Abstract
The ability of HIV to establish a latent infection causes life-long virus persistence, even after long-term highly active antiretroviral therapy (HAART). The role that latency is playing in preventing clearance of the virus infection has become evident in recent years. Patients who have been successfully treated with ART, having undetectable levels of viral RNA (below 50 copies/ml) in the plasma for years, experienced rapid virus rebound on withdrawal of therapy. Activation of latent proviruses from the infected cells in combination with ART is a therapeutic strategy that may lead to the complete elimination of HIV infection. We report here that suberoylanilide hydroxamic acid (SAHA), a histone deacetylase inhibitor that has been approved for the treatment of cutaneous T cell lymphoma (CTCL), can activate an HIV-1 vector provirus in a cell model system. Treatment of cells harboring a latent, HIV-1-derived provirus caused activation of both early and late viral gene expression, acetylation of nucleosome on the 5’ long terminal repeat (LTR), and remodeling of the chromatin at the 5’ LTR. Several compounds, including valproic acid, have been tested for their ability to activate latent HIV-1, but have met with disappointing results. SAHA, a relatively nontoxic, FDA-approved compound, should be considered for developing a strategy to eliminate HIV from patients.
Highly active antiretroviral therapy (HAART) has led to a significant improvement in the care and survival of patients infected with human immunodeficiency virus type 1 (HIV-1). However, even after long-term suppression of viral replication with HAART, the virus rapidly rebounds after therapy is discontinued.1,2 A key contributor to viral rebound appears to be a reservoir of latently infected cells. The half-life of the latently infected population is quite long, and it is estimated that it would take over 60 years of HAART to eliminate this population.3
Chromatin regulation plays an important role in HIV-1 latency. Several LTR-binding factors recruit the histone deacetylase HDAC1 including NF-κB subunit p50, c-Myc, Sp1, YY1, LSF, AP-4, and CBF-1.4–8 HDAC1 deacetylates nucleosome 1 (nuc1) of the HIV-1 LTR and inhibits Tat activation, potentially contributing to the maintenance of HIV-1 latency.9 Among the several compounds that have been identified that have the ability to activate latent reservoirs of HIV-1 are the histone deacetylase (HDAC) inhibitors valproic acid, trapoxin (TPX), and trichostatin A (TSA).10,11 When stimulated by phorbol esters or tumor necrosis factor (TNF-α), nuc1 is acetylated and remodeled, allowing the transcriptional machinery to access the DNA.11,12 Although initial studies treating patients with valproic acid with intensifying HAART suggested that it could be used to deplete the latent HIV-1 reservoir,13 recent studies found that HIV-1 patients who were prescribed valproic acid as an anticonvulsive showed no significant reduction in viral load.14,15
Suberoylanilide hydroxamic acid (SAHA, vorinostat) is an HDAC inhibitor approved for the treatment of cutaneous T cell lymphoma (CTCL) in patients with progressive, persistent, or recurrent disease.16 Moreover, SAHA can inhibit growth in a variety of transformed cells with little to no toxicity.17 We present data here that demonstrate that SAHA can effectively activate latent provirus in a model cell system developed for high-throughput screening of latency activating compounds.18 Furthermore, we demonstrate that SAHA results in an increased acetylation of the LTR histones and remodeling of the nucleosomes. This offers the promise that SAHA may be a potent, relatively nontoxic drug that can assist in the elimination of the latent reservoir in HIV+ patients.
