 (18–37) |
| CD4+ T cell count cells/μl | 486 (195–1655) | 428 (40–1479) |
| Viral load | 15940 (399–491000) | 11700 (399–466,000) |
Results are expressed as medians, with the range in parentheses.
HIV-specific CD4+ and CD8+ T cell responses to HIV-1 peptide pools to Gag, Env, Pol, Nef, and the regulatory region (Reg) were analyzed using a whole blood intracellular cytokine staining (ICS) assay that measures both IL-2 and interferon (IFN)-γ (both antibodies labeled with the same fluorochrome) as described previously.17 There was a greater number of positive responses (defined as responses ≥0.1% after subtraction of the background staining) in the women who had not received SD-NVP in comparison with those who had for CD4+ T cell Pol-, Nef-, and Reg-specific responses, and for CD8+ T cell responses to all peptide pools (Table 2). By contrast, detectable CD4+ T cell Env responses were more highly represented in the NVP group, with Gag CD4+ T cell responses seen in similar proportions of individuals in either group. However, differences in proportions of individuals responding in either group were not significant for any of the comparisons (p > 0.05).
Table 2.
Frequency of CD4+ and CD8+ T Cell Responses to HIV-1 Peptides
| Peptide pool | No NVP (%) | NVP (%) | p valuea |
|---|---|---|---|
| CD4+ T cell responses | |||
| Gag | 9/25 (36%) | 18/51 (35%) | P = 1.0 |
| Pol | 6/25 (24%) | 10/51 (20%) | P = 0.766 |
| Nef | 6/25 (24%) | 6/51 (12%) | P = 0.193 |
| Reg | 9/25 (36%) | 8/51 (16%) | P = 0.077 |
| Env | 7/25 (28%) | 20/51 (39%) | P = 0.446 |
| CD8+ T cell responses | |||
| Gag | 21/25 (84%) | 41/51 (80%) | P = 1.0 |
| Pol | 22/25 (88%) | 37/51 (73%) | P = 0.154 |
| Nef | 23/25 (92%) | 38/51 (75%) | P = 0.123 |
| Reg | 18/25 (72%) | 28/51 (55%) | P = 0.213 |
| Env | 19/25 (76%) | 28/51 (55%) | P = 0.085 |
Fisher's exact test. Results are not significant (p > 0.05).
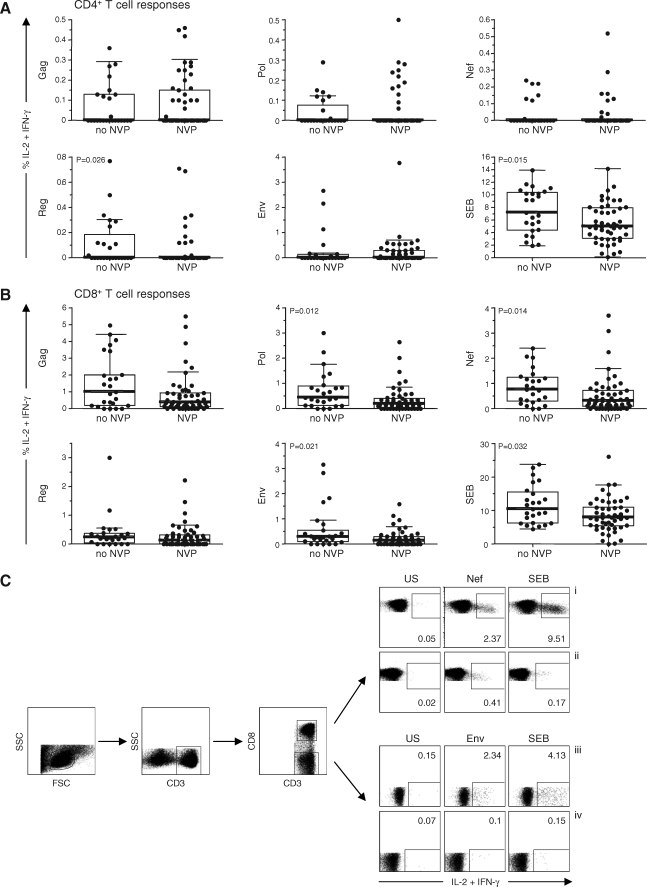
The magnitude of the CD4+ T cell responses to any of the HIV peptides was not significantly different between the women who had received SD-NVP and those who had not, with the exception of Reg-specific responses (p = 0.026) (Fig. 1A). Significant reductions in the magnitude of the CD8+ Pol- (p = 0.012), Nef- (p = 0.014), and Env-specific responses (p = 0.021) were evident, with Gag and Reg CD8+ T cell responses not significantly different between the two groups. Interestingly, there was a significant reduction in the magnitude of the CD4+ (p = 0.015) and CD8+(p = 0.032) T cell responses to SEB in the women who had received SD-NVP compared with those who had not. These findings suggest that a reduction in the magnitude of HIV-specific T cell responses is explained by immunomodulatory consequences of the drug, rather than through antiviral effects of the drug reducing viral load and so reducing anti-HIV T cell responses. Furthermore, the fact that results were not different when samples were tested within 48 h of SD-NVP administration compared to inclusion of samples up to 6 weeks (data not shown) suggests that if the effects of NVP wane this would occur at a time point beyond this.