For this study we used two cell lines harboring latent HIV-1 modified vectors. 24STNLESG cells are SupT1 T-lymphoblasts containing a latent HIV-1 vector provirus previously described in detail.18 The latent vector provirus contains insertions of seap, a secretable alkaline phosphatase that can be measured by a sensitive chemiluminescense assay, and egfp, a fluorescent reporter gene, in the env and nef positions, respectively. This configuration makes it possible to monitor early gene expression via GFP and late gene expression via SEAP with safety due to the lack of replication competence. Cells were treated with DMSO alone, TNF-α, SAHA (Cayman Chemical, Ann Arbor, MI), or valproic acid dissolved in DMSO. HeLa cell lines harboring latent HIV were established in a fashion similar to the SupT1-derived 24STNLESG cells.18 More specifically, the HeLa-based line was generated by infecting cells with NLRLucRFP, which is based on the HIV-1 NL4-3 reference strain and has been made replication incompetent by a 2.5-kb deletion of pol and a 1.0-kb deletion of env. The renilla luciferase and red fluorescence protein (RFP) genes were inserted into the env and nef positions, respectively. Pseudotyped virions were generated by cotransfection of packaging plasmids pCMVΔR8.2 and pMD.G (Addgene, MA).
To measure seap reporter gene expression, the Great EscAPe SEAP Reporter System (Clontech, Mountain View, CA) was used. 24STNLESG cells (2 × 105) were treated as indicated for 48 h.
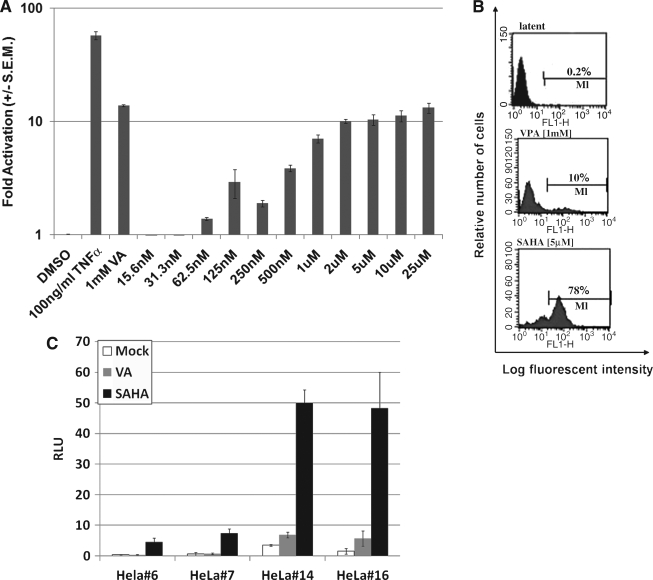
24STNLESG cells (2 × 105) were treated with the indicated amounts of DMSO, TNF-α, valproic acid, or SAHA. Forty-eight hours after treatment, the media were assayed for SEAP activity. In the TNF-α-treated cells, SEAP activity increased 58-fold as compared to cells treated with DMSO alone (Fig. 1A). Addition of valproic acid to a final concentration of 1.0 mM resulted in a 13-fold increase. Concentration-dependant activation of gene expression using SAHA was also observed, reaching a maximum of about 10-fold induction at approximately 2.0 μM.
FIG. 1.
SAHA-induced activation of 24STNLESG cells. (A) 24STNLESG cells (2 × 105) were plated in 24-well plates. Twenty-four hours later they were treated as indicated and assayed for SEAP activity 48 h posttreatment. Experiments were performed in triplicate and the results are presented relative to the values obtained for DMSO treatment. (B) The percentage of reactivated 24STNLSG cells was evaluated by flow cytometry 48 h on SAHA (5.0 μM) or VPA (1.0 mM) treatment. Latent reflects an untreated control. Data from one of two representative experiments are shown. (C) Individual clones of HeLa cells infected with the NLRLucRFP vector were untreated, treated with 1.0 mM valproic acid, or treated with 2.0 μM SAHA, and then assayed for activity. The experiment was performed in triplicate and results are presented as relative light units (RLU) as measured by the luminometer. Results in (A) and (C) are presented as ± SEM.