FIG. 1.
The magnitude of the CD4+ (A) and CD8+ (B) T cell responses for 25 women who received no NVP and 51 women who received single-dose NVP at the onset of labor. Y-axis: percentage of IL-2 and/or IFN-γ-expressing cells in response to peptide pools Gag, Pol, Nef, Reg (Tat, Rev, Vif, Vpu, Vpr peptide pools combined), and Env, and the positive control SEB. Data are presented as individual values (dots), medians (thick horizontal lines), 25 and 75th percentiles (boxes), and 10th and 90th percentiles (thin horizontal lines). Comparison of the magnitudes of the T cell responses between the study groups was done using the Mann–Whitney U test and SPSS software (SPSS, Chicago, IL). Significant differences between groups are indicated. NVP, nevirapine. (C) Representative flow cytometric plots show gating of lymphocytes according to side scatter (SSC) and forward scatter (FSC) characteristics, followed by gating CD3-positive lymphocytes and CD8+ and CD8− cell (CD4) populations. (i and iii) Flow plots showing CD3+CD8+ cells and CD3+CD8− (CD4) cells (y-axis) expressing IL-2 and/or IFN-γ (x-axis) of two different patients (no NVP group). (ii and iv) Flow plots from a patient, exposed to NVP, showing the proportions of CD3+CD8+ and CD3+CD8− (CD4) cells expressing IL-2 and/or IFN-γ (x-axis), respectively. Stimuli are indicated above CD3+CD8− and CD3+CD8− plots: US, unstimulated; Nef or Env peptide pools; SEB. Values indicated within plots are percentages of T cells (CD8 or CD4) expressing IL-2 and/or IFN-γ.
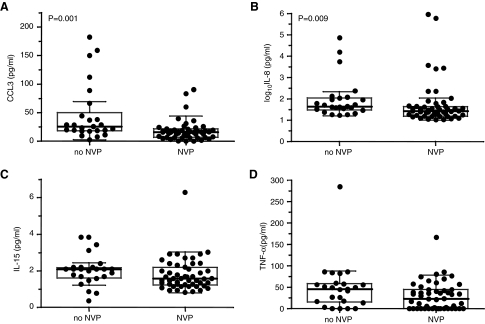
Consistent with reductions in T cell responses, circulating levels of particular soluble factors were also decreased in the group of HIV-1-infected women who received SD-NVP. Concentrations of Fas ligand (FasL), tumor necrosis factor alpha (TNF-α), interferon gamma (IFN-γ), interleukin (IL)-2, IL-4, IL-7, IL-8, IL-10, and IL-12, granulocyte macrophage colony-stimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF), macrophage inflammatory proteins 1-alpha (CCL3), 1-beta (CCL4), and CCL5 (formerly known as “regulated on activation, normal T cell expressed and secreted” or RANTES) (all using BD™ CBA Flex Set System and BD™ Human Soluble Protein Master Buffer Kit; BD, San Diego, CA) and IL-15 (Quantikine IL-15 ELISA kit; R&D Systems Inc., Minneapolis, MN) were determined from maternal plasma samples. There was only a significant reduction in CCL3 (p = 0.001) and IL-8 (p = 0.009) plasma levels in the women who had received SD-NVP compared with those who had not (Fig. 2), with a trend to that effect for IL-15 (p = 0.058) and TNF-α (p = 0.062) plasma levels. These data are consistent with our earlier findings of a reduction in spontaneously released and phytohemagglutinin-stimulated CCL3 in HIV-exposed uninfected infants whose mothers had been given SD-NVP, compared with infants whose mothers had not received antiretroviral therapy.18 Therefore, reductions in levels of certain cytokines can be detected with short exposure to nevirapine in the context of existing infection (HIV-1-infected mothers) and in the context of HIV-1 exposure (exposed-uninfected infant). It is also significant that host genotypes of CCL3 and CCR5 show associations with maternal–infant HIV-1 transmission only in the absence of antiretrovirals,18,19 suggesting that positive effects of antiretrovirals in preventing MTCT mask these relationships.
FIG. 2.