In addition, we analyzed the percentage of cells activated by counting the number of GFP+ cells by FACS. A total of 1 × 106 cells were washed twice with PBS and resuspended in 1 ml of 0.5% paraformaldehyde in phosphate-buffered saline (PBS) then analyzed by flow cytometry using a FACScan (Becton Dickinson) equipped with Cell Quest (Macintosh, Sunnyvale, CA). We observed activation of gene expression in 78% of the cells when treated with 5.0 μM SAHA as opposed to 10% activation with 1.0 mM valproic acid (Fig. 1B). It is interesting to note that we were able to observe levels of activation with SAHA similar or greater to that of valproic acid, but with three orders of magnitude lower concentration when measured by SEAP or GFP expression.
Finally, we tested the ability of SAHA to activate gene expression from a latent HIV-1-based vector in HeLa cell lines. In uninduced cells, the level of marker gene expression was similar to that of uninfected HeLa cells, indicated a latent phenotype (data not shown). However, in two different clonal cell lines (Fig. 1C), SAHA was able to induce expression of activation marker genes. These data show that the effect of SAHA is neither cell-type dependent nor integration-site dependent, but able to stimulate a general mechanism for activating virus in latently infected cells.
Posttranslational modification of the four core histones, H2A, H2B, H3, and H4, regulates many aspects of chromatin dynamics including transcription. Acetylation of the N-terminal tails of histones H3 and H4 is generally associated with actively transcribed regions of the genome.19 HDACs work to counteract histone acetylation, resulting in hypoacetylated chromatin and lower levels of transcription. Several HDAC inhibitors, including SAHA, are being developed as anticancer drugs due to their ability to kill cells that have lost cell-cycle control.20
To determine the acetylation status of the HIV-1 LTR in 24STNLESG cells, we performed chromatin immunoprecipitation (ChIP) assays. ChIP was performed essentially as described.21,22 24STNLESG cells (1 × 107) were treated for 2 h with either DMSO (0.1%), valproic acid (1.0 mM in DMSO), or SAHA (5.0 μM in DMSO). Then 20.0-μg chromatin aliquots were prepared and used for precipitations with antiacetylated H3 and anti-acetylated H4 antibodies (Millipore, Billerica, MA). For a negative control, no antibody was added. Recovered DNA was quantified relative to input using real-time PCR on an Opticon 2 detection system (Bio-Rad, Hercules, CA) utilizing a TaqMan probe that spans from the 5’ LTR into the gag coding sequence. The sequences of the primers and probe, respectively, are 5’-CCGTCTGTTGTGTGACTCTGGTAA-3’, 5’-GTCGAGAGATCTCCTCTGGCTTTACT-3’, and 5’-FAM-TTCGCTTTCAAGTCCCTGTTCGG-Iowa Black-FQ-3’ (Integrated DNA Technologies, Coralville, IA).
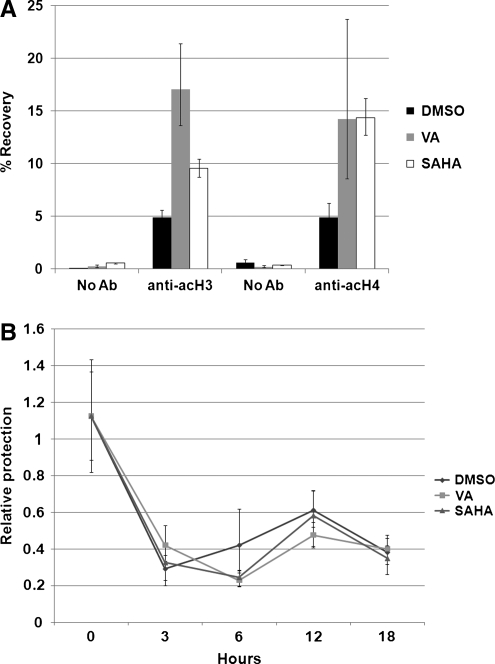
We found using ChIP that we were able to recover 10% and 15% of the DNA from SAHA-treated cells using the anti-acH3 and anti-acH4 antibodies, respectively (Fig. 2A). This is compared to 5% recovery from DMSO-treated cells and the ∼1% from the negative control. These data provide evidence that the HIV-1 5’ LTR becomes hyperacetylated when treated with the HDAC inhibitors valproic acid and SAHA. Again, SAHA was able to achieve effects similar to valproic acid, but at three orders lower magnitude concentration.
FIG. 2.
SAHA causes chromatin acetylation and remodeling at the HIV-1 LTR. (A) 24STNLESG cells (1 × 107) were treated with DMSO, valproic acid (1 mM), or SAHA (5 μM). Chromatin was extracted and precipitated with no antibody, anti-acH3, or anti-acH4. Precipitated DNA was recovered and quantified by real-time PCR performed in triplicate. Results are presents as percentage of DNA recovered relative to the input amount, p < 0.05. (B) 24STNLESG cells (1 × 107) were treated as described. The nuclei were isolated and then treated with 60 U micrococcal nuclease for 5 min. Genomic DNA was recovered and quantified by real-time PCR performed in triplicate, p < 0.05. Results are presented as amount of DNA recovered relative to undigested nuclei, normalized to untreated cells.
Another key component to chromatin-based regulation of transcription is remodeling in which nucleosomes are moved to allow regulators and the transcription machinery to access the DNA. This type of regulation plays a role in transcription from the HIV-1 LTR.23 To determine if treatment with SAHA causes remodeling of the HIV-1 LTR chromatin, we utilized chromatin accessibility real-time PCR (CHART-PCR). CHART-PCR evaluates the accessibility of genomic DNA by comparing the quantity of intact DNA from a nuclease-treated sample to that of an untreated sample.24
CHART-PCR was performed as described.22,24 24STNLESG cells (1 × 107) were treated for 2 h with DMSO (0.1%), valproic acid (1.0 mM in DMSO), or SAHA (5.0 μM in DMSO) for the indicated times. Isolated nuclei were then treated with 60 U micrococcal nuclease for 5 min at room temperature. Genomic DNA was isolated from the nuclei and quantified by real-time PCR using the primers and probe described above. Results are presented relative to untreated nuclei. As shown in Fig. 2B, within 3 h posttreatment with either valproic acid or SAHA, the nuc1 nucleosome is remodeled, reducing the protection from micrococcal nuclease by 65%. It is interesting to note that although DMSO treatment alone can cause nucleosome remodeling of nuc1, it does not lead to histone acetylation (Fig. 2A) or appreciable viral gene expression (Fig. 1). This indicates that histone modification is a key component of HDAC-mediated latent virus reactivation.
Due to the long-lived nature of resting memory T cells, it is anticipated that patients will need to remain on HAART for the remainder of their lives. Unfortunately, long-term HAART has the drawbacks of toxicity, cost, and the potential to generate resistant mutations. New compounds are needed that can effectively activate the virus without globally activating all T cells in order to develop protocols to eliminate the latent arm of the infection. Here, we present data showing that SAHA can effectively activate the latent virus in cell-based systems and induce chromatin changes on the latent HIV-1 LTR. While SAHA can activate viral gene expression at concentrations lower than valproic acid, its efficacy relative to valproic acid, which has met with disappointing results in the clinic, has yet to be established. Moreover, the anti-inflammatory action of SAHA has to be taken into consideration when given to patients infected with HIV.25,26 Although cell culture models have been used to characterize HIV-1 latency, questions remain as to the relevance of these cell lines to latency in patients, in part due to the fact that some models contain mutations in the viral genome. Clearly, cell culture models cannot fully recapitulate the complex and dynamic interplay of factors governing HIV-1 latency in patients. However, they can be utilized as a good first step in determining which compounds may have suitable activity for further studies potentially leading to clinical trials. Given that our ultimate goal is to purge the latent reservoir from infected individuals, SAHA, an FDA-approved chemotherapeutic agent, represents an enticing candidate to consider for further preclinical studies. It is noteworthy that Contreras et al.27 recently reported that SAHA could activate latent virus including virus from patient samples.