Comparison of plasma levels of CCL3 (A), IL-8 (B), IL-15 (C), and TNF-α (D) for 25 women who received no NVP and 51 women who received single dose NVP at the onset of labor. Data are presented as individual values (dots), medians (thick horizontal lines), 25 and 75th percentiles (boxes), and 10th and 90th percentiles (thin horizontal lines). Comparison of CCL3 and IL-8 plasma levels between the study groups was done using the Mann–Whitney U test and SPSS software (SPSS, Chicago, IL). Significant differences (p < 0.05) between groups are indicated.
As both nonpolarized and naive T cells can be induced by CCL3 to produce IFN-γ,20 the lower plasma levels of CCL3 in the women who had received SD-NVP compared with those who had not may contribute to the lower magnitude of T cell intracellular IFN-γ production in these women. The chemokine IL-8 has been predominantly characterized as a factor that induces chemotaxis of neutrophils that express the IL-8 receptors CXCR1 and CXCR2.21 However, effector CD8+ T cells have recently been found to express CXCR1, and could be induced to migrate by IL-8. Therefore the IL-8-CXCR1 axis is likely to be important in the homing of effector CD8+ T cells.22 The lower IL-8 plasma levels in the women who had received SD-NVP, compared with those who had not received any antiretroviral therapy, may affect the IL-8–CXCR1 axis and influence the development of T cell responses.
In conclusion, this study has demonstrated that SD-NVP has a suppressive effect on T cell responses induced by HIV-1 peptides and by SEB (therefore irrespective of T cell response specificity), and also reduces plasma levels of certain chemokines (CCL3 and IL-8). These data add further to our findings of increased levels of soluble plasma activation markers in infants in the presence of SD-NVP.16 Taken together these data suggest that nevirapine may act by enhancing some immune pathways yet suppressing others. These results should not be interpreted as discouraging the use of single-dose nevirapine for prevention of mother-to-child HIV transmission. Although SD-NVP is not as effective as longer regimens, it remains a useful strategy for low resource settings in which the infrastructure to provide more effective prevention strategies is not yet in place. Initial concerns about the detrimental effects of the intervention for later treatment outcomes among women proved to be overstated. Studies now show no demonstrable long-term effects on treatment outcomes if therapy is started more than a year after exposure.5,23 The immunomodulatory effects we demonstrate here may help explain why the intervention is effective in preventing mother-to-child HIV transmission and are unlikely to have adverse clinical effects on long-term disease progression in the mother. Understanding the mechanisms responsible for immunomodulatory effects of NVP or other antivirals remains important as certain drugs may act indirectly through induction/suppression of various immune pathways that serve to curb HIV-1 infection independently of direct antiviral drug effects.
Acknowledgments
This study was supported in part by the South African AIDS Vaccine Initiative (SAAVI) and by grants from NICHD 42402 and the Wellcome Trust. CTT is a Wellcome Trust International Senior Research Fellow (076352/Z/05/Z).
Disclosure Statement
No competing financial interests exist.
References
- 1.Guay LA. Musoke P. Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999;354:795–802. doi: 10.1016/S0140-6736(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 2.Jackson JB. Musoke P. Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: 18-month follow-up of the HIVNET 012 randomised trial. Lancet. 2003;362:859–868. doi: 10.1016/S0140-6736(03)14341-3. [DOI] [PubMed] [Google Scholar]
- 3.Stringer JS. Zulu I. Levy J, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: Feasibility and early outcomes. JAMA. 2006;296:782–793. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 4.Jourdain G. Ngo-Giang-Huong N. Le Coeur S, et al. Intrapartum exposure to nevirapine and subsequent maternal responses to nevirapine-based antiretroviral therapy. N Engl J Med. 2004;351:229–240. doi: 10.1056/NEJMoa041305. [DOI] [PubMed] [Google Scholar]
- 5.Lockman S. Shapiro RL. Smeaton LM, et al. Response to antiretroviral therapy after a single, peripartum dose of nevirapine. N Engl J Med. 2007;356:135–147. doi: 10.1056/NEJMoa062876. [DOI] [PubMed] [Google Scholar]
- 6.Pitcher CJ. Quittner C. Peterson DM, et al. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat Med. 1999;5:518–525. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- 7.Sester M. Sester U. Kohler H, et al. Rapid whole blood analysis of virus-specific CD4 and CD8 T cell responses in persistent HIV infection. AIDS. 2000;14:2653–2660. doi: 10.1097/00002030-200012010-00004. [DOI] [PubMed] [Google Scholar]