Acknowledgment
This work was done with the support of NIH Grant 5R01AI070039.
Disclosure Statement
No competing financial interests exist.
References
- 1.Chun TW. Davey RT., Jr Ostrowski M, et al. Relationship between pre-existing viral reservoirs and the re-emergence of plasma viremia after discontinuation of highly active anti-retroviral therapy. Nat Med. 2000;6:757–761. doi: 10.1038/77481. [DOI] [PubMed] [Google Scholar]
- 2.Davey RT., Jr Bhat N. Yoder C, et al. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc Natl Acad Sci USA. 1999;96:15109–15114. doi: 10.1073/pnas.96.26.15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finzi D. Blankson J. Siliciano JD, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 4.Jiang G. Espeseth A. Hazuda DJ. Margolis DM. c-Myc and Sp1 contribute to proviral latency by recruiting histone deacetylase 1 to the human immunodeficiency virus type 1 promoter. J Virol. 2007;81:10914–10923. doi: 10.1128/JVI.01208-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tyagi M. Karn J. CBF-1 promotes transcriptional silencing during the establishment of HIV-1 latency. EMBO J. 2007;26:4985–4995. doi: 10.1038/sj.emboj.7601928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams SA. Chen LF. Kwon H. Ruiz-Jarabo CM. Verdin E. Greene WC. NF-kappaB p50 promotes HIV latency through HDAC recruitment and repression of transcriptional initiation. EMBO J. 2006;25:139–149. doi: 10.1038/sj.emboj.7600900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coull JJ. Romerio F. Sun JM, et al. The human factors YY1 and LSF repress the human immunodeficiency virus type 1 long terminal repeat via recruitment of histone deacetylase 1. J Virol. 2000;74:6790–6799. doi: 10.1128/jvi.74.15.6790-6799.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imai K. Okamoto T. Transcriptional repression of human immunodeficiency virus type 1 by AP-4. J Biol Chem. 2006;281:12495–12505. doi: 10.1074/jbc.M511773200. [DOI] [PubMed] [Google Scholar]
- 9.He G. Margolis DM. Counterregulation of chromatin deacetylation and histone deacetylase occupancy at the integrated promoter of human immunodeficiency virus type 1 (HIV-1) by the HIV-1 repressor YY1 and HIV-1 activator Tat. Mol Cell Biol. 2002;22:2965–2973. doi: 10.1128/MCB.22.9.2965-2973.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ylisastigui L. Archin NM. Lehrman G. Bosch RJ. Margolis DM. Coaxing HIV-1 from resting CD4 T cells: Histone deacetylase inhibition allows latent viral expression. AIDS. 2004;18:1101–1108. doi: 10.1097/00002030-200405210-00003. [DOI] [PubMed] [Google Scholar]
- 11.Van Lint C. Emiliani S. Ott M. Verdin E. Transcriptional activation and chromatin remodeling of the HIV-1 promoter in response to histone acetylation. EMBO J. 1996;15:1112–1120. [PMC free article] [PubMed] [Google Scholar]
- 12.Verdin E. Paras P., Jr Van Lint C. Chromatin disruption in the promoter of human immunodeficiency virus type 1 during transcriptional activation. EMBO J. 1993;12:3249–3259. doi: 10.1002/j.1460-2075.1993.tb05994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lehrman G. Hogue IB. Palmer S, et al. Depletion of latent HIV-1 infection in vivo: A proof-of-concept study. Lancet. 2005;366:549–555. doi: 10.1016/S0140-6736(05)67098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ances BM. Letendre S. Buzzell M, et al. Valproic acid does not affect markers of human immunodeficiency virus disease progression. J Neurovirol. 2006;12:403–406. doi: 10.1080/13550280600981695. [DOI] [PubMed] [Google Scholar]
- 15.Siliciano JD. Lai J. Callender M, et al. Stability of the latent reservoir for HIV-1 in patients receiving valproic acid. J Infect Dis. 2007;195:833–836. doi: 10.1086/511823. [DOI] [PubMed] [Google Scholar]
- 16.Mann BS. Johnson JR. Cohen MH. Justice R. Pazdur R. FDA approval summary: Vorinostat for treatment of advanced primary cutaneous T-cell lymphoma. Oncologist. 2007;12:1247–1252. doi: 10.1634/theoncologist.12-10-1247. [DOI] [PubMed] [Google Scholar]
- 17.Marks PA. Discovery and development of SAHA as an anticancer agent. Oncogene. 2007;26:1351–1356. doi: 10.1038/sj.onc.1210204. [DOI] [PubMed] [Google Scholar]
- 18.Micheva-Viteva S. Pacchia AL. Ron Y. Peltz SW. Dougherty JP. Human immunodeficiency virus type 1 latency model for high-throughput screening. Antimicrob Agents Chemother. 2005;49:5185–5188. doi: 10.1128/AAC.49.12.5185-5188.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhaumik SR. Smith E. Shilatifard A. Covalent modifications of histones during development and disease pathogenesis. Nat Struct Mol Biol. 2007;14:1008–1016. doi: 10.1038/nsmb1337. [DOI] [PubMed] [Google Scholar]
- 20.Marks PA. Breslow R. Dimethyl sulfoxide to vorinostat: Development of this histone deacetylase inhibitor as an anticancer drug. Nat Biotechnol. 2007;25:84–90. doi: 10.1038/nbt1272. [DOI] [PubMed] [Google Scholar]
- 21.Edelstein LC. Lagos L. Simmons M. Tirumalai H. Gelinas C. NF-kappa B-dependent assembly of an enhanceosome-like complex on the promoter region of apoptosis inhibitor Bfl-1/A1. Mol Cell Biol. 2003;23:2749–2761. doi: 10.1128/MCB.23.8.2749-2761.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edelstein LC. Pan A. Collins T. Chromatin modification and the endothelial-specific activation of the E-selectin gene. J Biol Chem. 2005;280:11192–11202. doi: 10.1074/jbc.M412997200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El Kharroubi A. Piras G. Zensen R. Martin MA. Transcriptional activation of the integrated chromatin-associated human immunodeficiency virus type 1 promoter. Mol Cell Biol. 1998;18:2535–2544. doi: 10.1128/mcb.18.5.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rao S. Procko E. Shannon MF. Chromatin remodeling, measured by a novel real-time polymerase chain reaction assay, across the proximal promoter region of the IL-2 gene. J Immunol. 2001;167:4494–4503. doi: 10.4049/jimmunol.167.8.4494. [DOI] [PubMed] [Google Scholar]
- 25.Leoni F. Zaliani A. Bertolini G, et al. The antitumor histone deacetylase inhibitor suberoylanilide hydroxamic acid exhibits antiinflammatory properties via suppression of cytokines. Proc Natl Acad Sci USA. 2002;99:2995–3000. doi: 10.1073/pnas.052702999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reddy P. Maeda Y. Hotary K, et al. Histone deacetylase inhibitor suberoylanilide hydroxamic acid reduces acute graft-versus-host disease and preserves graft-versus-leukemia effect. Proc Natl Acad Sci USA. 2004;101:3921–3926. doi: 10.1073/pnas.0400380101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Contreras X. Schweneker M. Chen CS, et al. Suberoylanilide hydroxamic acid reactivates HIV from latently infected cells. J Biol Chem. 2009;284:6782–6789. doi: 10.1074/jbc.M807898200. [DOI] [PMC free article] [PubMed] [Google Scholar]