- 8.Casazza JP. Betts MR. Picker LJ. Koup RA. Decay kinetics of human immunodeficiency virus-specific CD8+ T cells in peripheral blood after initiation of highly active antiretroviral therapy. J Virol. 2001;75:6508–6516. doi: 10.1128/JVI.75.14.6508-6516.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalod M. Dupuis M. Deschemin JC, et al. Broad, intense anti-human immunodeficiency virus (HIV) ex vivo CD8(+) responses in HIV type 1-infected patients: Comparison with anti-Epstein-Barr virus responses and changes during antiretroviral therapy. J Virol. 1999;73:7108–7116. doi: 10.1128/jvi.73.9.7108-7116.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gray CM. Lawrence J. Schapiro JM, et al. Frequency of class I HLA-restricted anti-HIV CD8+ T cells in individuals receiving highly active antiretroviral therapy (HAART) J Immunol. 1999;162:1780–1788. [PubMed] [Google Scholar]
- 11.Kalams SA. Goulder PJ. Shea AK, et al. Levels of human immunodeficiency virus type 1-specific cytotoxic T-lymphocyte effector and memory responses decline after suppression of viremia with highly active antiretroviral therapy. J Virol. 1999;73:6721–6728. doi: 10.1128/jvi.73.8.6721-6728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Markowitz M. Vesanen M. Tenner-Racz K, et al. The effect of commencing combination antiretroviral therapy soon after human immunodeficiency virus type 1 infection on viral replication and antiviral immune responses. J Infect Dis. 1999;179:527–537. doi: 10.1086/314628. [DOI] [PubMed] [Google Scholar]
- 13.Ogg GS. Jin X. Bonhoeffer S, et al. Decay kinetics of human immunodeficiency virus-specific effector cytotoxic T lymphocytes after combination antiretroviral therapy. J Virol. 1999;73:797–800. doi: 10.1128/jvi.73.1.797-800.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuhn L. Meddows-Taylor S. Gray G, et al. Reduced HIV-stimulated T-helper cell reactivity in cord blood with short-course antiretroviral treatment for prevention of maternal-infant transmission. Clin Exp Immunol. 2001;123:443–450. doi: 10.1046/j.1365-2249.2001.01460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuhn L. Meddows-Taylor S. Gray G. Schramm D. Tiemessen CT. HIV-stimulated IL-2 production among exposed-uninfected infants of HIV-infected mothers given nevirapine prophylaxis. 10th Conference on Retroviruses and Opportunistic Infections; Boston, MA. 10–14 February; 2003. [Google Scholar]
- 16.Schramm DB. Kuhn L. Gray GE. Tiemessen CT. In vivo effects of HIV-1 exposure in the presence and absence of single-dose nevirapine on cellular plasma activation markers of infants born to HIV-1-seropositive mothers. J Acquir Immune Defic Syndr. 2006;42:545–553. doi: 10.1097/01.qai.0000225009.30698.ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shalekoff S. Meddows-Taylor S. Schramm DB, et al. Host CCL3L1 gene copy number in relation to HIV-1-specific CD4+ and CD8+ T-cell responses and viral load in South African women. J Acquir Immune Defic Syndr. 2008;48:245–254. doi: 10.1097/QAI.0b013e31816fdc77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuhn L. Schramm DB. Donninger S, et al. African infants' CCL3 gene copies influence perinatal HIV transmission in the absence of maternal nevirapine. AIDS. 2007;21:1753–1761. doi: 10.1097/QAD.0b013e3282ba553a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh KK. Hughes MD. Chen J, et al. Associations of chemokine receptor polymorphisms with HIV-1 mother-to-child transmission in sub-Saharan Africa: Possible modulation of genetic effects by antiretrovirals. J Acquir Immune Defic Syndr. 2008;49:259–265. doi: 10.1097/QAI.0b013e318186eaa4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karpus WJ. Lukacs NW. Kennedy KJ. Smith WS. Hurst SD. Barrett TA. Differential CC chemokine-induced enhancement of T helper cell cytokine production. J Immunol. 1997;158:4129–4136. [PubMed] [Google Scholar]
- 21.Yoshimura T. Matsushima K. Tanaka S, et al. Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc Natl Acad Sci USA. 1987;84:9233–9237. doi: 10.1073/pnas.84.24.9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takata H. Tomiyama H. Fujiwara M. Kobayashi N. Takiguchi M. Cutting edge: Expression of chemokine receptor CXCR1 on human effector CD8+ T cells. J Immunol. 2004;173:2231–2235. doi: 10.4049/jimmunol.173.4.2231. [DOI] [PubMed] [Google Scholar]
- 23.Coovadia A. Hunt G. Abrams EJ, et al. Persistent minority K103N mutations among women exposed to single-dose nevirapine and virologic response to nonnucleoside reverse-transcriptase inhibitor-based therapy. Clin Infect Dis. 2009;48:473–475. doi: 10.1086/596486. [DOI] [PMC free article] [PubMed] [Google Scholar]